Abstract
Elevated plasma numbers of atherogenic apoB-lipoproteins (apoB), mostly as low-density lipoproteins (LDL), predict diabetes risk by unclear mechanisms. Upregulation of the NLRP3 inflammasome/interleukin-1 beta (IL-1β) system in white adipose tissue (WAT) is implicated in type 2 diabetes (T2D); however, metabolic signals that stimulate it remain unexplored. We hypothesized that (1) subjects with high-apoB have higher WAT IL-1β-secretion than subjects with low-apoB, (2) WAT IL-1β-secretion is associated with T2D risk factors, and (3) LDL prime and/or activate the WAT NLRP3 inflammasome. Forty non-diabetic subjects were assessed for T2D risk factors related to systemic and WAT glucose and fat metabolism. Regulation of the NLRP3 inflammasome was explored using LDL without/with the inflammasome’s priming and activation controls (LPS and ATP). LDL induced IL1B-expression and IL-1β-secretion in the presence of ATP in WAT and macrophages. Subjects with high-apoB had higher WAT IL-1β-secretion independently of covariates. The direction of association of LDL-induced WAT IL-1β-secretion to T2D risk factors was consistently pathological in high-apoB subjects only. Adjustment for IL-1β-secretion eliminated the association of plasma apoB with T2D risk factors. In conclusion, subjects with high-apoB have higher WAT IL-1β-secretion that may explain their risk for T2D and may be related to LDL-induced priming of the NLRP3 inflammasome.
ClinicalTrials.gov (NCT04496154): Omega-3 to Reduce Diabetes Risk in Subjects With High Number of Particles That Carry "Bad Cholesterol" in the Blood—Full Text View—ClinicalTrials.gov.
Subject terms: Immunology, Biomarkers, Cardiology, Diseases, Endocrinology, Medical research, Molecular medicine, Pathogenesis, Risk factors, Signs and symptoms
Introduction
While the role of low-density lipoproteins (LDL) in the pathophysiology of cardiovascular disease is well established, their link to type 2 diabetes (T2D) has only been recently recognized. In 2006, we first proposed that high plasma numbers of apoB-lipoproteins (plasma apoB), more than 90% of which are LDL, may be a promotor and not a mere consequence of T2D as it predicted several plasma pro-inflammatory markers including C-reactive protein and IL-61. We further reported that plasma apoB, but not LDL-cholesterol (LDL-C), predicted white adipose tissue (WAT) dysfunction and related risk factors for T2D including elevated glucose-induced insulin secretion (GIIS), insulin resistance (IR), systemic inflammation, and postprandial hypertriglyceridemia independently of sex and adiposity1–6. Moreover, we showed that this may be mediated through an effect on WAT as native LDL inhibited murine2 and human7 adipocyte differentiation and function as well as human WAT function2,5. In line with our findings, epidemiological data emerging since 2007 confirmed that plasma apoB predicts the incidence of diabetes 3–21 years before its onset independently of traditional risk factors including adiposity and glycemia8–11. However, mechanisms linking LDL-particles to WAT-related risk factors for T2D remained unexplored.
Activation of the nucleotide-binding domain leucine-rich repeat containing a pyrin domain 3 (NLRP3) inflammasome and IL-1 beta (IL-1β) secretion in immune cells is protective in host defense to infection. However, its chronic activation has been linked to systemic subclinical inflammation, dysfunction of pancreatic beta cells, WAT and muscle and the promotion of T2D12–16. The activation of the NLRP3 inflammasome requires 2 signals. The first is a priming signal that is reported to act mostly through the nuclear factor-κB (NF-κB) pathway inducing transcriptional-upregulation of NLRP3 and mostly IL1B and an array of inflammatory cytokines including pro-IL-1β. Priming is also believed to be induced by post-translation modification of NLRP3 independent of its transcription17–19. The second signal is an activation signal that leads to the assembly of the NLRP3 inflammasome, activation of pro-caspase-1, cleavage of pro-IL-1β and secretion of IL-1β12,13. A wide range of endogenous danger signals, such as oxidized/modified LDL20,21, cholesterol crystals21,22, glucose23, ceramide15 and palmitate24,25 were described to prime and/or activate the NLRP3 inflammasome in macrophages and β-cells. However, metabolic signals that stimulate the NLRP3 inflammasomes in WAT triggering IL-1β-secretion remain unexplored. Moreover, studies exploring the relation of WAT NLRP3 inflammasome to T2D have focused on the inflammasome priming as NLRP3 and IL1B expressions15,26,27, and little to no evidence is garnered in relation to that of its activation as IL-1β-secretion.
We reported in subjects with obesity that plasma apoB predicts plasma IL-1 receptor antagonist (IL-1Ra)3, which is a marker of systemic activation of the IL-1 system that precedes the onset of diabetes by 10 years28. Statistical adjustment for plasma IL-1Ra eliminated the association of plasma apoB with hyperinsulinemia and IR in this cohort3. This suggested that the upregulation of the NLRP3 inflammasome may be a mechanism linking elevated plasma native LDL to the risk of T2D in humans. Thus, we tested the hypotheses that (1) Compared to subjects with low plasma apoB, subjects with high plasma apoB have higher WAT NLRP3 inflammasome activity indicated by higher WAT IL-1β secretion (primary), (2) WAT IL-1β secretion is associated with risk factors for T2D, and (3) native LDL prime and/or activate the NLRP3 inflammasome in subjects’ own WAT ex vivo.
Materials and methods
Study design, objectives, and population
This work represents baseline data of a clinical trial that was conducted at the Montréal Clinical Research Institute (IRCM). The trial's central hypothesis was that apoB-lipoproteins act as metabolic danger-associated molecular patterns that activate the NLRP3 inflammasome in WAT leading to WAT dysfunction and associated risks for T2D in humans, which can be treated by eicosapentaenoic and docosahexaenoic acids supplementation. The primary objective examined at baseline was to explore whether subjects with high plasma apoB (high-apoB) have higher WAT NLRP3 inflammasome activity indicated by higher WAT IL-1β secretion than subjects with low plasma apoB (low-apoB). The secondary objectives at baseline were to test whether, (1) WAT IL-1β secretion is associated with risk factors for T2D, and (2) native LDL prime and/or activate the NLRP3 inflammasome in subjects’ own WAT ex vivo. Diabetes risk factors measured were WAT dysfunction, systemic inflammation, postprandial hypertriglyceridemia, GIIS and IR.
Sample size was calculated based on the primary hypothesis/objective and was estimated from post-hoc analysis of 7 subjects with similar characteristics, where plasma apoB correlated with WAT IL-1β-secretion over 4-h (r = 0.85, p = 0.025). Using average IL-1β-secretion of 437 ± 225 pg/ml/g and assumed power of 80%, ∝ -value of 0.05 and an attrition rate of 20%, N = 20/group were needed to detect an effect size of 1 SD between subjects with high-apoB versus low-apoB.
Volunteers were recruited by advertisement with a similar criteria to a previous trial by our group29: males and postmenopausal females, BMI > 20 kg/m2, 45–74 years, non-smokers, sedentary, with low/moderate alcohol consumption. Exclusion criteria were elevated cardiovascular risk, chronic disease (i.e. diabetes, inflammatory, autoimmune, hepatic), cancer in the last 3-years, medications affecting metabolism, anemia, abnormal blood coagulation, cholecystectomy, seafood allergy, substance-abuse, sleep-apnea, claustrophobia, and other medical/psychological conditions as judged by the study physicians. Subjects were placed on a 4-week weight-stabilization period verified by weekly weighing (± 2 kg), after which metabolic measures were conducted. The Human Ethics Board of the IRCM approved the research protocol (study number 2013-14). All research was performed in accordance with relevant guidelines/regulations and in accordance with the Declaration of Helsinki. Informed consent was obtained from all participants prior to initiation of any testing. The study was registered retrospectively at ClinicalTrial.org (identifier: NCT04496154) on 03/08/2020. Sample analyses were blinded using subject identification numbers.
Body composition, energy intake and expenditure
Body composition was assessed by dual-energy Xray absorptiometry (GE Healthcare), basal metabolic rate and substrate oxidation by indirect calorimetry (Vmax Encore; Carefusion), and dietary intake using 3-day-food records and the Food Processor software (V11.3.285, ESHA Research)2,4,5,7,29,30.
Insulin sensitivity and secretion
Subjects followed a 3-day high-carbohydrate diet to maximize glycogen stores after which gold-standard Botnia clamps were conducted as published3–5,7,29–31. Briefly, first and second phase GIIS and C-peptide secretion were measured as the AUC of their plasma concentrations during the first 10 min and last 50 min of a 1-h IVGTT, respectively. Insulin sensitivity (IS) was measured during a 3-h hyperinsulinemic euglycemic clamp that followed and expressed as glucose infusion rate divided by steady-state plasma insulin (M/Iclamp). The disposition index (DI) was calculated as 1st phase or total C-peptide secretion multiplied by M/Iclamp.
Postprandial fat metabolism
On a second day of testing, 1–4 weeks after the Botnia clamp, subjects consumed a high-fat meal (600 kcal/m2, 68% fat, 18% carbohydrate) after which postprandial plasma clearance of fat and chylomicrons were measured as AUC6hr of plasma TG and apoB48, respectively. Fasting WAT needle-biopsies were collected under local anesthesia (Xylocaine, AstraZeneca) and washed with antibiotic/antifungal supplemented HBSS buffer. One portion was snap-frozen for WAT mRNA and protein expression, and another immediately used for experiments2,4,5,7,29,30.
WAT IL-1β-secretion
Given the absence of studies evaluating the regulation of human WAT NLRP3 inflammasome/IL-1β secretion, pilot kinetic studies were first conducted to establish positive controls for the WAT inflammasome of 4 subjects using LPS as a priming control (Sigma-Aldrich L4591, 0–30 μg/ml, 5 min–24 h) and ATP as an activation control (Sigma-Aldrich A2383, 0–30 mmol/L, 1–20 h) as standard in macrophages13. Interleukin-1β secretion from these subjects was not used in data analysis. Minimal concentrations and durations of LPS and ATP that induced maximal WAT IL-1β-secretion were set as positive controls, which corresponded to 0.3 μg/ml LPS for 4 h followed by 3 mmol/L ATP for 3 h (Supplemental Figure 1). Native LDL was isolated from fasting blood samples collected during the Botnia clamp by sequential ultracentrifugation in 0.01% EDTA, sterilized, and used within 4-weeks2,5,29. LDL concentration of 1.2 g/L apoB was used, which corresponds to that used to induce human WAT dysfunction2,5, the 75th percentile in Canadians32, and the average plasma levels in a similar cohort of high-apoB1,2,29,33. To assess WAT IL-1β-secretion, WAT samples were incubated for 4 h with medium alone or supplemented with LDL or LPS as priming signals. Medium was removed and WAT was washed and re-incubated for 3 h with medium alone or supplemented with LDL or ATP as activation signals. Medium accumulation of IL-1β was then quantified (termed WAT IL-1β-secretion). All WAT experiments were run in 5% FBS DMEM medium (Gibco/Thermo Fisher) using 5–10 mg WAT/well, 4 wells/condition2,5,7. Cell death was verified by a lactate dehydrogenase (LDH) kit (Invitrogen-ThermoFisher Scientific).
