Abstract
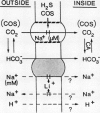
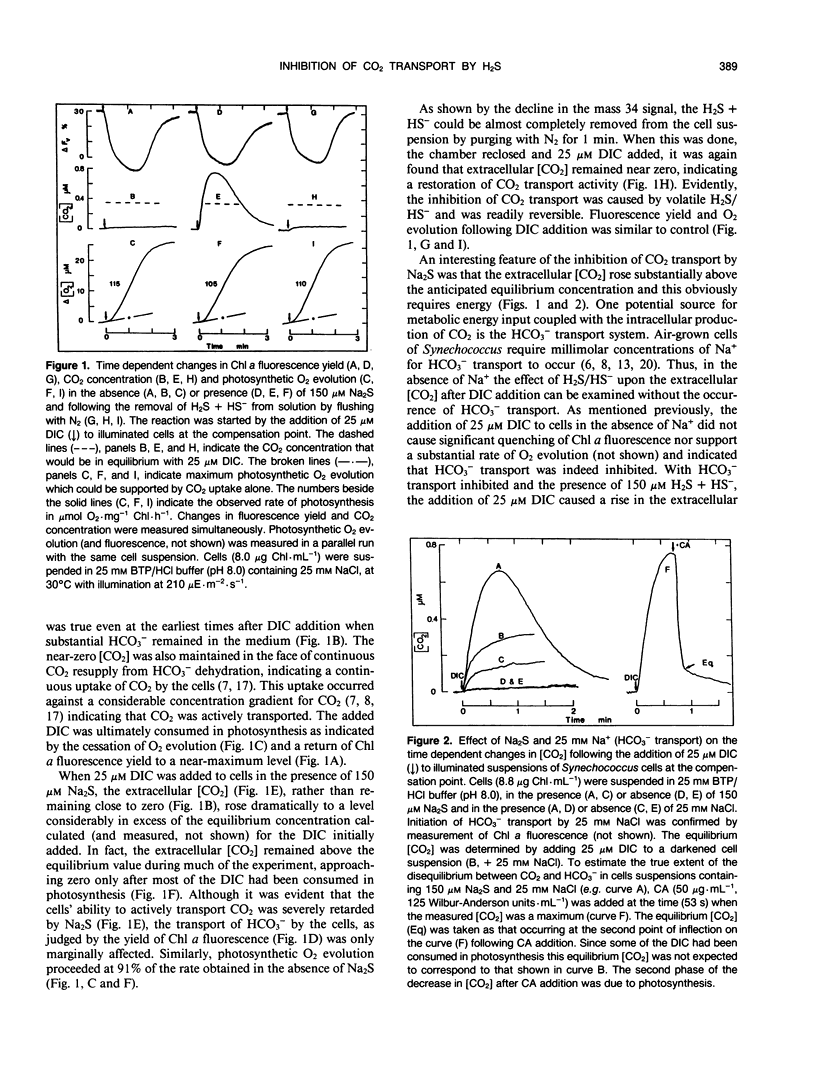
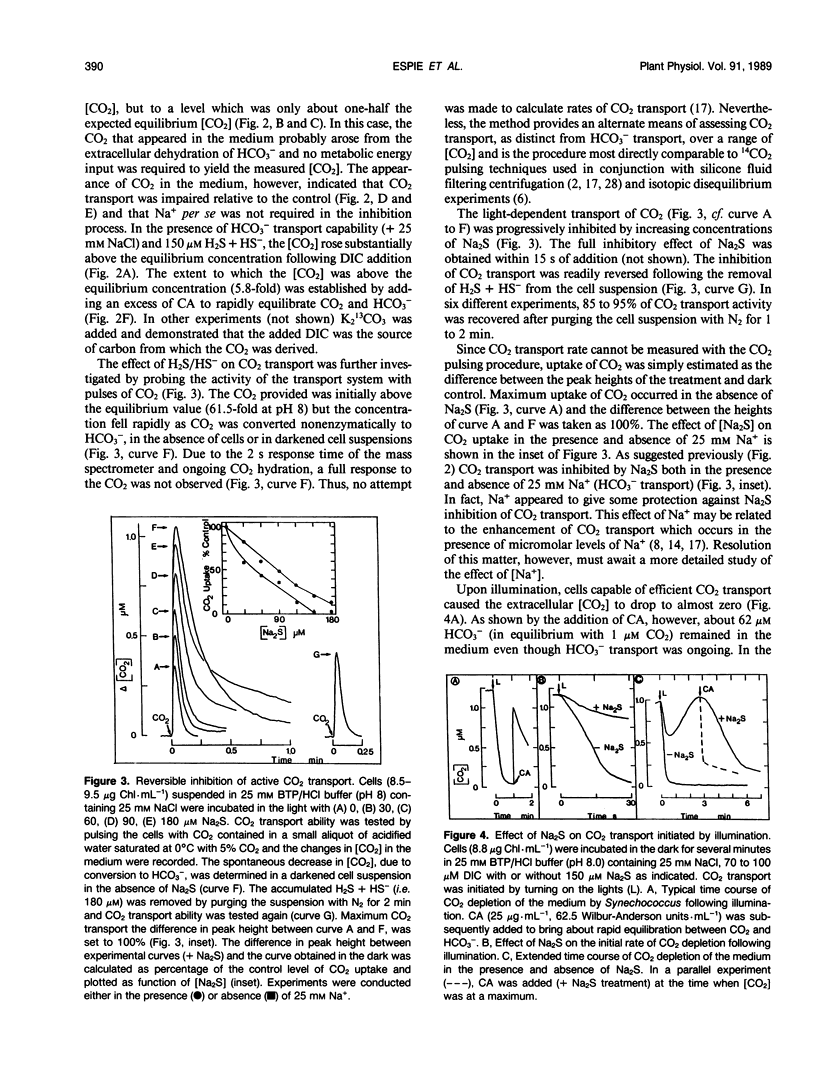
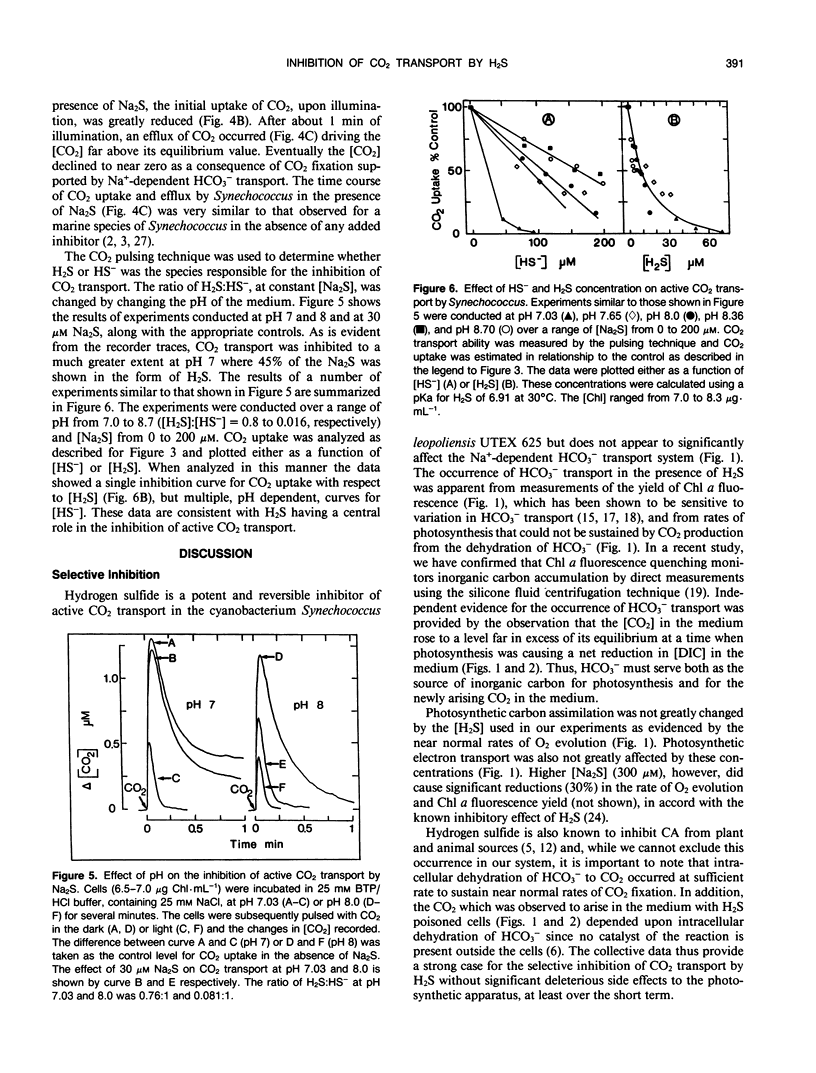
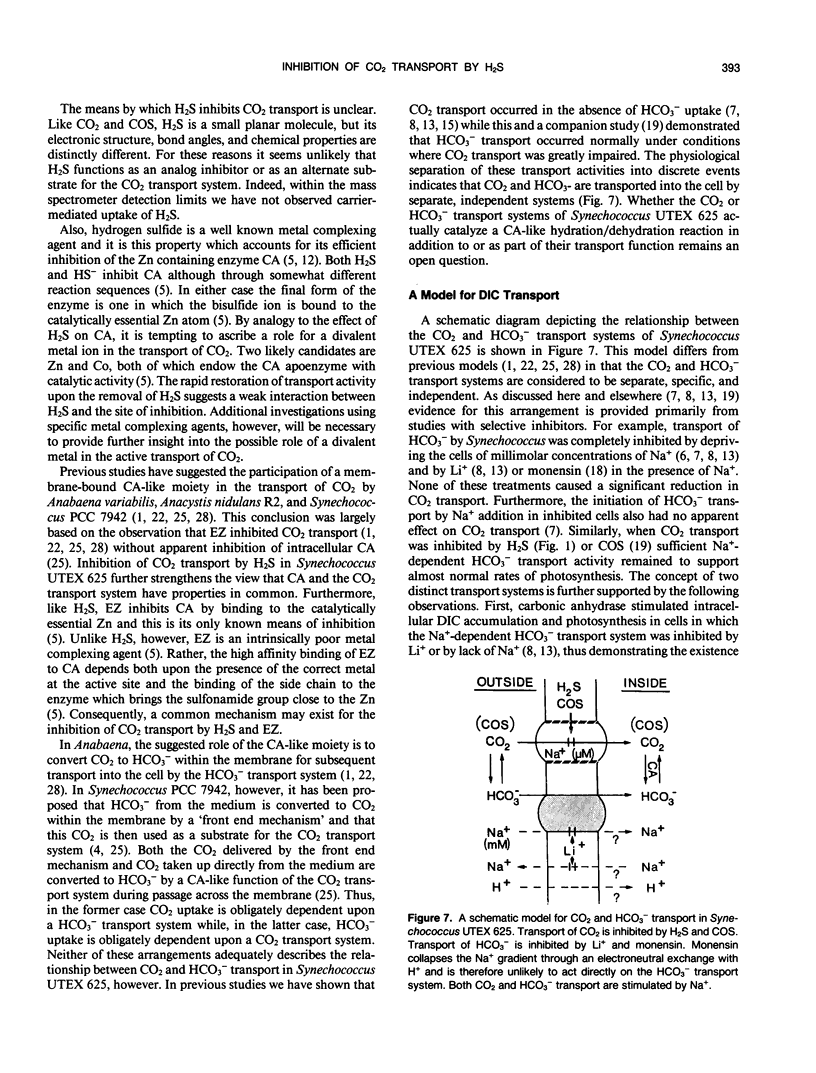
The active transport of CO2 in the cyanobacterium Synechococcus UTEX 625 was inhibited by H2S. Treatment of the cells with up to 150 micromolar H2S + HS− at pH 8.0 had little effect on Na+-dependent HCO3− transport or photosynthetic O2 evolution, but CO2 transport was inhibited by more than 90%. CO2 transport was restored when H2S was removed by flushing with N2. At constant total H2S + HS− concentrations, inhibition of CO2 transport increased as the ratio of H2S to HS− increased, suggesting a direct role for H2S in the inhibitory process. Hydrogen sulfide does not appear to serve as a substrate for transport. In the presence of H2S and Na+ -dependent HCO3− transport, the extracellular CO2 concentration rose considerably above its equilibrium level, but was maintained far below its equilibrium level in the absence of H2S. The inhibition of CO2 transport, therefore, revealed an ongoing leakage from the cells of CO2 which was derived from the intracellular dehydration of HCO3− which itself had been recently transported into the cells. Normally, leaked CO2 is efficiently transported back into the cell by the CO2 transport system, thus maintaining the extracellular CO2 concentration near zero. It is suggested that CO2 transport not only serves as a primary means of inorganic carbon acquisition for photosynthesis but also serves as a means of recovering CO2 lost from the cell. A schematic model describing the relationship between the CO2 and HCO3− transport systems is presented.