Abstract
Polydactyly is the most common limb malformation that occurs in 1.6–10.6 per one thousand live births, with incidence varying with ancestry. The underlying gene has been identified for many of the ~100 syndromes that include polydactyly. While for the more common form, nonsydromic polydactyly, eleven candidate genes have been reported. We investigated the underlying genetic cause of autosomal recessive nonsyndromic postaxial polydactyly in four consanguineous Pakistani families. Some family members with postaxial polydactyly also present with syndactyly, camptodactyly, or clinodactyly. Analysis of the exome sequence data revealed two novel homozygous frameshift deletions in EFCAB7: [c.830delG;p.(Gly277Valfs*5)]; in three families and [c.1350_1351delGA;p.(Asn451Phefs*2)] in one family. Sanger sequencing confirmed that these variants segregated with postaxial polydactyly, i.e., family members with postaxial polydactyly were found to be homozygous while unaffected members were heterozygous or wild type. EFCAB7 displays expressions in the skeletal muscle and on the cellular level in cilia. IQCE-EFCAB7 and EVC-EVC2 are part of the heterotetramer EvC complex, which is a positive regulator of the Hedgehog (Hh) pathway, that plays a key role in limb formation. Depletion of either EFCAB7 or IQCE inhibits induction of Gli1, a direct Hh target gene. Variants in IQCE and GLI1 have been shown to cause nonsyndromic postaxial polydactyly, while variants in EVC and EVC2 underlie Ellis van Creveld and Weyers syndromes, which include postaxial polydactyly as a phenotype. This is the first report of the involvement of EFCAB7 in human disease etiology.
Subject terms: Bone, Consanguinity, Medical genetics
Introduction
Digit malformation is one of the most common skeletal disorders. Gross reduction deficits and more subtle abnormalities in the number, length, and structure of the digits are all examples of digit anomalies. Polydactyly characterized by the presence of extra fingers or toes, is the most common type of digit anomaly. There are several forms of polydactyly that include postaxial, preaxial, and central, with central polydactyly being the rarest form. Polydactyly can be an isolated entity or may be part of a syndrome. It often segregates in families and has an incidence of 1.6–10.6 per one thousand live births, with individuals of African ancestry having the highest occurrence [1–4]. To date, eleven nonsyndromic polydactyly genes (DACH1, FAM92A, GLI1, GLI3, IQCE, KIAA0825, MIPOL1, PITX, SHH, STKLD1, and ZNF141) have been reported [5–13]. In this study, we identified single and two base-pair deletions in EF-hand calcium binding domain 7 (EFCAB7), located on 1p31.3, that segregate with nonsyndromic postaxial polydactyly.
Hedgehog (Hh) signaling pathway plays a key role to regulate the digits pattern in limb formation. A heterotetramer EvC complex, composed of two sub-complexes (IQCE-EFCAB7 and EVC-EVC2), is a positive regulator of Hh pathway. EFCAB7 binds to the C-terminal region of EVC2 and N-terminus of IQCE thus acting as a critical link between IQCE and the EVC-EVC2 complex. Variants in EVC-EVC2 lead to Ellis van Creveld and Weyers syndromes, which include a variety of skeletal phenotypes including postaxial polydactyly. Depletion of either EFCAB7 or IQCE inhibits induction of Gli1 (transcriptional activator), a direct Hh target gene [14]. Pathogenic variants in interaction partners of EFCAB7 including EVC, EVC2, IQCE, and GLI1 have been reported to underlie polydactyly, but EFCAB7 has not been previously reported.
In the present study, we have investigated four consanguineous Pakistani families exhibiting nonsyndromic postaxial polydactyly segregating with an autosomal recessive mode of inheritance. Using exome sequencing we have identified two different homozygous variants in EFCAB7 that segregate with postaxial polydactyly in the families understudy.
Methods
Study approval
This study was performed according to the declaration of Helsinki protocols and approved by the Institutional Review Board (IRB) of Quaid-i-Azam University (IRB-QA-176), Islamabad, COMSATS University Islamabad, Islamabad, University of Balochistan, Quetta, Pakistan, Columbia University Medical Center (IRB-AAAS3421), New York, NY, USA, University of Tübingen (313/2023 A), Tübingen, Germany. Written informed consent was obtained from all participating members over the age of 18. Parents provided written informed consent for their children who were less than 18 years of age at the time of study. For children over the age of 8 years, assent was obtained.