Experiments in human monocyte-derived-macrophages (hMDM) and differentiated THP-1 macrophages
Blood mononuclear cells (PBMCs) were isolated using Ficoll-Paque Plus (Sigma Aldrich), plated (1 × 106 cells/mL in 24-well plates) and incubated for 2-h with RPMI-1640 medium supplemented with 10% heat-inactivated FBS (Wisent), 1% of penicillin–streptomycin and 2 mM L-Glutamine (Gibco/Thermo Fisher) (baseline medium). Medium was removed and adherent monocytes were differentiated for 6-days in fresh baseline medium with 50 ng/mL macrophage colony-stimulating factor (MCSF, Biolegend)34. THP-1 cells (ATCC) were cultured in baseline medium with 0.05 mM 2-mercaptoethanol, and medium was replaced every 2–3 days keeping cell-density between 1 × 105 and 8 × 105. Cells were differentiated for 48-h between passages 15–25 and plated (4 × 105 cells/mL) in baseline medium with 100 nM Phorbol 12-Myristate 13-Acetate (Sigma Aldrich). Macrophages were rested in medium for 48-h before being used35. Macrophages were incubated with LDL or LPS (10 ng/mL34) and gene expression and IL-1β-secretion were quantified or re-incubated with ATP (3 mmol/L for 3 h) and IL-1β-secretion was quantified.
mRNA and protein expressions
Gene expression in WAT and macrophages were analyzed by RT-PCR using RotorGene Q (Qiagen) with HPRT as an internal standard. WAT proteins were extracted in RIPA buffer and quantified by western blot using an internal control made from pooled WAT from 5 subjects7,30. mRNA expression in macrophages was calculated relative to their respective control using the 2−ΔΔCT method. The list of primers and antibodies were previously published7,30.
Plasma and WAT metabolic parameters
Plasma lipids, apoB and apoA1 were analyzed by an automated analyzer (Cobas Integra 400, Roche Diagnostics), glucose by YSI (2300 STAT Plus), apoB48 and PCSK9 (proprotein convertase subtilisin/ kexin type 9) by ELISA kits (BioVendor and CircuLex MBL International), insulin and C-peptide by RIA kits (Millipore Corporation) and IL-1Ra and IL-1β by alpha-LISA kits (Perkin Elmer Canada) and plasma phospholipid-fatty acid composition (PL-FA) by gas-chromatography/mass spectrometry2,4,5,7,29–31.
Statistical analysis
Baseline data in Tables 1 and 2 are presented as mean ± SD and compared by unpaired t-test. Data with large intersubject-variability were LOG10-transformed before being used. Non-parametric Friedman or Wilcoxon rank sum tests were used when data could not be transformed (negative or zero values). As WAT and/or LDL from some subjects were insufficient to complete all experiments, mixed-model analyses were used with interaction and Geisser-Greenhouse correction and with controlling for false-discovery rate. When interaction was significant, inter or intra-subject differences were further analyzed. Pearson correlation was used to examine the association between variables in the low-apoB and high-apoB groups separately and data was pooled when no group-differences in the regression lines existed. Partial correlation was used to adjust the association of plasma apoB with diabetes risk factors for covariates. Analysis was performed using SPSS (V26) and GraphPad Prism (V 9.4) with significance set at p < 0.05.
Table 1.
Anthropometric and metabolic parameters of the study population.
| Females (N = 27) | Males (N = 13) | p value | |
|---|---|---|---|
| Anthropometric parameters | |||
| Age (years) | 58.7 ± 7.3 | 56.0 ± 9.4 | 0.326 |
| Weight (kg) | 76.8 ± 14.5 | 94.1 ± 20.7 | 0.004 |
| BMI (kg/m2) | 30.1 ± 5.5 | 30.4 ± 6.1 | 0.876 |
| Waist circumference (cm)a | 95.4 ± 14.8 | 105.6 ± 15.3 | 0.051 |
| Hip circumference (cm) | 108.3 ± 15.8 | 108.5 ± 14.1 | 0.961 |
| Fat mass (kg) | 32.9 ± 10.4 | 29.6 ± 13.1 | 0.395 |
| Android fat mass (kg) | 2.96 ± 1.24 | 3.06 ± 1.60 | 0.839 |
| Gynoid fat mass (kg) | 5.53 ± 1.56 | 4.44 ± 2.25 | 0.082 |
| Android/gynoid ratio | 0.52 ± 0.13 | 0.68 ± 0.15 | 0.001 |
| Fasting metabolic parameters | |||
| SBP (mm Hg) | 121 ± 14 | 122 ± 9 | 0.775 |
| DBP (mm Hg) | 75 ± 8 | 75 ± 7 | 0.879 |
| Plasma apoB (g/L) | 1.03 ± 0.21 | 1.05 ± 0.30 | 0.797 |
| Plasma apoB48 (mg/L) | 6.07 ± 2.74 | 7.39 ± 4.19 | 0.242 |
| Plasma apoA-I (g/L) | 1.72 ± 0.23 | 1.43 ± 0.20 | < 0.001 |
| Plasma cholesterol (mmol/L) | 5.35 ± 0.81 | 4.89 ± 0.90 | 0.111 |
| Plasma LDL-C (mmol/L) | 3.18 ± 0.74 | 3.06 ± 0.77 | 0.649 |
| Plasma HDL-C (mmol/L) | 1.66 ± 0.35 | 1.13 ± 0.26 | < 0.001 |
| Plasma TG (mmol/L) | 1.11 ± 0.47 | 1.51 ± 0.93 | 0.075 |
| Plasma NEFA (mmol/L) | 0.633 ± 0.211 | 0.494 ± 0.244 | 0.070 |
| Plasma PCSK9 (ng/mL) | 227.7 ± 74.4 | 181.7 ± 56.6 | 0.056 |
| Plasma apoB/PCSK9 ratio (mg/µg) | 4.90 ± 1.64 | 5.94 ± 1.56 | 0.065 |
| Plasma IL-1Ra (pg/ml) | 473 ± 357 | 334 ± 189 | 0.198 |
| Energy intake and expenditure | |||
| Daily energy intake (kcal/day)b | 2022 ± 426 | 2689 ± 505 | < 0.001 |
| % fat intakeb | 37.3 ± 4.9 | 31.2 ± 6.5 | 0.003 |
| % saturated fat intakeb | 12.2 ± 3.5 | 10.3 ± 4.8 | 0.188 |
| Cholesterol intake (mg/day)a | 310 ± 144 | 313 ± 162 | 0.960 |
| % carbohydrate intakeb | 46.3 ± 4.9 | 50.9 ± 10.2 | 0.069 |
| Fiber intake (g/day)b | 21.4 ± 9.4 | 33.0 ± 13.1 | 0.004 |
| % protein intakeb | 16.5 ± 2.9 | 16.3 ± 3.4 | 0.861 |
| % alcohol intakeb | 1.12 ± 1.9 | 3.51 ± 4.1 | 0.019 |
| Basal metabolic rate (kcal/day) | 1337 ± 204 | 1755 ± 322 | < 0.001 |
| % carbohydrate oxidation | 16.3 ± 12.6 | 12.5 ± 14.6 | 0.423 |
| % fat oxidation | 63.1 ± 13.8 | 71.8 ± 13.7 | 0.082 |
| Botnia clamp measures | |||
| Fasting plasma glucose (mmol/L) | 5.11 ± 0.62 | 4.94 ± 0.31 | 0.329 |
| Fasting plasma insulin (µU/mL)c | 11.2 ± 7.7 | 12.9 ± 9.0 | 0.535 |
| Fasting plasma C-peptide (ng/ml)c | 1.76 ± 0.92 | 1.67 ± 0.73 | 0.768 |
| HOMA-IR (mmol/L) × (µU/mL)c | 2.64 ± 2.37 | 2.90 ± 2.31 | 0.752 |
| 1st phase GIISIVGTT (µU/mL)d | 458 ± 380 | 566 ± 301 | 0.407 |
| 2nd phase GIISIVGTT (µU/mL)c | 1703 ± 1529 | 2268 ± 1943 | 0.334 |
| Total GIISIVGTT (µU/mL)d | 2167 ± 1863 | 2628 ± 2121 | 0.510 |
| 1st phase C-peptideIVGTT (ng/mL)d | 34.1 ± 17.5 | 38.3 ± 10.5 | 0.466 |
| 2nd phase C-peptideIVGTT (ng/mL)d | 209 ± 112 | 208 ± 70 | 0.986 |
| Total C-peptideIVGTT (ng/mL)d | 243 ± 128 | 246 ± 79 | 0.933 |
| M/Iclamp (mg/kg*min)/(µU/ml))c | 0.092 ± 0.048 | 0.097 ± 0.046 | 0.756 |
| Fasting WAT IL-1β-secretion and postprandial plasma fat clearance | |||
| Baseline WAT IL-1β-secretion (pg/mg)e | 0.022 ± 0.028 | 0.012 ± 0.008 | 0.220 |
| LPS/ATP-induced WAT IL-1β-secretion (pg/mg)e | 9.01 ± 4.75 | 14.29 ± 20.06 | 0.235 |
| AUC6h plasma TG (mmol/L) | 10.9 ± 3.9 | 16.6 ± 9.1 | 0.008 |
| AUC6h plasma apoB48 (mg/L) | 66.5 ± 23.0 | 77.3 ± 37.3 | 0.267 |
Significant values are in bold.
Data are presented as mean ± SD and analyzed using unpaired Student's t test.
aFor N = 26 females.
bFor N = 25 females and N = 12 males.
cFor N = 12 males.
dFor N = 11 males.
eFor N = 23 females and N = 11 males for missing data.
Table 2.