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badger M. R., Andrews T. J. Photosynthesis and Inorganic Carbon Usage by the Marine Cyanobacterium, Synechococcus sp. Plant Physiol. 1982 Aug;70(2):517–523. doi: 10.1104/pp.70.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badger M. R., Bassett M., Comins H. N. A Model for HCO(3) Accumulation and Photosynthesis in the Cyanobacterium Synechococcus sp: Theoretical Predictions and Experimental Observations. Plant Physiol. 1985 Feb;77(2):465–471. doi: 10.1104/pp.77.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman J. E. Chemical reactions of sulfonamides with carbonic anhydrase. Annu Rev Pharmacol. 1975;15:221–242. doi: 10.1146/annurev.pa.15.040175.001253. [DOI] [PubMed] [Google Scholar]
- Espie G. S., Canvin D. T. Evidence for Na-Independent HCO(3) Uptake by the Cyanobacterium Synechococcus leopoliensis. Plant Physiol. 1987 May;84(1):125–130. doi: 10.1104/pp.84.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espie G. S., Miller A. G., Birch D. G., Canvin D. T. Simultaneous Transport of CO(2) and HCO(3) by the Cyanobacterium Synechococcus UTEX 625. Plant Physiol. 1988 Jul;87(3):551–554. doi: 10.1104/pp.87.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espie G. S., Miller A. G., Canvin D. T. Characterization of the na-requirement in cyanobacterial photosynthesis. Plant Physiol. 1988 Nov;88(3):757–763. doi: 10.1104/pp.88.3.