Abstract
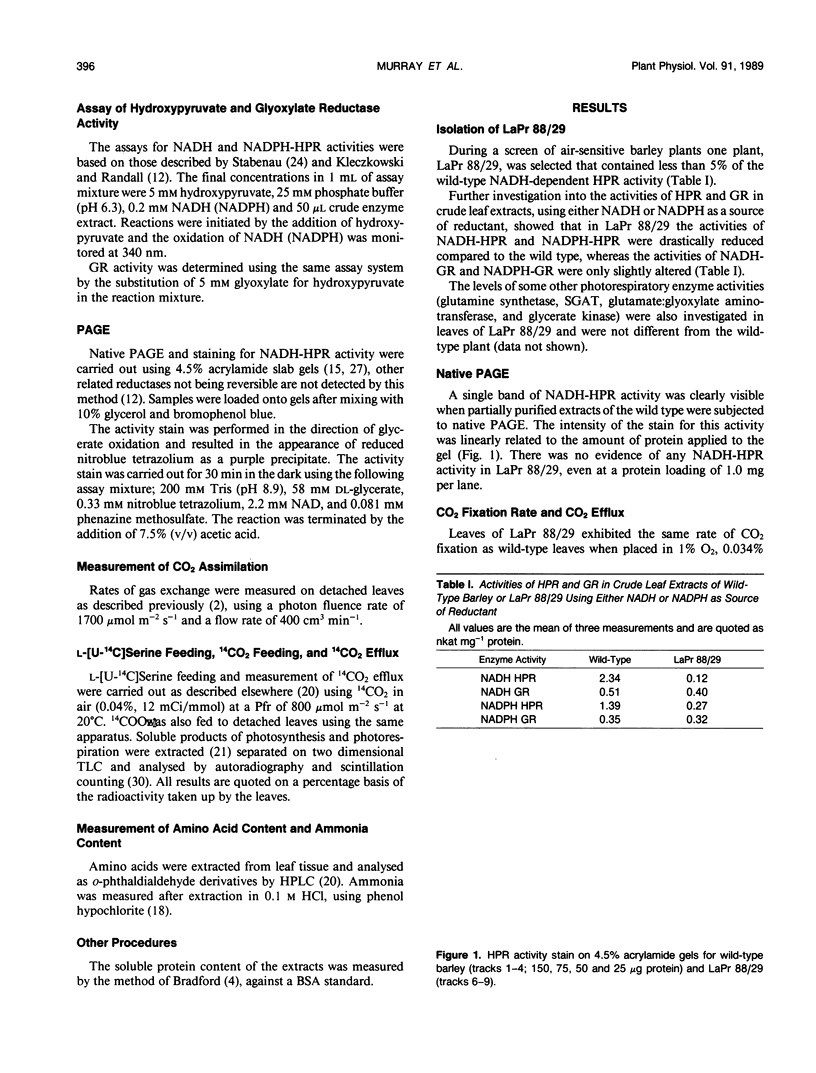
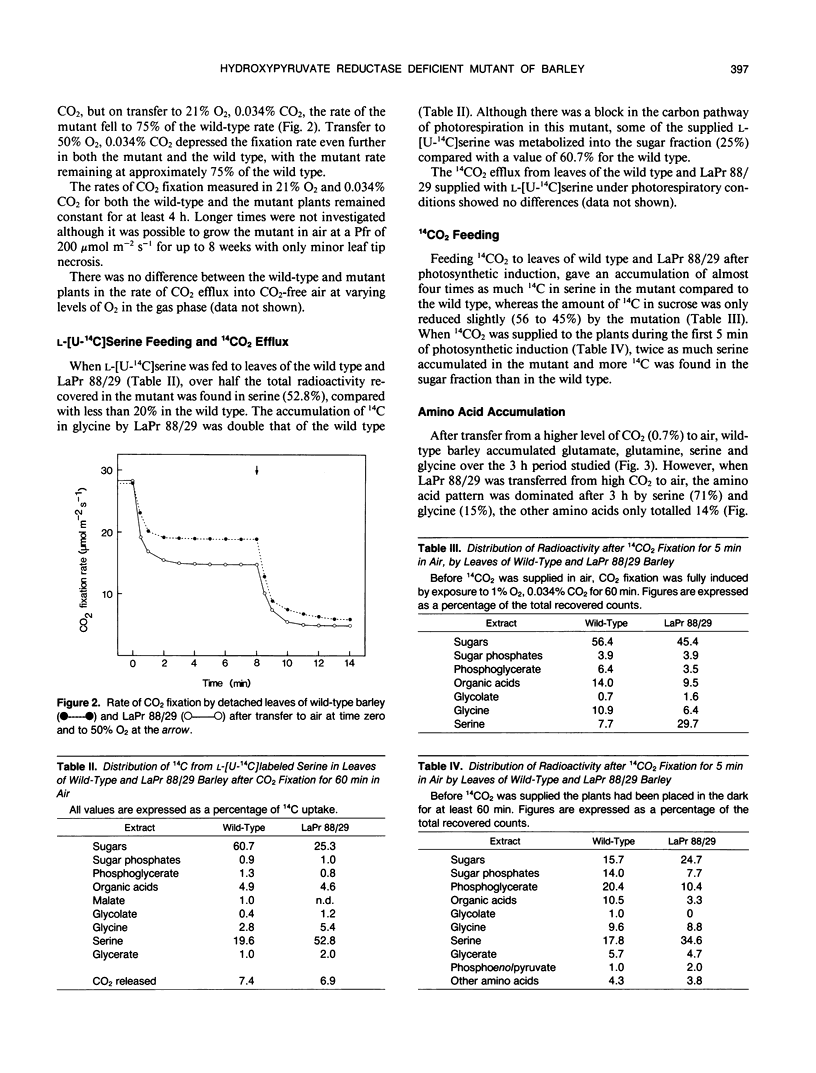
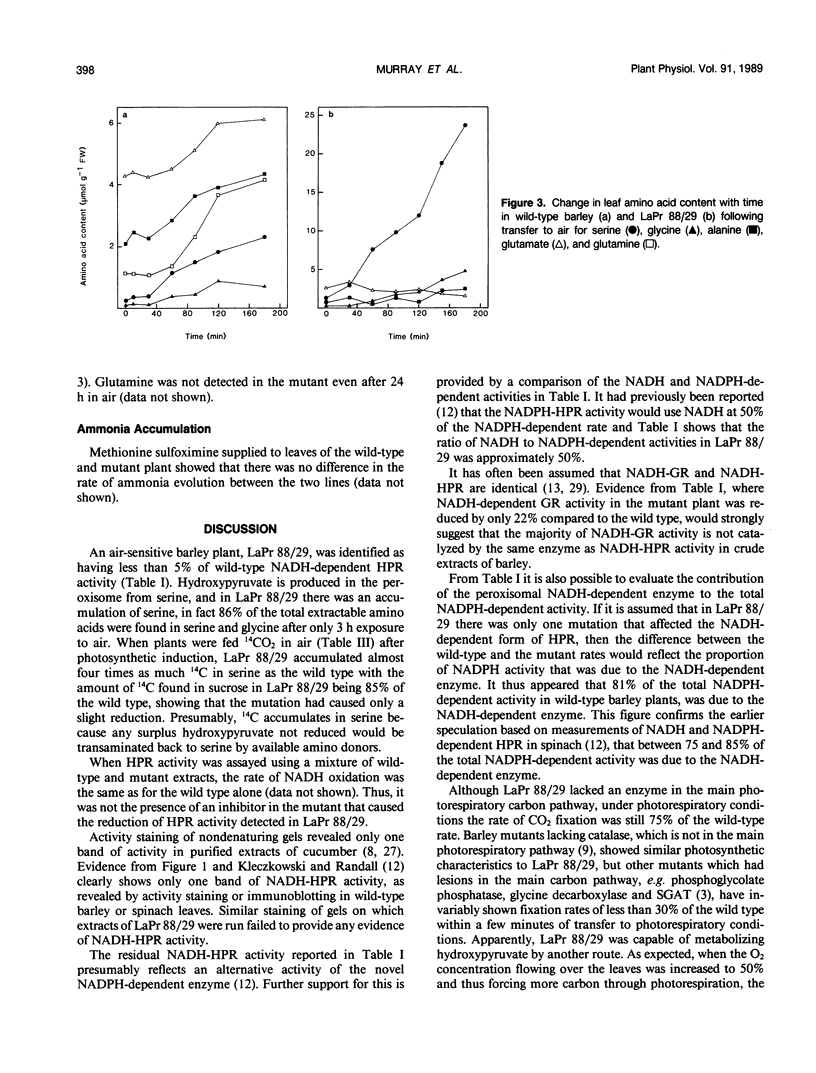
A mutant of barley (Hordeum vulgare L.), LaPr 88/29, deficient in NADH-dependent hydroxypyruvate reductase (HPR) activity has been isolated. The activities of both NADH (5%) and NADPH-dependent (19%) HPR were severely reduced in this mutant compared to the wild type. Although lacking an enzyme in the main carbon pathway of photorespiration, this mutant was capable of CO2 fixation rates equivalent to 75% of that of the wild type, in normal atmospheres and 50% O2. There also appeared to be little disruption to the photorespiratory metabolism as ammonia release, CO2 efflux and 14CO2 release from l-[U-14C]serine feeding were similar in both mutant and wild-type leaves. When leaves of LaPr 88/29 were fed either [14C]serine or 14CO2, the accumulation of radioactivity was in serine and not in hydroxypyruvate, although the mutant was still able to metabolize over 25% of the supplied [14C]serine into sucrose. After 3 hours in air the soluble amino acid pool was almost totally dominated by serine and glycine. LaPr 88/29 has also been used to show that NADH-glyoxylate reductase and NADH-HPR are probably not catalyzed by the same enzyme in barley and that over 80% of the NADPH-dependent HPR activity is due to the NADH-dependent enzyme. We also suggest that the alternative NADPH activity can metabolise a proportion, but not all, of the hydroxypyruvate produced during photorespiration and may thus form a useful backup to the NADH-dependent enzyme under conditions of maximal photorespiration.