DNA extraction
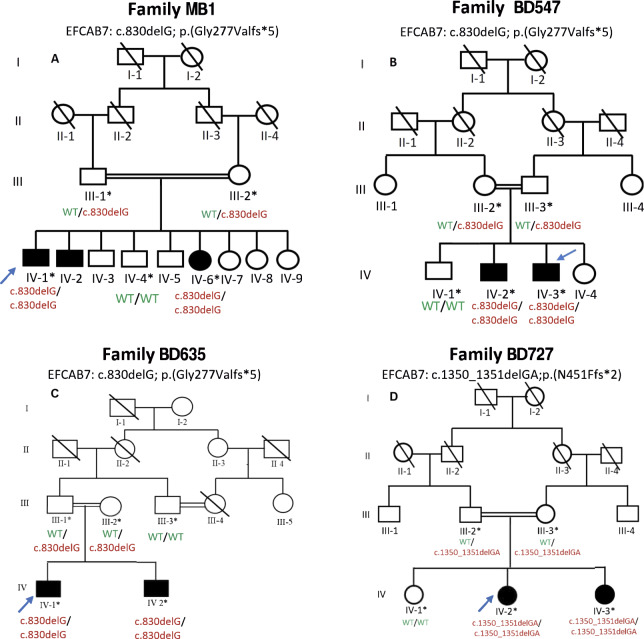
Venipuncture was performed and blood samples were collected in EDTA-containing Vacutainer tubes (Becton Dickinson, Franklin Lakes, NJ, USA) from two affected (IV-1 and IV-6) and three unaffected (III-1, III-2, and IV-4) members of Family MB1; two affected (IV-2 and IV-3) and three unaffected (III-2, III-3, and IV-1) members of Family BD547; two affected (IV-1 and IV-2) and three unaffected (III-1, III-2, and III-3) members of Family BD635; and two affected (IV-2 and IV-3) and three unaffected (III-2, III-3, and IV-1) members of Family BD727 (Fig. 1A-D). Genomic DNA was extracted using GenEluteTM Blood Genomic DNA Kit (Sigma-Aldrich, MO, USA). Extracted DNA was quantified using Nanodrop-1000 spectrophotometer (Thermo Fisher Scientific, Wilmington, DE, USA).
Fig. 1. Pedigrees of postaxial polydactyly families MB1, BD547, BD635, and BD727.
Circles represent females and squares males. Filled symbols designate individuals with postaxial polydactyly. An asterisk indicates a DNA sample was obtained from the family member and a blue arrow indicates that the DNA sample underwent exome sequencing. Below every individual with an available DNA sample is shown their genotype for the EFCAB7 variant segregating in the family. A Family MB1 that segregates c.830delG;p.(Gly277Valfs*5). B Family BD547 that segregates c.830delG;p.(Gly277Valfs*5). C Family BD635 that segregates c.830delG;p.(Gly277Valfs*5). D Family BD727 that segregates c.1350_1351delGA;p.(Asn451Phefs*2).
Exome sequencing and analysis
DNA samples obtained from four affected family members [Family MB1 (IV-1), BD547 (IV-3), Family BD635 (IV-1), and Family BD727 (IV-2)] underwent exome sequencing (Fig. 1A–D). Enrichment of coding sequences was carried out using Agilent SureSelect kit (Family MB1: kit version 11 and Families BD547, BD635, and BD727: kit version 6; Agilent Technologies, Santa Clara, CA, USA). For families MB1 and BD547 sequencing was performed on a NovaSeq 6000 systems (Illumina Inc., San Diego, CA, USA) while for families BD635 and BD727 sequencing was done on an Illumina Hiseq 2000 (Illumina Inc., San Diego, CA, USA). Using the Burrows-Wheeler Aligner-MEM (BWAv0.7.15), reads with low quality were removed and the remaining reads were aligned to the human reference genome (GRCh38/Hg38) [15]. Duplicate reads were marked using Picard-tools (v2.5.0). Single nucleotide variants and insertions/deletions were called using Genome Analysis Toolkit (GATK) v3.7 HaplotypeCaller. Base quality score recalibration and insertion/deletion-realignment were also performed using GATK [16]. Variants were annotated using ANNOVAR [17], dbscSNV1.1, and dbnsfp35a and in-house custom scripts were used to filter to identify candidate variants. Given the pedigree structures (Fig. 1A-D) it was assumed that the mode of inheritance was autosomal recessive. Homozygous or potentially compound heterozygous variants that were exonic, splice region (+/- 12 bp), predicted to have an effect on pre-mRNA splicing or protein function (nonsense, missense, start-loss, frameshift, splice region, etc.), Combined Annotation-Dependent Depletion (CADD) c-score >15 (nonsynonymous variants) [18] with a population-specific minor allele frequency (MAF) < 0.005 in each Genome Aggregation Database (gnomAD) v2.1.1 and v3.1.2 [19] population were retained (Supplementary Table 1).