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren T. H., Sanyal G. The activity of sulfonamides and anions against the carbonic anhydrases of animals, plants, and bacteria. Annu Rev Pharmacol Toxicol. 1983;23:439–459. doi: 10.1146/annurev.pa.23.040183.002255. [DOI] [PubMed] [Google Scholar]
- Miller A. G., Canvin D. T. Na-Stimulation of Photosynthesis in the Cyanobacterium Synechococcus UTEX 625 Grown on High Levels of Inorganic Carbon. Plant Physiol. 1987 May;84(1):118–124. doi: 10.1104/pp.84.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. G., Colman B. Active transport and accumulation of bicarbonate by a unicellular cyanobacterium. J Bacteriol. 1980 Sep;143(3):1253–1259. doi: 10.1128/jb.143.3.1253-1259.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. G., Espie G. S., Canvin D. T. Active Transport of CO(2) by the Cyanobacterium Synechococcus UTEX 625 : Measurement by Mass Spectrometry. Plant Physiol. 1988 Mar;86(3):677–683. doi: 10.1104/pp.86.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. G., Espie G. S., Canvin D. T. Chlorophyll a Fluorescence Yield as a Monitor of Both Active CO(2) and HCO(3) Transport by the Cyanobacterium Synechococcus UTEX 625. Plant Physiol. 1988 Mar;86(3):655–658. doi: 10.1104/pp.86.3.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. G., Espie G. S., Canvin D. T. Use of Carbon Oxysulfide, a Structural Analog of CO(2), to Study Active CO(2) Transport in the Cyanobacterium Synechococcus UTEX 625. Plant Physiol. 1989 Jul;90(3):1221–1231. doi: 10.1104/pp.90.3.