Anthropometric and metabolic parameters of subjects with low and high plasma apoB.
| Low-apoB | High-apoB | p value | |
|---|---|---|---|
| Females: males | 13:7 | 14:6 | |
| Plasma apoB (g/L) | 0.84 ± 0.14 | 1.23 ± 0.15 | < 0.001 |
| Anthropometric parameters | |||
| Age (years) | 56.4 ± 7.5 | 59.3 ± 8.5 | 0.268 |
| Weight (kg) | 79.3 ± 12.6 | 85.6 ± 22.7 | 0.279 |
| BMI (kg/m2) | 28.7 ± 3.6 | 31.6 ± 7.0 | 0.112 |
| Waist circumference (cm)a | 97.0 ± 9.6 | 100.7 ± 20.1 | 0.460 |
| Hip circumference (cm) | 106.9 ± 6.6 | 109.8 ± 20.5 | 0.558 |
| Fat mass (kg) | 28.8 ± 7.4 | 34.8 ± 13.7 | 0.093 |
| Android fat mass (kg) | 2.59 ± 0.79 | 3.40 ± 1.66 | 0.058 |
| Gynoid fat mass (g) | 4.77 ± 1.29 | 5.57 ± 2.26 | 0.176 |
| Android/gynoid (g/g) | 0.55 ± 0.15 | 0.59 ± 0.16 | 0.430 |
| Fasting metabolic parameters | |||
| SBP (mm Hg) | 119 ± 9 | 124 ± 14 | 0.218 |
| DBP (mm Hg) | 75 ± 8 | 76 ± 8 | 0.741 |
| Plasma apoB48 (mg/L) | 5.71 ± 3.28 | 7.29 ± 3.17 | 0.128 |
| Plasma apoA-I (g/L) | 1.57 ± 0.22 | 1.68 ± 0.28 | 0.168 |
| Plasma cholesterol (mmol/L) | 4.61 ± 0.55 | 5.78 ± 0.69 | < 0.001 |
| Plasma LDL-C (mmol/L) | 2.60 ± 0.44 | 3.68 ± 0.56 | < 0.001 |
| Plasma HDL-C (mmol/L) | 1.56 ± 0.43 | 1.42 ± 0.38 | 0.271 |
| Plasma TG (mmol/L) | 0.99 ± 0.50 | 1.50 ± 0.72 | 0.013 |
| Plasma NEFA (mmol/L) | 0.599 ± 0.230 | 0.577 ± 0.233 | 0.769 |
| Plasma PCSK9 (ng/mL) | 212 ± 84 | 214 ± 59 | 0.944 |
| Plasma apoB/PCSK9 (mg/µg) | 4.42 ± 1.48 | 6.06 ± 1.45 | 0.001 |
| Plasma LDL-C/apoB (mmol/g) | 3.11 ± 0.07 | 3.00 ± 0.08 | 0.341 |
| Fasting plasma glucose (mmol/L) | 5.09 ± 0.68 | 5.03 ± 0.38 | 0.741 |
| Fasting plasma insulin (µU/mL)b | 11.3 ± 8.3 | 12.1 ± 8.0 | 0.745 |
| Fasting plasma C-peptide (ng/ml)b | 1.58 ± 0.90 | 1.87 ± 0.80 | 0.285 |
| HOMA-IR (mmol/L) × (µU/mL)b | 2.70 ± 2.70 | 2.74 ± 1.97 | 0.957 |
| Energy intake and expenditure | |||
| Daily energy intake (kcal/day)c | 2161 ± 560 | 2312 ± 539 | 0.410 |
| % fat intakec | 32.3 ± 5.0 | 38.2 ± 5.8 | 0.002 |
| % saturated fat intakec | 10.1 ± 2.9 | 13.0 ± 4.4 | 0.027 |
| Cholesterol intake (mg/day)c | 266 ± 137 | 354 ± 149 | 0.070 |
| % carbohydrate intakec | 51.7 ± 6.9 | 44.0 ± 5.5 | 0.001 |
| Fiber intake (g/day)c | 25.5 ± 12.9 | 24.9 ± 11.1 | 0.883 |
| % protein intakec | 16.2 ± 2.2 | 16.7 ± 3.7 | 0.616 |
| % alcohol intakec | 1.46 ± 1.87 | 2.31 ± 3.70 | 0.392 |
| Basal metabolic rate (kcal/day) | 1418 ± 260 | 1528 ± 359 | 0.274 |
| % carbohydrate oxidation | 18.4 ± 14.0 | 12.3 ± 12.1 | 0.165 |
| % fat oxidation | 62.0 ± 13.9 | 69.2 ± 13.9 | 0.126 |
Significant values are in bold.
Data are presented as mean ± SD. Data analyzed using unpaired Student's t test.
aFor N = 13 females in the high-apoB.
bFor N = 6 males in the low-apoB.
cFor N = 12 females and N = 6 males in the low-apoB and N = 13 females in the high-apoB groups for missing data.
Ethics approval and consent to participate
Subjects signed an informed consent form approved by the IRCM Human Ethics Board prior to participation in the study, which was conducted in accordance with the Declaration of Helsinki.
Consent for publication
Not applicable.
Results
This report represents the baseline data of a 3-month clinical trial with omega-3 fatty acid supplementation. Subject screening was completed between 2013 and 2019, during which 930 subjects were screened and 27 females and 14 males who met the inclusion/exclusion criteria were enrolled. Common exclusion criteria were related to lack time to participate in all the study, high level of physical activity and plasma metabolic measures outside the inclusion criteria. One man with unreported sleep-apnea was excluded at baseline for safety. The baseline characteristics of the 40 subjects included in this analysis are presented in Table 1. Males had higher weight and central obesity, basal metabolic rate, and energy, fiber and alcohol intake but lower fat intake. Males also had lower fasting plasma HDL-C and apoA1 and delayed postprandial plasma TG clearance.
Native LDL upregulate IL-1β-secretion from human WAT
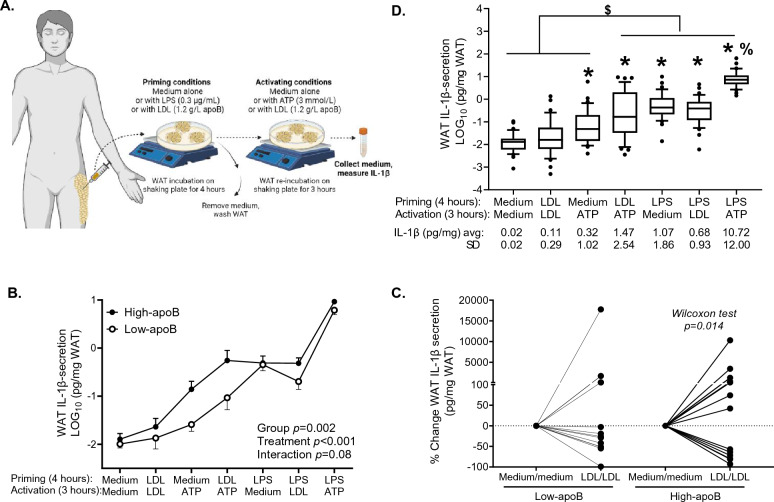
To quantify IL-1β-secretion from subjects’ WAT while assessing if and where are native LDL acting in the WAT NLRP3 inflammasome, we assessed priming versus activation using standard positive controls for priming (LPS) and activation (ATP) of the NLRP3 inflammasome in murine and human macrophages13,36 (Fig. 1A).
Figure 1.
Regulation of WAT IL-1β-secretion ex vivo by LDL, LPS and/or ATP in subjects with high-apoB and low-apoB: experimental design to assess WAT IL-1β-secretion (A), WAT IL-1β-secretion induced by the 7-incubation-conditions in all subjects (B) WAT IL-1β-secretion in subjects with high-apoB versus subjects with low-apoB (C), and % change in WAT IL-1β-secretion induced by LDL/LDL compared to baseline in subjects with low-apoB and high-apoB (D). Data was analyzed by mixed-method analyses and presented as boxes with whiskers representing the 10th–90th percentile and a line at the average in panel B, as average +/− SEM in panel C and by Wilcoxon rank sum test in panel D. N = 23 females and N = 11 males except with LDL/LDL where N = 21 females and N = 10 males for panel B, and N = 15 for low-apoB and N = 19 for high-apoB except with LDL/LDL where N = 14 for low-apoB and N = 17 for high-apoB for panel C and D for missing data. *for p < 0.001 compared to baseline or LDL/LDL, $for p ≤ 0.01 compared to baseline, LDL/LDL, or medium/ATP incubations, and %for p < 0.001 compared to all other conditions.
As presented in Fig. 1B, unstimulated baseline WAT IL-1β-secretion (medium/medium for priming/activation) was close to the detection limit (kit = 0.58 ± 0.40 pg/ml vs subject = 0.61 ± 2.50 pg/mL) and maximal induction was attained with the positive controls LPS/ATP that was higher than all other incubation-conditions (10.7 ± 12.0 pg/mg, ~ 1000-fold vs baseline). Stimulating subject WAT with their own LDL (LDL/LDL) increased IL-1β-secretion with large inter-subject variability that did not attain significance. Stimulating WAT with medium/ATP, LDL/ATP, LPS/medium and LPS/LDL significantly increased IL-1β-secretion above baseline and LDL/LDL levels. Stimulating WAT with LDL before activation with ATP (LDL/ATP) increased IL-1β-secretion above that with ATP alone (p = 0.015), suggesting that LDL are priming signals of the NLRP3 inflammasome. Conversely, LDL had no effect on IL-1β-secretion when added after LPS compared to LPS alone, suggesting that LDL have no effect on the inflammasome activation. There were no sex-differences in WAT IL-1β-secretion under all incubation-conditions (Supplemental Figure 2). Moreover, LDH release, as a surrogate marker of lytic cell death, was equivalent to baseline under all incubation-conditions indicating that IL-1β-secretion was independent of cell death (Supplemental Figure 3). WAT IL-1β-secretion was correlated with total and central adiposity with LPS stimulation only (Supplemental Figure 4).
Subjects with high-apoB have higher WAT IL-1β-secretion
To test the primary hypothesis, we separated the 40 subjects following their enrolment around median plasma apoB per sex (Table 2). Average plasma apoB for the low-apoB group (0.84 ± 0.14 g/L) and the high-apoB group (1.23 ± 0.15 g/L) corresponded to < 50th and > 75th percentile in a Canadian population, respectively32, similar to values obtained in previous cohorts where the relation of plasma apoB to T2D risk factors was first established1,2,29. Subjects with high-apoB had higher fasting plasma total cholesterol, LDL-C, TG and apoB/PCSK9 ratio, higher % total and saturated fat intake and lower % carbohydrate intake. There was no other group-difference including in plasma PL-FA profile, which suggests similar LDL PL-FA profile between the two apoB-groups (Supplemental Figure 5). There were also no significant group-differences in the expression of genes related to WAT differentiation and function (ADIPOQ, PPARG), lipid metabolism and sensing (HMGCR, SREBP1C, SREBP2), LDL and FA receptors (LDLR, CD36), inflammation including MCP1 (monocyte chemoattractant), ADGRE1 (macrophage marker), anti-inflammatory IL10 and the inflammasome-subunits (NLRP3, CASP1, IL1B) (Supplemental Figure 6).
As hypothesized, subjects with high-apoB had higher WAT IL-1β-secretion than those with low-apoB (group-effect p = 0.002, Fig. 1C), most evident with ATP incubations without/with LDL. Moreover, LDL/LDL induced WAT IL-1β-secretion above baseline in subjects with high-apoB only (Fig. 1D) explaining the large inter-subjects’ variability in Fig. 1B. Statistical adjustment for several covariates related to body composition, lipoproteins, energy intake and expenditure, WAT mRNA, WAT pro-IL-1β, or plasma PL-FA did not eliminate group-differences in WAT IL-1β-secretion (Table 3).
