Abstract
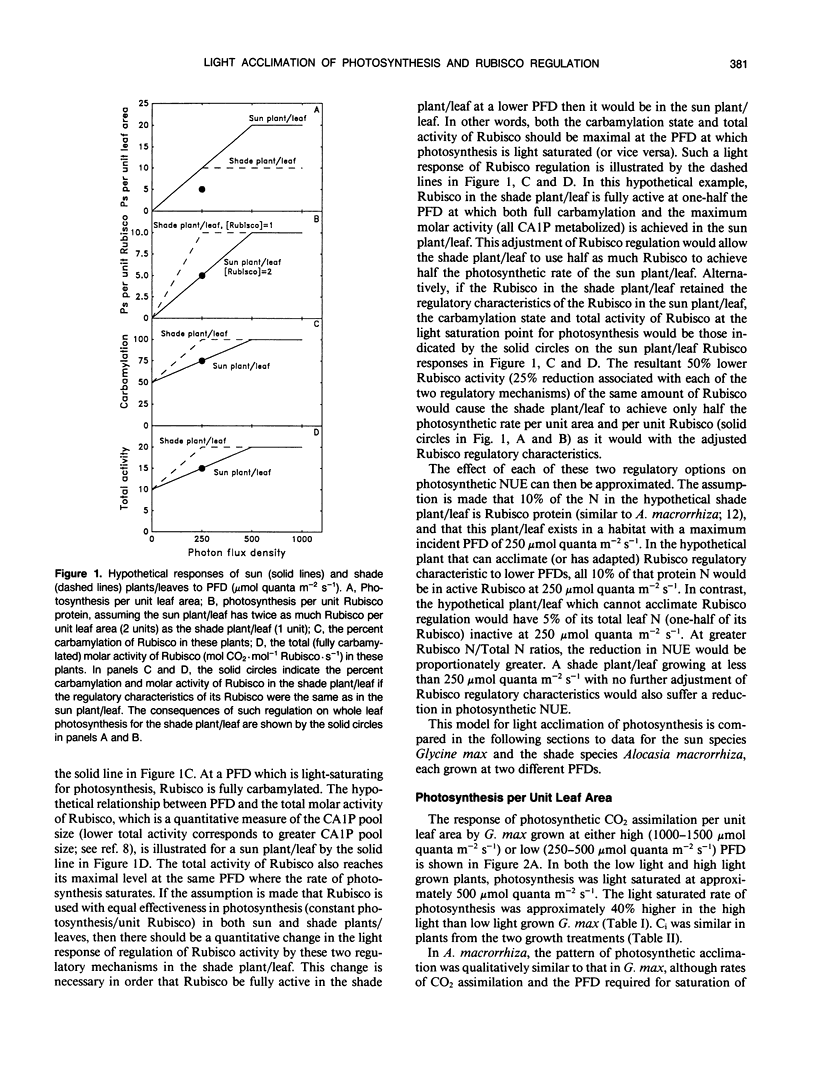
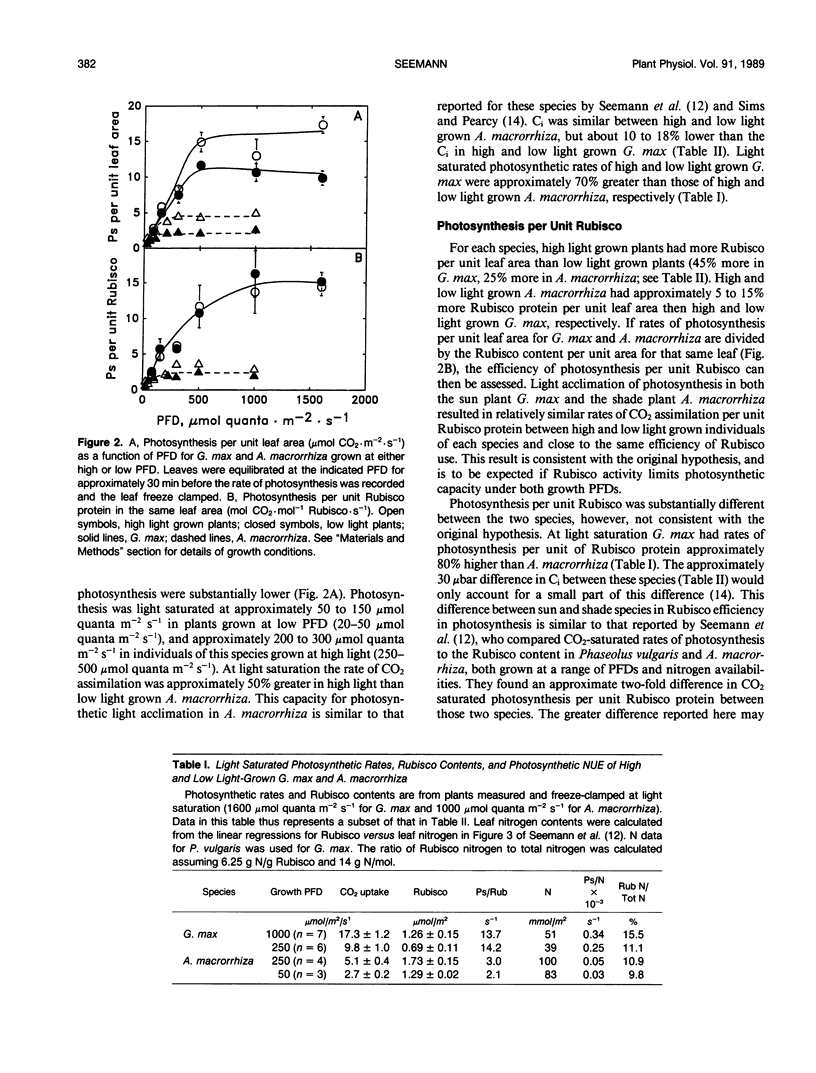
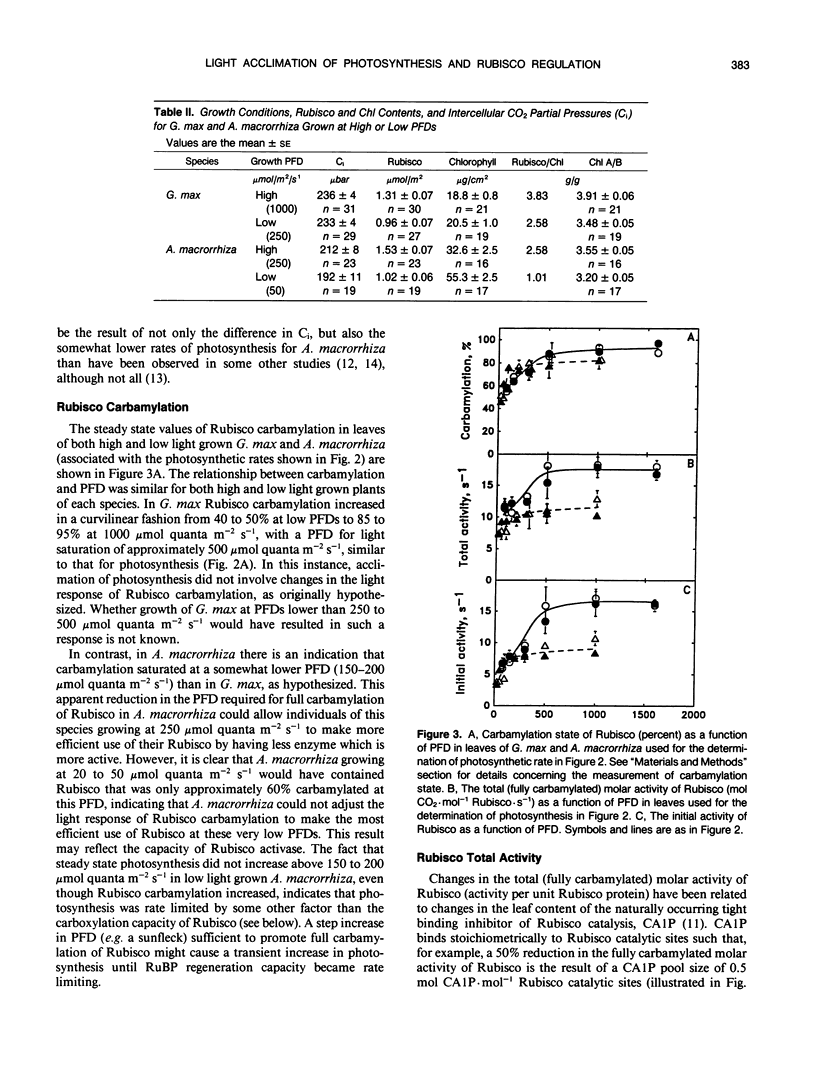
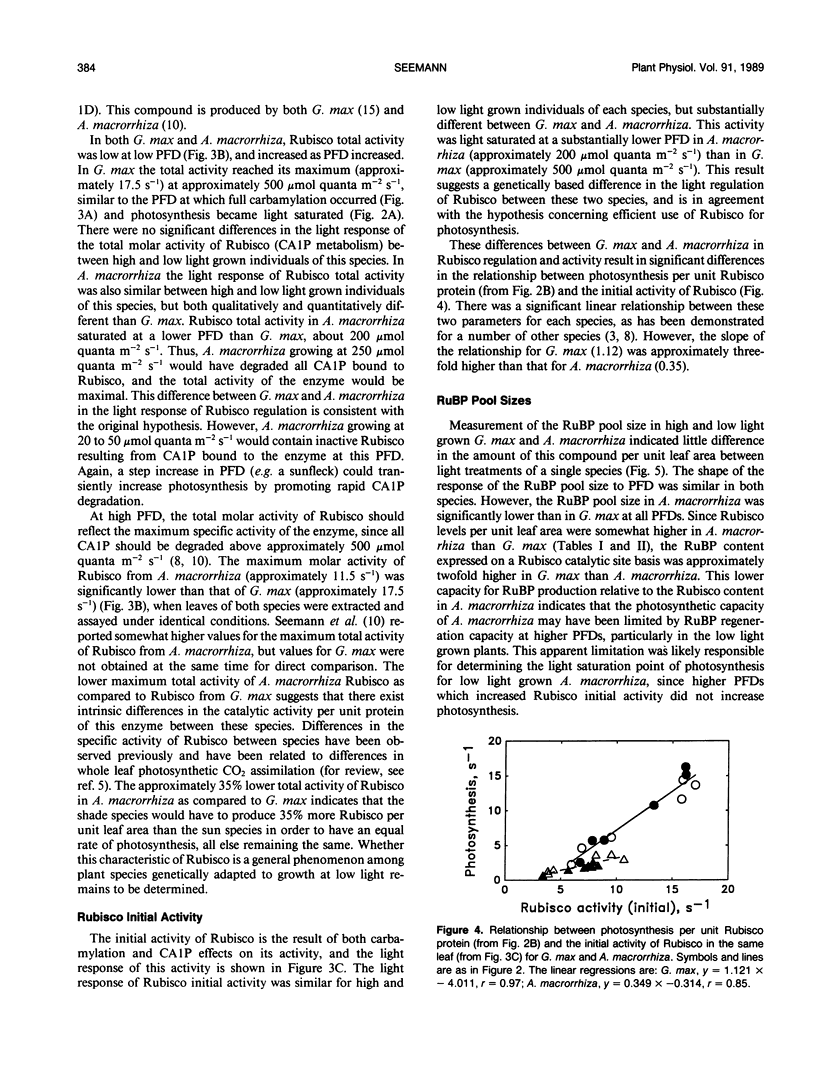
The consequences of light adaptation and acclimation of photosynthesis on photosynthetic nitrogen use efficiency (NUE), particularly as it relates to the efficiency of ribulose-1,5-bisphosphate carboxylase (Rubisco) use in photosynthetic CO2 assimilation, was studied in the sun species Glycine max and the shade species Alocasia macrorrhiza. Both G. max and A. macrorrhiza were found to possess the capacity for light acclimation of CO2 assimilation, but over distinctly different ranges of photon flux density (PFD). For each species, light acclimation of photosynthesis had little effect on the rate of photosynthesis per unit Rubisco protein or the light response of Rubisco carbamylation and CA 1P metabolism. In contrast, photosynthesis per unit Rubisco protein was significantly higher in G. max than in A. macrorrhiza, due in part to a lower total (fully carbamylated) molar activity (activity per unit enzyme) of A. macrorrhiza Rubisco than that of G. max. Comparison