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. G., Turpin D. H., Canvin D. T. Na+ requirement for growth, photosynthesis, and pH regulation in the alkalotolerant cyanobacterium Synechococcus leopoliensis. J Bacteriol. 1984 Jul;159(1):100–106. doi: 10.1128/jb.159.1.100-106.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T., Kaplan A. The Stoichiometry between CO(2) and H Fluxes Involved in the Transport of Inorganic Carbon in Cyanobacteria. Plant Physiol. 1987 Apr;83(4):888–891. doi: 10.1104/pp.83.4.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T., Togasaki R. K. Carbonyl sulfide: an inhibitor of inorganic carbon transport in cyanobacteria. Plant Physiol. 1988 Nov;88(3):800–804. doi: 10.1104/pp.88.3.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price G. D., Badger M. R. Ethoxyzolamide Inhibition of CO(2) Uptake in the Cyanobacterium Synechococcus PCC7942 without Apparent Inhibition of Internal Carbonic Anhydrase Activity. Plant Physiol. 1989 Jan;89(1):37–43. doi: 10.1104/pp.89.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu C., Spiller H., Wynns G. C., Silverman D. N. Carbonic Anhydrase and the Uptake of Inorganic Carbon by Synechococcus sp. (UTEX-2380). Plant Physiol. 1987 Sep;85(1):72–77. doi: 10.1104/pp.85.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volokita M., Zenvirth D., Kaplan A., Reinhold L. Nature of the Inorganic Carbon Species Actively Taken Up by the Cyanobacterium Anabaena variabilis. Plant Physiol. 1984 Nov;76(3):599–602. doi: 10.1104/pp.76.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]