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Gerbaud A., Andre M. An evaluation of the recycling in measurements of photorespiration. Plant Physiol. 1987 Apr;83(4):933–937. doi: 10.1104/pp.83.4.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Givan C. V., Joy K. W., Kleczkowski L. A. A decade of photorespiratory nitrogen cycling. Trends Biochem Sci. 1988 Nov;13(11):433–437. doi: 10.1016/0968-0004(88)90217-4. [DOI] [PubMed] [Google Scholar]
- Kleczkowski L. A., Givan C. V., Hodgson J. M., Randall D. D. Subcellular Location of NADPH-Dependent Hydroxypyruvate Reductase Activity in Leaf Protoplasts of Pisum sativum L. and Its Role in Photorespiratory Metabolism. Plant Physiol. 1988 Dec;88(4):1182–1185. doi: 10.1104/pp.88.4.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleczkowski L. A., Randall D. D., Blevins D. G. Purification and characterization of a novel NADPH(NADH)-dependent glyoxylate reductase from spinach leaves. Comparison of immunological properties of leaf glyoxylate reductase and hydroxypyruvate reductase. Biochem J. 1986 Nov 1;239(3):653–659. doi: 10.1042/bj2390653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleczkowski L. A., Randall D. D. Purification and characterization of a novel NADPH(NADH)-dependent hydroxypyruvate