Sanger validation
Segregation of selected sequence variants was examined using DNA samples obtained from all family members (Fig. 1A–D) that participated in this study by performing bidirectional Sanger sequencing. Primers, for PCR-amplification of the variants, were designed using Primer3.
Results
Clinical description of families
The families were all ascertained from Pakistan. Families MB1 and BD547 hail from Sindh province, family BD635 from Dera Murad Jamali, Balochistan, and family BD727 from Sohbatpur, Balochistan.
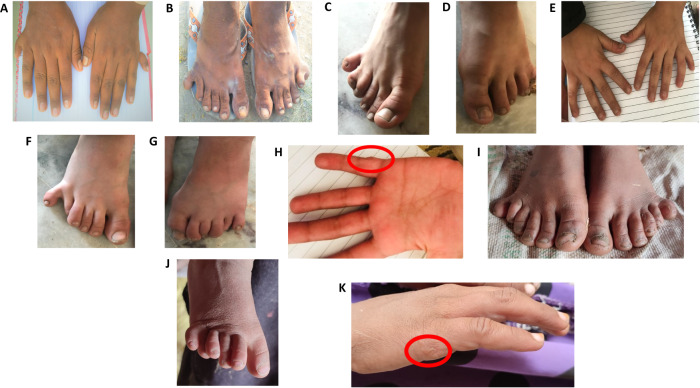
Family MB1: A consanguineous pedigree with two affected (IV-1 male 22 years of age and IV-6 female 16 years of age) and three unaffected (III-1, III-2, and IV-4) members that participated in the study (Fig. 1A). Clinical examinations were performed at the local government hospital, and a diagnosis of nonsyndromic postaxial polydactyly was made for the affected members. The mother (III-2) of the two affected family members had normal pregnancies and the children’s limb abnormalities were apparent at birth. IV-1 presented with a well-developed duplication of a little finger and toe in the hands and feet, respectively (Fig. 2A, B). IV-6 who did not consent to clinical photography, manifests bilateral postaxial polydactyly in hands and feet. Nails were found to be normal for both individuals. No other abnormalities were observed in affected family members.
Fig. 2. Photographs of hands and feet of affected pedigree members.
A Hands of affected individual (IV-1) from Family MB1 who has postaxial polydactyly of both hands. B Feet of affected individual (IV-1) from Family MB1 showing postaxial polydactyly. C–E Hands and feet of affected individual (IV-1) from Family BD635 manifesting postaxial polydactyly with bilateral syndactyly in feet only. F–H Affected individual (IV-2) from BD635 exhibiting bilateral postaxial polydactyly in the feet. Extra digits of the hands that were surgically removed are circled in red. I, J Feet of affected individual (IV-3) from Family BD727 manifesting bilateral postaxial polydactyly, polydactyly of the hands was surgically corrected. K Affected individual (IV-2) from Family BD727 exhibiting bilateral postaxial polydactyly in feet only.
Family BD547: A consanguineous pedigree with two affected males (I1:2, 25 years of age and II:3, 21 years of age) and three unaffected (III-1, III-2, and IV- I) members that participated in the study (Fig. 1B). Both affected members had nonsyndromic postaxial polydactyly and presented with bilateral well-developed duplication of fifth digit in the hands and feet. Affected individual (IV-3) also manifested clinodactyly, and camptodactyly of the little fingers of the hands. Affected members of this family did not wish to provide clinical photographs of their hands and feet for publication.