of the light response of Rubisco regulatory mechanisms between G. max and A. macrorrhiza indicated some degree of adaptation, such that carbamylation was higher and CA 1P levels lower at lower PFDs in the shade species than the sun species. However, this adjustment was not sufficient for Rubisco in low light grown A. macrorrhiza to be fully active at the growth PFD. Photosynthesis in A. macrorrhiza appeared to become RuBP regeneration-limited at lower PFDs than G. max, and this was probably the determinant of the light saturated rate of photosynthesis in the shade species. The low efficiency of Rubisco use in A. macrorrhiza was a major contributing factor to its five- to sixfold lower photosynthetic NUE than G. max. Shade species such as A. macrorrhiza appear to make far from maximal use of Rubisco protein N.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobza J., Seemann J. R. Mechanisms for light-dependent regulation of ribulose-1,5-bisphosphate carboxylase activity and photosynthesis in intact leaves. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3815–3819. doi: 10.1073/pnas.85.11.3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seemann J. R., Kirschbaum M. U., Sharkey T. D., Pearcy R. W. Regulation of Ribulose-1,5-Bisphosphate Carboxylase Activity in Alocasia macrorrhiza in Response to Step Changes in Irradiance. Plant Physiol. 1988 Sep;88(1):148–152. doi: 10.1104/pp.88.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seemann J. R., Sharkey T. D., Wang J., Osmond C. B. Environmental effects on photosynthesis, nitrogen-use efficiency, and metabolite pools in leaves of sun and shade plants. Plant Physiol. 1987 Jul;84(3):796–802. doi: 10.1104/pp.84.3.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey T. D., Seemann J. R., Pearcy R. W. Contribution of Metabolites of Photosynthesis to Postillumination CO(2) Assimilation in Response to Lightflects. Plant Physiol. 1986 Dec;82(4):1063–1068. doi: 10.1104/pp.82.4.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu J. C., Allen L. H., Bowes G. Dark/Light modulation of ribulose bisphosphate carboxylase activity in plants from different photosynthetic categories. Plant Physiol. 1984 Nov;76(3):843–845. doi: 10.1104/pp.76.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]