Table 3.
Differences in WAT IL-1β-secretion induced by the 7-incubation conditions with medium, LDL, LPS and/or ATP in subjects with low-apoB (N = 13) and high-apoB (N = 16) before and after adjustment for covariates.
| Adjusted for | Group-effect p value |
|---|---|
| No adjustment | 0.003 |
| Body composition | |
| BMI | 0.005 |
| Total body fat | 0.006 |
| BMI and waist/hip circumference ratio | 0.007 |
| Total body fat and android/gynoid ratio | 0.008 |
| Fasting plasma lipoprotein-related parameters | |
| Plasma cholesterol | 0.009 |
| Plasma LDL-C | 0.005 |
| Plasma HDL-C | 0.003 |
| Plasma NEFA | 0.004 |
| LOG10 plasma TG | 0.006 |
| Plasma apoB | 0.079 |
| Plasma PCSK9 | 0.004 |
| Plasma apoB/PCSK9 | 0.029 |
| Plasma LDL-C/apoB | 0.006 |
| Energy intake and expenditure | |
| Basal metabolic rate | 0.003 |
| Daily energy intake | 0.001 |
| % carbohydrate intake | 0.022 |
| % fat intake | 0.011 |
| % saturated fat intake | 0.005 |
| LOG10 fasting baseline WAT mRNA | |
| PPARG | 0.005 |
| ADIPOQ | 0.002 |
| HMGCR | 0.005 |
| SREBP1C | 0.004 |
| SREBP2 | 0.009 |
| CD36 | 0.007 |
| LDLR | 0.004 |
| MCP1 | 0.005 |
| ADGRE1 | 0.008 |
| NLRP3 | 0.007 |
| IL1B | 0.003 |
| CASP1 | 0.002 |
| IL10 | 0.005 |
| Fasting baseline WAT protein | |
| Pro-IL-1β | 0.003 |
| % plasma phospholipid FA | |
| % total saturated FA | 0.007 |
| % total monounsaturated FA | 0.008 |
| % total omega-3 FA | 0.007 |
| % total omega-6 FA | 0.009 |
| % total polyunsaturated FA | 0.009 |
Significant values are in bold.
Data analyzed by 2-way RM-ANOVA. N.B. Only subjects with complete WAT IL-1β-secretion profiles induced by the 7-incubation conditions were used in this analysis.
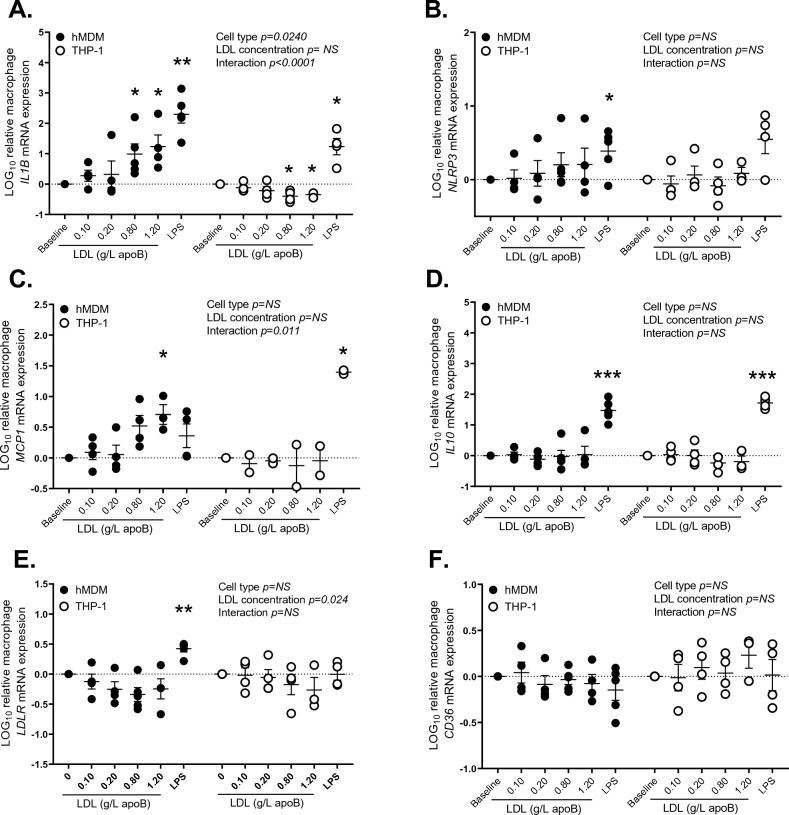
Native LDL upregulate IL1B mRNA and IL-1β-secretion in human primary macrophages
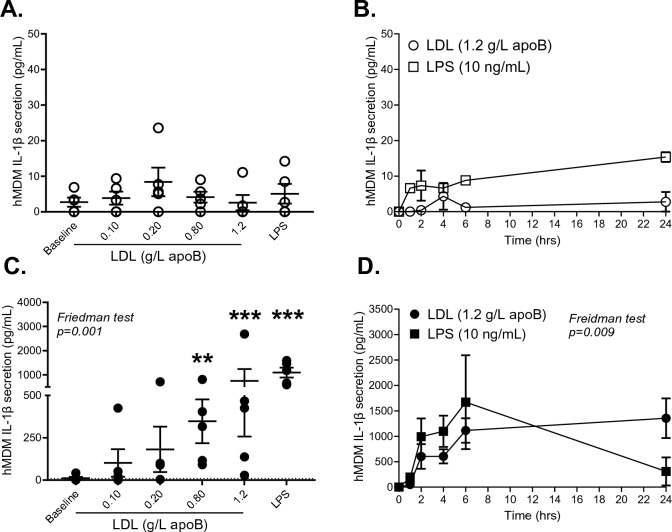
As the yield from WAT needle-biopsies limited the number of experiments that can be conducted, we further examined the effects of LDL on the inflammasome priming in hMDM over 4 h as conducted in WAT. hMDM were used given the detection of macrophage marker ADGRE1 in subject WAT and that we did not detect NLRP3 expression in a human model of primary adipocytes7. As presented in Fig. 2A, similar to LPS, LDL increased IL1B expression in a concentration-dependant manner in hMDM. However, unlike LPS, the same LDL preparation decreased IL1B expression in THP-1- macrophages, suggesting that this cell-line may not be a suitable model of unpolarized hMDM. LDL also increased MCP1 expression in hMDM (Fig. 2C) but had no significant effect on NLRP3, IL10, or LDLR, while both LDL and LPS had no affect on CD36 expression in either macrophage-model (Fig. 2B, D–F). To explore if LDL-induced increase in IL1B expression translates into IL-1β-secretion, IL-1β-secretion before and after activation of the NLRP3 inflammasome by ATP was measured. Neither LDL nor LPS alone triggered IL-1β-secretion in hMDM using various LDL-concentrations (Fig. 3A) and up to 24 h without ATP (Fig. 3B). However, when followed by ATP, LDL induced IL-1β-secretion in a dose- (Fig. 3C) and time-dependent manner (Fig. 3D). LDL did not induce IL-1β-secretion from THP-1-derived macrophages without or with ATP (data not shown).
Figure 2.
Effects of LDL versus LPS on gene expression in human macrophage models: the effect of native LDL on the mRNA expression of IL1B (A), NLRP3 (B), MCP1 (C), IL10 (D), LDLR (E) and CD36 (F) in hMDM and THP-1 derived macrophages using baseline medium supplemented with subject own LDL (0.1–1.2 g/L apoB) or LPS (0.01 μg/mL) over 4 h. Data was analyzed by mixed-method analyses, *for p < 0.05 and **for p < 0.01 compared to baseline medium alone.
Figure 3.
Effects of LDL versus LPS on IL-1β-secretion from hMDM: IL-1β-secretion from hMDM from 5 subjects after incubation with their own native LDL (0.1–1.2 g/L apoB) over 4 h (A) or with 1.2 g/L LDL over 1–24 h (B) without ATP. Experiments in panel A following removal of priming medium and re-incubation with ATP (3 mmol/L) over 3 h (C), and experiments in panel B following removal of medium and re-incubation with ATP (3 mmol/L) over 3 h (D). LPS (10 ng/mL) was used as a priming control in all experiments. Data was analyzed by non-parametric Friedman test separately for LDL. **for p < 0.01 and ***for p < 0.001 versus baseline.