reductase from spinach leaves. Comparison of immunological properties of leaf hydroxypyruvate reductases. Biochem J. 1988 Feb 15;250(1):145–152. doi: 10.1042/bj2500145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liang Z., Huang A. H. Metabolism of glycolate and glyoxylate in intact spinach leaf peroxisomes. Plant Physiol. 1983 Sep;73(1):147–152. doi: 10.1104/pp.73.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough H. The determination of ammonia in whole blood by a direct colorimetric method. Clin Chim Acta. 1967 Aug;17(2):297–304. doi: 10.1016/0009-8981(67)90133-7. [DOI] [PubMed] [Google Scholar]
- Redgwell R. J. Fractionation of plant extracts using ion-exchange Sephadex. Anal Biochem. 1980 Sep 1;107(1):44–50. doi: 10.1016/0003-2697(80)90489-3. [DOI] [PubMed] [Google Scholar]
- Stafford H. A., Magaldi A. A Developmental Study of D-Glyceric Acid Dehydrogenase. Plant Physiol. 1954 Nov;29(6):504–508. doi: 10.1104/pp.29.6.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titus D. E., Hondred D., Becker W. M. Purification and characterization of hydroxypyruvate reductase from cucumber cotyledons. Plant Physiol. 1983 Jun;72(2):402–408. doi: 10.1104/pp.72.2.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolbert N. E., Yamazaki R. K., Oeser A. Localization and properties of hydroxypyruvate and glyoxylate reductases in spinach leaf particles. J Biol Chem. 1970 Oct 10;245(19):5129–5136. [PubMed] [Google Scholar]