Family BD635: A consanguineous pedigree with two affected males (IV-1, 11 years of age and IV-2, 10 years of age) and three unaffected (III-1, III-2, and III-3) members who participated in the study (Fig. 1C). IV-1 was born via normal delivery and at birth he was diagnosed with nonsydromic bilateral postaxial polydactyly of the hands. His right-hand underwent surgery to correct the polydactyly (Fig. 2C). He was also diagnosed with bilateral postaxial polydactyly of feet: right foot syndactyly of 4th and 5th toes and left foot syndactyly of 4th, 5th, and 6th toes (Fig. 2D, E). IV-2 who was also born via normal delivery and at birth also exhibited nonsyndromic bilateral postaxial polydactyly of the hands that were surgically corrected (Fig. 2F) and bilateral postaxial polydactyly of the feet with the little toe of right foot displaying slight radial drift (Fig. 2G, H).
Family BD727: A consanguineous family with two affected female members (IV-2, 15 years of age and IV-3, 8 years of age) and three unaffected members (III-1, III-2, and IV-1) that participated in the study (Fig. 1D). The two affected females were diagnosed with nonsyndromic postaxial polydactyly. IV-3 exhibited bilateral postaxial polydactyl in both hands and feet. The extra digits from her hands were surgically excised (Fig. 2I, J). IV-2 displayed bilateral postaxial polydactyl in the feet (Fig. 2K). No polydactyly was observed in her hands.
Molecular analysis
Cross-checking of the prioritized variants in exomes of the four families segregating polydactyly led to the identification of EFCAB7 as the likely underlying genetic cause of the postaxial polydactyly segregating in the families. Two different homozygous frameshift variants were identified in EFCAB7: [c.830delG;p.(Gly277Valfs*5); (families MB1, BD547, and BD635)] and [c.1350_1351delGA;p.(Asn451Phefs*2); (family BD727)]. EFCAB7 is an interacting partner of a previously identified polydactyly gene IQCE, EFCAB7-IQCE module anchors the EVC-EVC2 complex, and EVC and EVC2 both underlie syndromes which include polydactyly. Therefore, EFCAB7 is a strong candidate for postaxial polydactyly and the only gene for which all four families were found to carry potentially disease-causing variants. Variant c.830delG; p.(Gly277Valfs5) is present in gnomAD v2.1.1 with a MAF = 3.997 ×10-6 but it is absent in gnomAD v3.1.2 [17], the Trans-Omics for Precision Medicine (TOPMed) BRAVO database [20], the Greater Middle East Variome project database [21], and the All of Us research program data [22]. Variant c.1350_1351delGA; p.(Asn451Phefs2) has a MAF = 7.114 ×10-5 in gnomAD v2.1.1, MAF = 1.317 ×10-5 in gnomAD v3.1.2, and MAF = 1.889 ×10-5 in TOPMed BRAVO, but it is absent in All of Us and the Greater Middle East Variome project database. None of the other variants which passed the filtering criteria are involved in skeletal development (Supplementary Table 1).
Sanger sequencing of DNA samples obtained from all available family members validated the frameshift variants and confirmed segregation with postaxial polydactyly in each family (Fig. 1A–D).
Discussion
The four consanguineous families that were studied all segregated nonsyndromic postaxial polydactyly, but some affected family members had additional features. In family BD547, affected individual IV-3 in addition to presenting with postaxial polydactyly also manifested clinodactyly and camptodactyly and in family BD727 affected individual IV-1 had postaxial polydactyly and bilateral syndactyly of feet (Fig. 2C, D). In all the affected individuals, the postaxial polydactyly was not restricted to any specific type. Phenotypic variability was observed intrafamilial as well as interfamilial. Clinical variability reported in families with polydactyly might be due to epigenetics, genetic modifiers, and environmental factors [23].
The two frameshift variants that were identified likely initiates nonsense-medicated decay. If a stop codon is established >50-55 bases upstream of the 3’ last exon-exon junction it is predicted to initiate nonsense-mediated decay in mammals [24]. It is highly likely that the mutant mRNA is degraded through nonsense-mediated mRNA decay, with no or little expression of the protein.
EFCAB7 functions as an adaptor to link the EVC-EVC2 complex to IQCE, using ECH2 to engage the W-peptide in EVC2 [9, 14]. Dorn et al. (2012) have suggested that EVC–EVC2 complex is involved in regulation of SMO-GLI step in Hh signaling [25]. Pusapati et al. (2014) have verified experimentally that IQCE-EFCAB7 complex plays an important role in the step between SMO-GLI proteins and depletion of both IQCE and EFCAB7 affects the signaling via SMO which in turn affects the Hh signaling [14]. Hh signaling is an evolutionary conserved pathway of intercellular communication which is one of the major regulators of vertebrate development [26].