WAT IL-1β secretion are consistently associated with higher diabetes risk factors in subjects with high-apoB
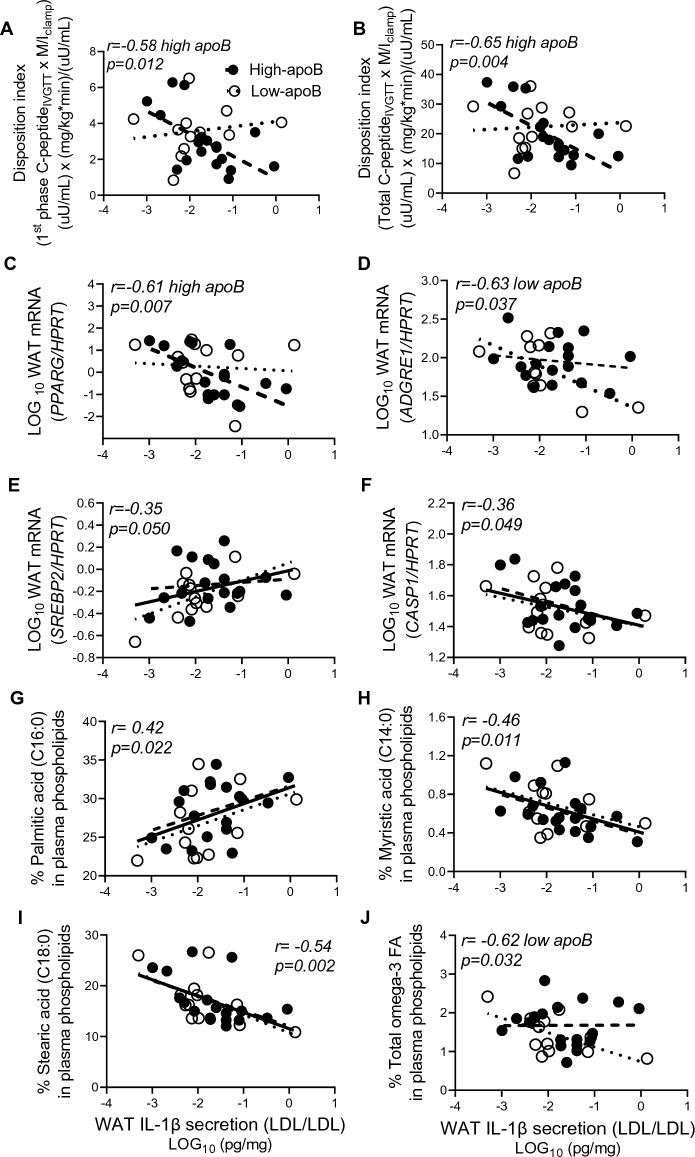
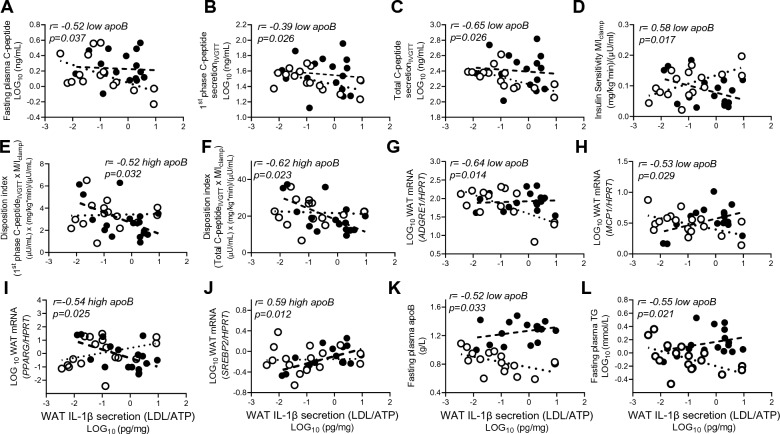
Confirming our previous findings in a similar cohort2–5,29, plasma apoB was associated positively with measures of GIISIVGTT, IR, plasma IL-1Ra, postprandial hypertriglyceridemia and hyperchylomicronemia (Supplemental Figure 7). Here, we tested whether WAT IL-1β-secretion was associated with these risk factors. Indeed, LDL-induced WAT IL-1β-secretion was associated to metabolic risk in the high-apoB group as it was inversely correlated with 1st phase and total DI, which is a predictor of diabetes risk37 and with WAT PPARG, which is a marker of adipocyte differentiation (Fig. 4A–C). Conversely, these associations were absent in the low-apoB group, in whom IL-1β-secretion was inversely related to macrophage infiltration (Fig. 4D). In both groups, LDL-induced IL-1β-secretion was positively associated with WAT SREBP2, which is upregulated upon NLRP3 inflammasome activation13,38, and negatively with CASP1, which is required for adipocyte differentiation14 (Fig. 4E, F). Importantly, the profile of LDL-FA appears to modulate WAT IL-1β-secretion, as higher palmitate but lower myristate and stearate were associated with higher IL-1β-secretion in both groups, while higher omega-3 associated with lower IL-1β-secretion in the low-apoB group (Fig. 4G–J).
Figure 4.
Correlations of LDL/LDL-induced WAT IL-1β-secretion with diabetes risk factors and % fasting plasma PL-FAs: Correlation of WAT IL-1β-secretion with 1st phase disposition index (A), total disposition index (B), WAT PPARG mRNA (C), WAT ADGRE1 mRNA (D), WAT SREBP2 mRNA (E), and WAT CASP1 mRNA (F) normalized for HPRT, and % plasma PL-palmitic acid (G), myristic acid (H), stearic acid (I), and total omega-3 FA (J) in subjects with low-apoB (N = 14) and high-apoB (N = 17). Solid regression line represents correlation in all subjects.
Similarly, LDL/ATP-induced WAT IL-1β-secretion was related to metabolic health in the low-apoB group as it associated inversely with fasting plasma C-peptide and glucose-induced 1st phase and total C-peptide secretionIVGTT and positively with IS (Fig. 5A–D). Conversely in high-apoB, WAT IL-1β remained inversely associated with 1st phase and total DI (Fig. 5E, F). WAT IL-1β-secretion was also inversely associated with WAT inflammation in the low-apoB group (negatively with ADGRE1 and MCP1) but with WAT dysfunction in the high-apoB group (negatively with PPARG and positively with SREBP2) (Fig. 5G–J). Interestingly in the group with low-apoB, the lower was plasma apoB and TG the higher was IL-1β-secretion induced by LDL, suggesting higher sensitivity to LDL effects (Fig. 5K–L). Notably, the contradictory associations of WAT IL-1β-secretion with diabetes risk factors in the low-apoB and high-apoB groups cancelled each other when all subjects were combined (Fig. 6A). Thus, the physiological and diabetogenic associations of WAT IL-1β-secretion would have been missed.
Figure 5.
Correlations of LDL/ATP-induced WAT IL-1β-secretion with diabetes risk factors: Correlation of WAT IL-1β-secretion with fasting plasma C-peptide (A), 1st phase C-peptide secretionIVGTT (B), total C-peptide secretionIVGTT, (C), insulin sensitivity as M/Iclamp (D), 1st phase disposition index (E), total disposition index (F), WAT ADGRE1 mRNA (G), WAT MCP1 mRNA (H), WAT PPARG mRNA (I), and WAT SREBP2 mRNA (J) normalized for HPRT, and fasting plasma apoB (K) and TG (L) in subjects with low-apoB (N = 15) and high-apoB (N = 19). Solid regression line represents correlation in all subjects.
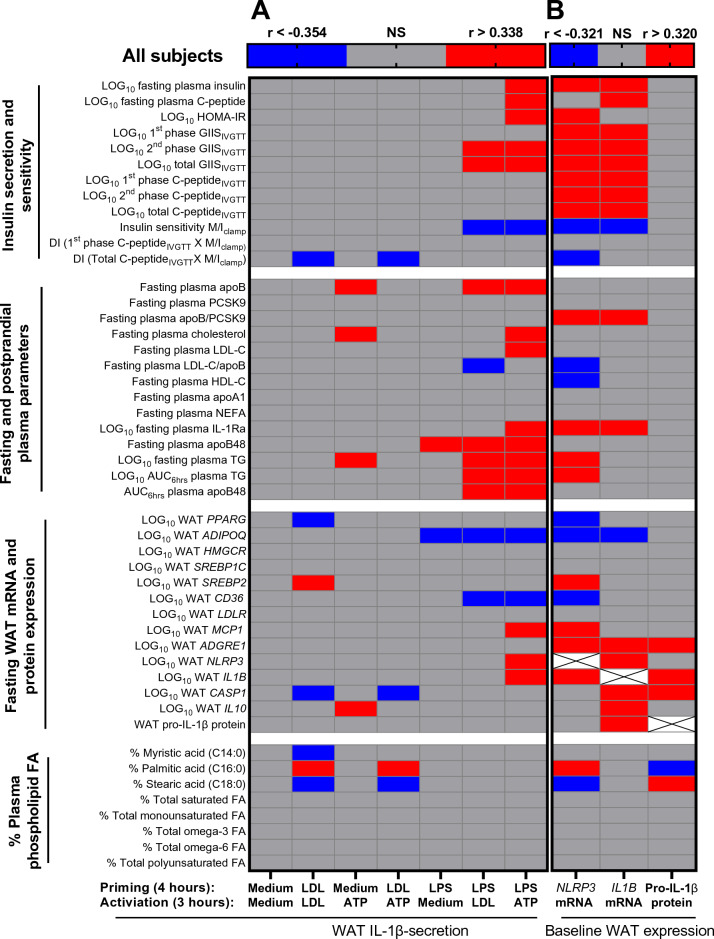
Figure 6.
Heat-map representing correlations of WAT NLRP3 inflammasome priming and activation measures with outcome measures: Correlations of WAT IL-1β-secretions induced by the 7-incubation conditions (A) and of WAT NLRP3 mRNA, WAT IL1B mRNA, and WAT pro-IL-1β protein (B) with fasting and Botnia clamp measures of insulin sensitivity and secretion, fasting and postprandial plasma lipoprotein-related parameters, fasting baseline WAT mRNA expression of genes related to WAT function and inflammation, and % plasma PL-FA in all subjects. Grey cells represent insignificant, blue cells represent significant negative associations, and red cells represent significant positive associations.
On the other hand, higher LPS-induced IL-1β-secretion was associated with diabetes risk factors without a group-differences. As presented in Fig. 6A and Supplemental Figure 8, WAT IL-1β-secretion, particularly when maximized by LPS/ATP, was associated with higher fasting and clamp-measures of insulin secretion and resistance, delayed fasting and postprandial plasma clearance of TG and apoB48, higher plasma apoB, total and LDL-C and/or IL-1Ra, lower LDL size (estimated from LDL-C/apoB ratio), and/or with markers of WAT dysfunction (inversely with ADIPOQ and CD36) and WAT inflammation (positively with MCP1, NLRP3 and IL1B).
WAT NLRP3 and IL1B mRNA and pro-IL-1β protein are associated with diabetes risk factors in all subjects
Unlike WAT IL-1β-secretion, the expression of WAT NLRP3 and to a lesser extent IL1B was positively associated with all diabetes risk factors examined with no group-differences (Fig. 6B, Supplemental Figure 9 ). Moreover, it was associated with higher plasma apoB/PCSK9 ratio, which is a predictor of WAT surface-expression of LDLR and7 and lower estimated LDL size and plasma HDL-C. It was also associated with a proinflammatory plasma PL-FA profile (positively with palmitate and arachidonate and negatively with stearate) and markers of WAT dysfunction/inflammation (inversely with PPARG, ADIPOQ, CD36 and positively with SREBP2, MCP1, ADGRE1, IL1B). WAT pro-IL-1β protein was also positively associated with WAT ADGRE1, IL1B and CASP1 but inversely with palmitate, suggesting higher cleavage of pro-IL-1β to active IL-1β with this inflammatory plasma PL-FA profile.
Statistical adjustment for WAT IL-1β-secretion attenuates the association of plasma apoB with diabetes risk factors
To explore whether the WAT NLRP3 inflammasome/IL-1β pathway is related to higher diabetes risk factors in subjects with high-apoB, we used partial correlation analysis. Adjusting for WAT IL-1β-secretion induced by LDL, LPS and/or ATP gradually eliminated the association of plasma apoB with measures of insulin secretion and resistance, plasma IL-1Ra and delayed postprandial chylomicron clearance reaching a maximum with LPS/ATP (Table 4). Adjusting for WAT NLRP3, WAT IL1B or total and central adiposity had less effects than maximal LPS/ATP-induced IL-1β-secretion, while adjusting for WAT pro-IL-1β protein or waist/hip ratio had little to no effect (Table 4).
Table 4.
