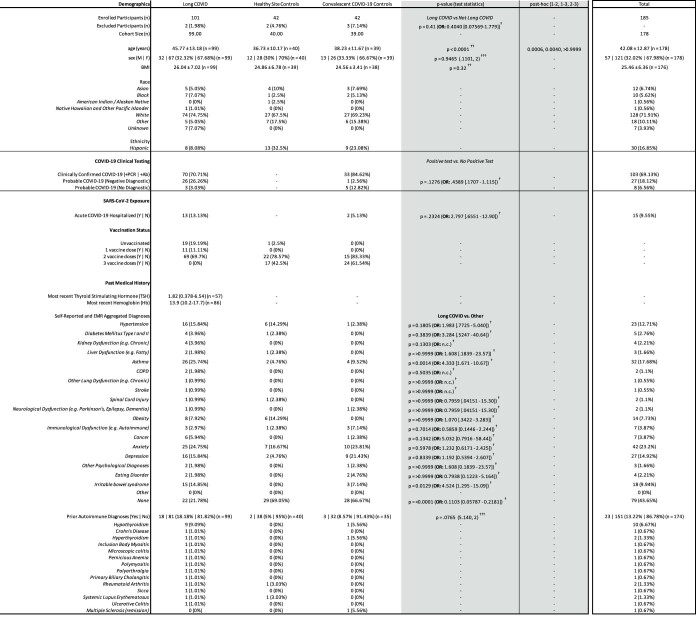
Extended Data Table 1.
Clinical Demographics of MY-LC Cohort
Summary demographic and clinical characteristics for the MY-LC Study. Participants were stratified into three study arms at enrollment: (1) Long COVID (prior SARS-CoV-2 infection with persistent, unexplained symptoms); (2) healthy study site cohort (no prior SARS-CoV-2 infection); or (3) convalescent COVID-19 cohort (prior SARS-CoV-2 infection without persistent symptoms). Various demographic features and clinical characteristics are reported by row for each cohort (row measurement units are specified in parentheses). Within each cell, counts or clinical feature averages are reported, with sample standard deviations, relative cohort percentages, and participant numbers reported where pertinent. Results from statistical tests are reported as p-values and accompanying test statistics: † Chi-square test p-value (Chi-square test statistic, degrees of freedom (df)); †† Kruskal-Wallis ANOVA p-value; ††† Fisher’s exact test p-value (Odd’s Ratio: [95% Confidence Interval (Baptista-Pike)]); ‡ Mann-Whitney U test p-value. Post-hoc comparisons were conducted using Dunn’s test with Tukey’s correction for multiple comparison (column comparison order left-right: 1-2, 1-3, 2-3). Participant medical histories were collected and collated from binary self-reports of prior medical history and review of electronic medical records by study staff (positive responses in either participant self-report or EMR review were considered an overall binary positive response). Abbreviations: n, number; M, male; F, female; BMI, body mass index; +PCR, positive result from SARS-CoV-2 nucleic acid test; +Ab, positive result from SARS-CoV-2 antibody test; Y, Yes; N, No.