Partial correlation of plasma apoB with risk factors for type 2 diabetes before and after adjustment for LOG10 fasting WAT IL-1β-secretion induced by the 7-incubation conditions with medium, LDL, LPS and/or ATP, WAT NLRP3 mRNA, WAT IL1B mRNA and WAT pro-IL-1β protein expression, or body composition measures.
| Adjustment for | LOG10 2nd phase GIISIVGTT | LOG10 2nd phase C- peptide secretionIVGTT | LOG10 total C-peptide secretionIVGTT | M/Iclamp | LOG10 plasma IL-1Ra | LOG10 AUC6hrs TG | AUC6hrs apoB48 |
|---|---|---|---|---|---|---|---|
| p values | |||||||
| None | 0.038 | 0.025 | 0.031 | 0.001 | 0.032 | < 0.001 | 0.029 |
| WAT IL-1β (LDL/LDL) | – | 0.024 | 0.029 | 0.006 | – | < 0.001 | 0.042 |
| WAT IL-1β (medium/ATP) | – | – | – | 0.007 | 0.040 | < 0.001 | 0.031 |
| WAT IL-1β (LDL/ATP) | 0.044 | 0.014 | 0.018 | 0.003 | – | < 0.001 | 0.033 |
| WAT IL-1β (LPS/medium) | – | 0.043 | – | 0.004 | – | < 0.001 | – |
| WAT IL-1β (LPS/LDL) | – | – | – | 0.031 | – | 0.002 | – |
| WAT IL-1β (LPS/ATP) | – | – | – | – | – | 0.002 | – |
| WAT NLRP3 mRNA | – | – | – | 0.010 | – | < 0.001 | – |
| WAT IL1B mRNA | – | – | – | 0.004 | – | < 0.001 | 0.036 |
| WAT pro-IL-1β protein | 0.036 | 0.033 | 0.041 | 0.002 | 0.035 | < 0.001 | 0.025 |
| BMI | – | – | – | 0.018 | – | < 0.00 | 0.044 |
| Total fat mass | – | – | – | 0.047 | – | < 0.001 | 0.042 |
| Waist/hip ratio | – | 0.034 | 0.043 | 0.003 | 0.043 | < 0.001 | – |
| Android/gynoid fat ratio | – | – | – | 0.012 | – | < 0.001 | – |
N = 27 females and N = 13 males for WAT mRNA and protein and anthropometric parameters, N = 23 females and N = 11 males for WAT IL-1β-secretion conditions except for LDL/LDL condition where and N = 21 females and N = 10 males for missing data.
Discussion
This study uncovered novel findings regarding the regulation of the human WAT NLRP3 inflammasome/IL-1β pathway by native LDL. We showed that native LDL are metabolic stimuli of the NLRP3 inflammasome in human WAT (Fig. 1) and primary macrophages (Figs. 2, 3) by acting as priming signals inducing IL1B expression and IL-1β-secretion in the presence of an activation signal. Accordingly, subjects with high-apoB have higher WAT IL-1β-secretion than those with low-apoB (Fig. 1C) independently of anthropometric and metabolic covariates (Table 3). While WAT NLRP3 and IL1B mRNA expressions were associated with all examined risk factors for T2D without apoB-group differences (Fig. 6B, Supplemental Figure 9), there was a dichotomy in the association of WAT IL-1β-secretion to these factors dependent on the WAT stimuli, IL-1β-secretion levels, and the study group. Higher WAT IL-1β-secretion induced by metabolic stimuli (LDL ± ATP) were positively associated with risk factors for T2D in subjects with high-apoB, while an inverse association was revealed with lower WAT IL-1β-secretion in subjects with low-apoB (Figs. 4, 5). On the other hand, replacing priming with LDL by microbial LPS induced IL-1β-secretion in subjects with low-apoB to levels similar to those in the high-apoB and revealed a diabetological relationship in all subjects, particularly with maximal LPS/ATP-induced stimulation (Fig. 6A, Supplemental Figure 8). It is important to underscore that these results were generated in a healthy population without chronic or infectious disease in an attempt to dissect out LDL-effects in vivo and ex vivo. Whether these findings translate to other populations needs to be examined.
To the best of our knowledge, only one human study examined 1L-1β-secretion from unstimulated subcutaneous WAT, where very low secretion levels were also reported16, while none examined its regulation in any human fat depot. Here we report that native LDL, LPS or ATP alone induce IL-1β-secretion from human WAT (Fig. 1). As our findings in hMDM confirm that, for IL-1β to be secreted, 2 signals for priming and activation of the NLRP3 inflammasome are needed (Figs. 2, 3), the induction of IL-1β-secretion from WAT by LDL, LPS or ATP alone implies that an endogenous WAT-released signal(s) must have provided the other missing signal. This signal is likely palmitate, given its release by endogenous lipolysis39 and effects on the inflammasome priming and activating in macrophages24,25. Endogenous ATP release, which increases with IR in WAT and would further stimulate lipolysis40, may have also provided an activation signal, especially in subjects with high-apoB and higher IR. While alone this endogenous signal(s) was insufficient to induce WAT IL-1β-secretion at baseline to detectable levels (Fig. 1), its effects together with the mass of WAT cannot be ignored when evaluating the physiological effects of LDL, ATP and LPS particularly with obesity and IR. Moreover, IL-1β-secretion was independent of cell-death (Supplemental Figure 3). This is in line with recent data demonstrating that IL-1β maturation by caspase-1 triggers its transport to the plasma membrane for both Gasdermin-dependent and -independent release in live cells41. The secretion of IL-1β is also proposed to occur in a continuum with secretion-levels depending on the stimulus strength (i.e. type, dose and duration) and extracellular IL-1β requirements36.
Many lipoprotein-related components and byproducts mostly studied in macrophages using oxidized or modified LDL in the context of atherosclerosis have been described to regulate the NLRP3 inflammasome. Accumulation of free cholesterol, PL and palmitate was shown to induce lysosomal dysfunction and the leakage of its content (e.g. Ca2+ and cathepsin B) that prime and/or activate the NLRP3 inflammasome in macrophages and tubular endothelial cells25,38,42,43. Moreover, fatty acid β-oxidation in the mitochondria and/or ceramide synthesis in the ER increase the production of reactive oxygen species that prime and activate the inflammasome44,45. More recently, ceramide46 and diacyl glycerol17, were shown to induce post-translational phosphorylation of NLRP3 needed for subsequent inflammasome activation17,19,46. Post-translational modification of NLRP3 may also explain significant induction of IL1B expression by native LDL in the absence of an effect on NLRP3 expression in this study (Fig. 2A, B). Alternatively, the upregulation of NLRP3 expression may have preceded that of IL1B as previously demonstrated in MCSF-differentiated hMDM stimulated by LPS alone or with cholesterol crystals (peaking by 1 h vs 3–6 h, respectively)22.
Moreover, intersubject variation in LDL quality may have translated into the observed variations in WAT and macrophage responses to LDL (Figs. 1, 2, 3). While native LDL was used without in vitro modification, the presence of variable content of oxLDL47, LDL-bound LPS48 or LDL-ceramide49 in subject LDL preparations cannot be excluded. These components are measurable in the plasma of subjects without chronic disease and would increase the priming and/or activation of the NLRP3 inflammasome. Here we extend these findings to report that smaller LDL size, higher LDL-enrichment with palmitate and arachidonate and lower enrichment with myristate, stearate and omega-3 FA (in low-apoB) are associated with higher expression of WAT NLRP3, depletion of pro-IL-1β and/or secretion of IL-1β (Figs. 4G–J, 6, Supplemental Figure 9S-U). Smaller denser LDL characterize subjects with high-apoB as previously reported using gel electrophoresis2,3. Moreover, we reported that normo-cholesterolemic subjects with lower plasma PCSK9 and higher WAT surface-expression of LDLR and CD36 have higher WAT IL-1β-secretion and risk factors for T2D7. Thus, receptor-mediated uptake of the whole LDL-particle together with all its components may be needed to mediate the effect of LDL reported here. And while counter-regulatory mechanisms exist to block excessive LDL-uptake via LDLR, such as by inhibiting SREBP2, the activation of the NLRP3 inflammasome in immune cells was shown to activate SREBP2, which in turn can act in a positive feed-back loop promoting further inflammation and cholesterol accumulation38. Consistently, there was a strong association between WAT NLRP3 mRNA (Fig. 6B) or IL-1β-secretion (Figs. 4E, 5J, 6) with WAT SREBP2 mRNA especially in subjects with high-apoB. Moreover, unlike previously reported temporal effects of LPS50 and as shown here, LDL-induced IL-1β-secretion from hMDM remained significant even up to 24-h (Fig. 3D). The intracellular mechanisms regulated by native LDL and their receptors, LDLR and CD36, are further being investigated in an ongoing study on WAT NLRP3 inflammasome (https://classic.clinicaltrials.gov/ct2/show/NCT04485871).
Adjusting for WAT NLRP3 and IL1B mRNA and mostly for maximal LPS/ATP-induced IL-1β-secretion attenuated the association of plasma apoB with diabetes risk factors (Table 4). This supports that WAT NLRP3 inflammasome/IL-1β pathway is a mechanism linking hyperapoB to diabetes risk, however; it does not exclude other mechanisms. We previously reported that LDL decrease the differentiation of a human model of adipocyte in an NLRP3-independent manner7. Moreover, other groups have reported that receptor-mediated uptake of apoB-lipoproteins in myocytes51 and β-cells52 induces their metabolic dysfunction.
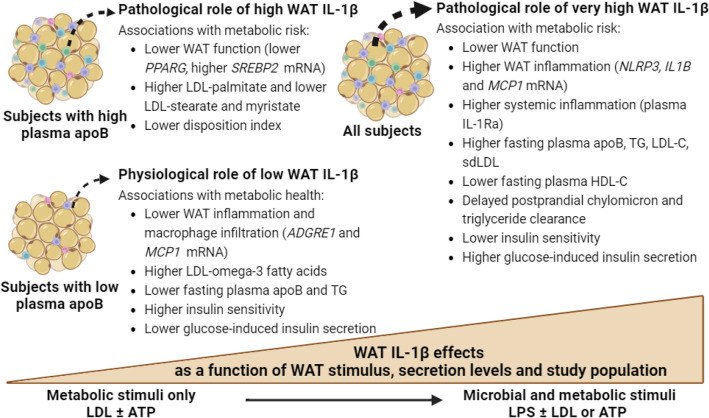
Intriguingly, there was a dichotomy in the association of WAT IL-1β-secretion to diabetes risk factors depending on the apoB-group and WAT stimulus (Figs. 4, 5). Conflicting outcomes were also reported regarding targeting the IL-1β pathway in humans. Interleukin-1Ra53 or anti-IL-1β54 were demonstrated to improve plasma glucose, β-cell function, IR, and/or inflammation in diabetic patients. Conversely anti-IL-1β therapy did not decrease diabetes incidence in cardiovascular patients on statins in the CANTOS trial55. This led to the proposal that targeting the IL-1β-pathway may reduce diabetes complications rather than prevent its incidence56. However, several lines of in vivo and ex vivo evidence indicate that IL-1β has hypoglycemic actions that are independent of insulin action and of IL-1β pro-inflammatory and pyroptotic effects (for review57). In vitro, IL-1β was reported to increase glucose-uptake in several cell type including adipocytes, while intraperitoneal injection of nanogram amounts into mice was shown to deplete hepatic glycogen, inhibits gluconeogenesis and reduces plasma glucose by 50% for over 24-h57. These physiological and pathological effects of IL-1β are dependent on its amount, and can be overridden with overproduction such as in infectious disease where IL-1β is believed to induce IR in non-immune tissue to deviate glucose-utilization to the immune cells57. Thus, taking together published data together with our findings suggest that under non-infectious conditions, WAT IL-1β-secretion may not be pathological in all subjects and should not be targeted in all subjects. The effects of WAT IL-1β may be dependent on the stimulus inducing its secretion, IL-1β secretion levels, and the metabolic health of the population examined encompassing the quality of LDL and WAT (Fig. 7).
Figure 7.
Proposed pathological and physiological roles of WAT IL-1β as a function of WAT stimulus, IL-1β secretion levels, and metabolic health of the study population: In the presence of metabolic stimuli such as LDL ± ATP, higher WAT IL-1β-secretion in subjects with high-apoB has a diabetological pro-inflammatory effect while lower WAT IL-1β-secretion in subjects with low-apoB has a physiological hypoglycemic effect. Microbial stimuli such as LPS maximize the WAT IL-1β-secretion overriding the metabolic effects of IL-1β and unraveling its pathological pro-inflammatory effects and associations with T2D risk factors in all subjects.
In conclusion, we report novel findings that native LDL are priming signals of the human WAT and macrophage NLRP3 inflammasome leading to IL-1β-secretion in the presence of an activation signal. Accordingly, subjects with high plasma apoB have higher WAT IL-1β-secretion, which is associated with higher risk factors for T2D. We propose that subjects with high-apoB may be an ideal population to target the IL-1β pathway given higher WAT IL-1β-secretion in response to both metabolic (LDL and ATP) and microbial (LPS) stimuli and higher risk for T2D in this population.
Supplementary Information
Acknowledgements
We gratefully acknowledge the contribution of Drs. Alexis Baass and late Robert Dufour in subject screening. MF is the guarantor of this work and has full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Abbreviations
- ADGRE1
Adhesion G protein-coupled receptor E1
- Apo
Apolipoprotein
- ATP
Adenosine triphosphate
- AUC6hrs
Area under the 6-h postprandial curve
- BMI
Body mass index
- BSA
Bovine serum albumin
- CASP1
Caspase-1
- CD36
Cluster of differentiation 36
- CTL
Control
- DI
Disposition index
- EDTA
Ethylenediaminetetraacetic acid
- FA
Fatty acids
- GIIS
Glucose-induced insulin secretion
- HBSS
Hanks’ balanced salt solution
- HDL-C
High-density lipoprotein cholesterol
- HMGCR
3-Hydroxy-3-methylglutaryl-CoA reductase
- HPRT
Hypoxanthine phosphoribosyltransferase 1
- IL-1β
Interleukin 1 beta
- IL-1Ra
Interleukin 1 receptor antagonist
- IS
Insulin sensitivity
- IR
Insulin resistance
- IVGTT
Intravenous glucose tolerance test
- LDL
Low-density lipoprotein
- LDLR
Low-density lipoprotein receptor
- LPS
Lipopolysaccharide
- MCP-1
Monocyte chemoattractant protein 1
- MCSF
Macrophage colony-stimulating factor
- M/I
Glucose infusion rate divided plasma insulin at steady state
- NEFA
Non-esterified fatty acids
- NF-κB
Nuclear factor-κB
- NLRP3
Nucleotide-binding domain leucine-rich repeat containing a pyrin domain 3
- PBMC
Peripheral blood mononuclear cells
- PCSK9
Proprotein convertase subtilisin-kexin type 9
- PL
Phospholipids
- RT-PCR
Real time PCR
- SREBP
Sterol regulatory element binding protein
- T2D
Type 2 diabetes
- TG
Triglyceride
- WAT
White adipose tissue
Author contributions
M.F., M.S. and S.B. designed the study; S.B., V.L., Y.C., B.O. and M.F. analyzed the data, M.F. and S.B. wrote the main manuscript text and prepared Figs. 1, 2, 3, 4, 5, 6 and 7, all authors reviewed he manuscript.
Funding
This study was supported by an operating grant from Canadian Institutes of Health Research (CIHR) to MF and MS (#123409). SB was supported by CIHR, VL by CIHR Vanier and BO and YC by Fonds de recherches du Québec (FRQ) doctoral scholarships. MC is funded by Richard and Edith Strauss Research Foundation.
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available due to ethical restrictions but are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-45870-1.
References
- 1.Faraj M, Messier L, Bastard JP, Tardif A, Godbout A, Prud'homme D, Rabasa-Lhoret R. Apolipoprotein B: A predictor of inflammatory status in postmenopausal overweight and obese women. Diabetologia. 2006;49(7):1637–1646. doi: 10.1007/s00125-006-0259-7. [DOI] [PubMed] [Google Scholar]
- 2.Bissonnette S, Salem H, Wassef H, Saint-Pierre N, Tardif A, Baass A, et al. Low density lipoprotein delays clearance of triglyceride-rich lipoprotein by human subcutaneous adipose tissue. J. Lipid Res. 2013;54(5):1466–1476. doi: 10.1194/jlr.P023176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bissonnette S, Saint-Pierre N, Lamantia V, Cyr Y, Wassef H, Faraj M. Plasma IL-1Ra: Linking hyperapoB to risk factors for type 2 diabetes independent of obesity in humans. Nutr. Diabetes. 2015;5:e180. doi: 10.1038/nutd.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wassef H, Bissonnette S, Saint-Pierre N, Lamantia V, Cyr Y, Chretien M, Faraj M. The apoB/ PCSK9 ratio: A new index for metabolic risk in humans. J. Clin. Lipidol. 2015;9:664–675. doi: 10.1016/j.jacl.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 5.Lamantia V, Bissonnette S, Wassef H, Cyr Y, Baass A, Dufour R, et al. ApoB-lipoproteins and dysfunctional white adipose tissue; relation to risk factors for type 2 diabetes in humans. J. Clin. Lipidol. 2017;11(1):34–45. doi: 10.1016/j.jacl.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 6.Faraj M. LDL, LDL receptors, and PCSK9 as modulators of the risk for type 2 diabetes: A focus on white adipose tissue. J. Biomed. Res. 2020;34(4):251–259. doi: 10.7555/JBR.34.20190124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cyr Y, Lamantia V, Bissonnette S, Burnette M, Besse-Patin A, Demers A, et al. Lower plasma PCSK9 in normocholesterolemic subjects is associated with upregulated adipose tissue surface-expression of LDLR and CD36 and NLRP3 inflammasome. Physiol. Rep. 2021;9(3):e14721. doi: 10.14814/phy2.14721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ley SH, Harris SB, Connelly PW, Mamakeesick M, Gittelsohn J, Wolever TM, et al. Association of apolipoprotein B with incident type 2 diabetes in an aboriginal Canadian population. Clin. Chem. 2010;56(4):666–670. doi: 10.1373/clinchem.2009.136994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Onat A, Can G, Hergenc G, Yazici M, Karabulut A, Albayrak S. Serum apolipoprotein B predicts dyslipidemia, metabolic syndrome and in women, hypertension and diabetes, independent of markers of central obesity and inflammation. Int. J. Obes. 2007;31(7):1119–1125. doi: 10.1038/sj.ijo.0803552. [DOI] [PubMed] [Google Scholar]
- 10.Pencina KM, Pencina MJ, Dufresne L, Holmes M, Thanassoulis G, Sniderman AD. An adverse lipoprotein phenotype-hypertriglyceridaemic hyperapolipoprotein B-and the long-term risk of type 2 diabetes: A prospective, longitudinal, observational cohort study. Lancet Healthy Longev. 2022;3(5):e339–e346. doi: 10.1016/S2666-7568(22)00079-4. [DOI] [PubMed] [Google Scholar]
- 11.Richardson TG, Wang Q, Sanderson E, Mahajan A, McCarthy MI, Frayling TM, et al. Effects of apolipoprotein B on lifespan and risks of major diseases including type 2 diabetes: A mendelian randomisation analysis using outcomes in first-degree relatives. Lancet Healthy Longev. 2021;2(6):e317–e326. doi: 10.1016/S2666-7568(21)00086-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Masters SL, Latz E, O'Neill LAJ. The inflammasome in atherosclerosis and type 2 diabetes. Sci. Transl. Med. 2011;3(81):81ps17-81ps17. doi: 10.1126/scitranslmed.3001902. [DOI] [PubMed] [Google Scholar]
- 13.Skeldon AM, Faraj M, Saleh M. Caspases and inflammasomes in metabolic inflammation. Immunol. Cell Biol. 2014;92(4):304–313. doi: 10.1038/icb.2014.5. [DOI] [PubMed] [Google Scholar]
- 14.Stienstra R, Joosten LAB, Koenen T, van Tits B, van Diepen JA, van den Berg SAA, et al. The inflammasome-mediated caspase-1 activation controls adipocyte differentiation and insulin sensitivity. Cell Metab. 2010;12(6):593–605. doi: 10.1016/j.cmet.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat. Med. 2011;17(2):179–188. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Esser N, L’homme L, De Roover A, Kohnen L, Scheen AJ, Moutschen M, et al. Obesity phenotype is related to NLRP3 inflammasome activity and immunological profile of visceral adipose tissue. Diabetologia. 2013;56(11):2487–2497. doi: 10.1007/s00125-013-3023-9. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Z, Meszaros G, He WT, Xu Y, de Fatima MH, Mailly L, et al. Protein kinase D at the Golgi controls NLRP3 inflammasome activation. J. Exp. Med. 2017;214(9):2671–2693. doi: 10.1084/jem.20162040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelley N, Jeltema D, Duan Y, He Y. The NLRP3 inflammasome: An overview of mechanisms of activation and regulation. Int. J. Mol. Sci. 2019;20(13):3328. doi: 10.3390/ijms20133328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song N, Liu ZS, Xue W, Bai ZF, Wang QY, Dai J, et al. NLRP3 phosphorylation is an essential priming event for inflammasome activation. Mol. Cell. 2017;68(1):185–197.e186. doi: 10.1016/j.molcel.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 20.Miller YI, Viriyakosol S, Worrall DS, Boullier A, Butler S, Witztum JL. Toll-like receptor 4-dependent and -independent cytokine secretion induced by minimally oxidized low-density lipoprotein in macrophages. Arterioscler. Thromb. Vasc. Biol. 2005;25(6):1213–1219. doi: 10.1161/01.ATV.0000159891.73193.31. [DOI] [PubMed] [Google Scholar]
- 21.Sheedy FJ, Grebe A, Rayner KJ, Kalantari P, Ramkhelawon B, Carpenter SB, et al. CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nat. Immunol. 2013;14:812. doi: 10.1038/ni.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rajamaki K, Lappalainen J, Oorni K, Valimaki E, Matikainen S, Kovanen PT, Eklund KK. Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: A novel link between cholesterol metabolism and inflammation. PLoS ONE. 2010;5(7):e11765. doi: 10.1371/journal.pone.0011765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol. 2010;11(2):136–140. doi: 10.1038/ni.1831. [DOI] [PubMed] [Google Scholar]
- 24.Wen H, Gris D, Lei Y, Jha S, Zhang L, Huang MT-H, et al. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat. Immunol. 2011;12(5):408–415. doi: 10.1038/ni.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weber K, Schilling JD. Lysosomes integrate metabolic-inflammatory cross-talk in primary macrophage inflammasome activation. J. Biol. Chem. 2014;289(13):9158–9171. doi: 10.1074/jbc.M113.531202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moschen A, Molnar C, Enrich B, Geiger S, Ebenbichler C, Tilg H. Adipose and liver expression of IL-1 family members in morbid obesity and effects of weight loss. Mol. Med. 2011;17(7–8):840–845. doi: 10.2119/molmed.2010.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rheinheimer J, de Souza BM, Cardoso NS, Bauer AC, Crispim D. Current role of the NLRP3 inflammasome on obesity and insulin resistance: A systematic review. Metab. Clin. Exp. 2017;74:1–9. doi: 10.1016/j.metabol.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 28.Herder C, Brunner EJ, Rathmann W, Strassburger K, Tabík AG, Schloot NC, Witte DR. Elevated levels of the anti-inflammatory interleukin-1 receptor antagonist precede the onset of type 2 diabetes. Diabetes Care. 2009;32(3):421–423. doi: 10.2337/dc08-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bissonnette S, Saint-Pierre N, Lamantia V, Leroux C, Provost V, Cyr Y, et al. High plasma apolipoprotein B identifies obese subjects who best ameliorate white adipose tissue dysfunction and glucose-induced hyperinsulinemia after a hypocaloric diet. Am. J. Clin. Nutr. 2018;108(1):62–76. doi: 10.1093/ajcn/nqy070. [DOI] [PubMed] [Google Scholar]
- 30.Cyr Y, Bissonnette S, Lamantia V, Wassef H, Loizon E, Ngo Sock ET, et al. White adipose tissue surface expression of LDLR and CD36 is associated with risk factors for type 2 diabetes in adults with obesity. Obesity (Silver Spring) 2020;28(12):2357–2367. doi: 10.1002/oby.22985. [DOI] [PubMed] [Google Scholar]
- 31.Lamantia V, Bissonnette S, Provost V, Devaux M, Cyr Y, Daneault C, et al. The association of polyunsaturated fatty acid delta-5-desaturase activity with risk factors for type 2 diabetes is dependent on plasma apoB-lipoproteins in overweight and obese adults. J. Nutr. 2018;149(1):57–67. doi: 10.1093/jn/nxy238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Connelly PW, Poapst M, Davignon J, Lussier-Cacan S, Reeder B, Lessard R, et al. Reference values of plasma apolipoproteins A-I and B, and association with nonlipid risk factors in the populations of two Canadian provinces: Quebec and Saskatchewan. Canadian Heart Health Surveys Research Group. Can. J. Cardiol. 1999;15(4):409–418. [PubMed] [Google Scholar]
- 33.Faraj M, Lavoie M-E, Messier L, Bastard JP, Prud'homme D. Reduction in serum apoB is associated with reduced inflammation and insulin resistance in post-menopausal women: A MONET study. Atherosclerosis. 2010;211(2):682–688. doi: 10.1016/j.atherosclerosis.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 34.Ito S, Hara Y, Kubota T. CARD8 is a negative regulator for NLRP3 inflammasome, but mutant NLRP3 in cryopyrin-associated periodic syndromes escapes the restriction. Arthritis Res. Ther. 2014;16(1):R52. doi: 10.1186/ar4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lund ME, To J, O'Brien BA, Donnelly S. The choice of phorbol 12-myristate 13-acetate differentiation protocol influences the response of THP-1 macrophages to a pro-inflammatory stimulus. J. Immunol. Methods. 2016;430:64–70. doi: 10.1016/j.jim.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 36.Lopez-Castejon G, Brough D. Understanding the mechanism of IL-1β secretion. Cytokine Growth Factor Rev. 2011;22(4):189–195. doi: 10.1016/j.cytogfr.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lorenzo C, Wagenknecht LE, Rewers MJ, Karter AJ, Bergman RN, Hanley AJG, Haffner SM. Disposition index, glucose effectiveness, and conversion to type 2 diabetes: the insulin resistance atherosclerosis study (IRAS) Diabetes Care. 2010;33(9):2098–2103. doi: 10.2337/dc10-0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tall AR, Yvan-Charvet L. Cholesterol, inflammation and innate immunity. Nat. Rev. Immunol. 2015;15:104. doi: 10.1038/nri3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rittig N, Bach E, Thomsen HH, Pedersen SB, Nielsen TS, Jorgensen JO, et al. Regulation of lipolysis and adipose tissue signaling during acute endotoxin-induced inflammation: A human randomized crossover trial. PLoS ONE. 2016;11(9):e0162167. doi: 10.1371/journal.pone.0162167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tozzi M, Hansen JB, Novak I. Pannexin-1 mediated ATP release in adipocytes is sensitive to glucose and insulin and modulates lipolysis and macrophage migration. Acta Physiol. (Oxford, England) 2020;228(2):e13360. doi: 10.1111/apha.13360. [DOI] [PubMed] [Google Scholar]
- 41.Monteleone M, Stanley AC, Chen KW, Brown DL, Bezbradica JS, von Pein JB, et al. Interleukin-1β maturation triggers its relocation to the plasma membrane for gasdermin-D-dependent and -independent secretion. Cell Rep. 2018;24(6):1425–1433. doi: 10.1016/j.celrep.2018.07.027. [DOI] [PubMed] [Google Scholar]
- 42.Moore KJ, Sheedy FJ, Fisher EA. Macrophages in atherosclerosis: A dynamic balance. Nat. Rev. Immunol. 2013;13:709. doi: 10.1038/nri3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rampanelli E, Orsó E, Ochodnicky P, Liebisch G, Bakker PJ, Claessen N, et al. Metabolic injury-induced NLRP3 inflammasome activation dampens phospholipid degradation. Sci. Rep. 2017;7(1):2861. doi: 10.1038/s41598-017-01994-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Camell C, Goldberg E, Dixit VD. Regulation of Nlrp3 inflammasome by dietary metabolites. Semin. Immunol. 2015;27(5):334–342. doi: 10.1016/j.smim.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Camell CD, Nguyen KY, Jurczak MJ, Christian BE, Shulman GI, Shadel GS, Dixit VD. Macrophage-specific de novo synthesis of ceramide is dispensable for inflammasome-driven inflammation and insulin resistance in obesity. J. Biol. Chem. 2015;290(49):29402–29413. doi: 10.1074/jbc.M115.680199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J. Clin. Investig. 2006;116(7):1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cicero AFG, Kuwabara M, Johnson R, Bove M, Fogacci F, Rosticci M, et al. LDL-oxidation, serum uric acid, kidney function and pulse-wave velocity: Data from the Brisighella Heart Study cohort. Int. J. Cardiol. 2018;261:204–208. doi: 10.1016/j.ijcard.2018.03.077. [DOI] [PubMed] [Google Scholar]
- 48.Verges B, Duvillard L, Lagrost L, Vachoux C, Garret C, Bouyer K, et al. Changes in lipoprotein kinetics associated with type 2 diabetes affect the distribution of lipopolysaccharides among lipoproteins. J. Clin. Endocrinol. Metab. 2014;99(7):E1245–1253. doi: 10.1210/jc.2013-3463. [DOI] [PubMed] [Google Scholar]
- 49.Wiesner P, Leidl K, Boettcher A, Schmitz G, Liebisch G. Lipid profiling of FPLC-separated lipoprotein fractions by electrospray ionization tandem mass spectrometry. J. Lipid Res. 2009;50(3):574–585. doi: 10.1194/jlr.D800028-JLR200. [DOI] [PubMed] [Google Scholar]
- 50.Gurung P, Li B, Subbarao Malireddi RK, Lamkanfi M, Geiger TL, Kanneganti TD. Chronic TLR stimulation controls NLRP3 inflammasome activation through IL-10 mediated regulation of NLRP3 expression and caspase-8 activation. Sci. Rep. 2015;5:14488. doi: 10.1038/srep14488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pedrini MT, Kranebitter M, Niederwanger A, Kaser S, Engl J, Debbage P, et al. Human triglyceride-rich lipoproteins impair glucose metabolism and insulin signalling in L6 skeletal muscle cells independently of non-esterified fatty acid levels. Diabetologia. 2005;48(4):756–766. doi: 10.1007/s00125-005-1684-8. [DOI] [PubMed] [Google Scholar]
- 52.Rutti S, Ehses JA, Sibler RA, Prazak R, Rohrer L, Georgopoulos S, et al. Low- and high-density lipoproteins modulate function, apoptosis, and proliferation of primary human and murine pancreatic beta-cells. Endocrinology. 2009;150(10):4521–4530. doi: 10.1210/en.2009-0252. [DOI] [PubMed] [Google Scholar]
- 53.Larsen CM, Faulenbach M, Vaag A, Volund A, Ehses JA, Seifert B, et al. Interleukin-1B receptor antagonist in type 2 diabetes mellitus. N. Engl. J. Med. 2007;356(15):1517–1526. doi: 10.1056/NEJMoa065213. [DOI] [PubMed] [Google Scholar]
- 54.Sloan-Lancaster J, Abu-Raddad E, Polzer J, Miller JW, Scherer JC, De Gaetano A, et al. Double-blind, randomized study evaluating the glycemic and anti-inflammatory effects of subcutaneous LY2189102, a neutralizing IL-1β antibody, in patients with type 2 diabetes. Diabetes Care. 2013;36(8):2239–2246. doi: 10.2337/dc12-1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Everett BM, Donath MY, Pradhan AD, Thuren T, Pais P, Nicolau JC, et al. Anti-inflammatory therapy with canakinumab for the prevention and management of diabetes. J. Am. Coll. Cardiol. 2018;71(21):2392–2401. doi: 10.1016/j.jacc.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 56.Chen X, Zhang D, Li Y, Wang W, Bei W, Guo J. NLRP3 inflammasome and IL-1β pathway in type 2 diabetes and atherosclerosis: Friend or foe? Pharmacol. Res. 2021;173:105885. doi: 10.1016/j.phrs.2021.105885. [DOI] [PubMed] [Google Scholar]
- 57.Besedovsky HO, Del Rey A. Physiologic versus diabetogenic effects of interleukin-1: A question of weight. Curr. Pharm. Des. 2014;20(29):4733–4740. doi: 10.2174/1381612820666140130204401. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available due to ethical restrictions but are available from the corresponding author upon reasonable request.