In tissues, EFCAB7 is expressed in the testis, fallopian tubes, retina, and skeletal muscle and shows a prominent expression in cilia. EFCAB7 is also expressed in the mouse forelimb during embryonic development [27]. Efcab7 knockout mice have abnormal auditory response and decreased total protein level [28]. The affected family members in this study did not present with hearing impairment. Additionally, to our knowledge this is the first report of EFCAB7 being involved in human disease etiology.
EVC, EVC2, EFCAB7, and IQCE form a protein complex. EFCAB7-IQCE module anchors the EVC-EVC2 complex in a signaling microdomain at the base of cilia [29]. EVC and EVC2 variants are involved in underlying Ellis-van Creveld syndrome and Weyers acrofacial dysostosis. Both these syndromes include polydactyly [30] and IQCE variants have been shown to cause nonsyndromic postaxial polydactyly type A7. Additionally, TTC23 protein is associated with IQCE and EFCAB7 in the EvC complex [29], with variants in the TTC23 gene being associated with orofacial digital syndrome that also includes polydactyly as a feature [31, 32]. EFCAB7 also alters interaction and expression of ciliary proteins of EVC-EVC2 complex [33]. Taken together, these findings suggest that polydactyly is one of the prominent features associated with EvC complex gene variants.
In conclusion, we have presented evidence that EFCAB7 underlies postaxial polydactyly in humans. Polydactyly in humans is genetically and phenotypically heterogeneous disorder. Identification of novel genes implicated in the congenital polydactyly is important to understand development of limbs for both patient management and the development of therapeutic strategies.
Web resources
Bravo TOPMed, https://bravo.sph.umich.edu/freeze8/hg38/
Burrows-Wheeler Aligner, http://bio-bwa.sourceforge.net
The Human Protein Atlas, https://www.proteinatlas.org/humanproteome/tissue
Genome Analysis Toolkit (GATK), https://gatk.broadinstitute.org/hc/en-us
Greater Middle East (GME) Variome project, http://igm.ucsd.edu/gme/
Genome Aggregation Database (gnomAD), https://gnomad.broadinstitute.org/
Primer3, https://primer3.ut.ee/
Supplementary information
Acknowledgements
We are highly obliged to members of the families for their cooperation and participation in this study. Muhammad Bilal, Hammal Khan and Muhammad Javed Khan were supported by Indigenous PhD and IRSIP fellowships from Higher Education Commission (HEC), Islamabad, Pakistan.
Author contributions
MB, HK, and MJK performed the laboratory experiments, analyzed the genomic data, and drafted the manuscript. IS, KL, AA, TB, N, HA, SA, KU, MSH, and AU collected samples and analyzed clinical and genomic data. WA and SML designed the project, analyzed the data, edited the manuscript, and provided funds for the study.
Funding
This research was funded by Higher Education Commission of Pakistan, Pediatric Genomics Discovery Program, and the Department of Neurology, Columbia University Medical Center.
Data availability
The identified variants have been submitted to the ClinVar database [Accession numbers: SCV003804323 and SCV003804374].
Competing interests
The authors declare no competing interest.
Ethical approval
Written informed consent for presentation and publication of this study was obtained from all the participating members.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41431-023-01450-5.
References
- 1.Ahmad Z, Liaqat R, Palander O, Bilal M, Zeb S, Ahmad F, et al. Genetic overview of postaxial polydactyly: Updated classification. Clin Genet. 2023;103:3–15. doi: 10.1111/cge.14224. [DOI] [PubMed] [Google Scholar]
- 2.Umair M, Ahmad F, Bilal M, Ahmad W, Alfadhel M. Clinical genetics of polydactyly: An updated review. Front Genet. 2018;9:447. doi: 10.3389/fgene.2018.00447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pompe van Meerdervoort HF. Congenital musculoskeletal malformation in South Africa Blakcs-a study of incidence. S Afr Med J. 1976;50:1853–5. [PubMed] [Google Scholar]
- 4.Woolf CM, Myrianthopoulos NC. Polydactyly in American negroes and whites. American. J Hum Genet. 1973;25:397. [PMC free article] [PubMed] [Google Scholar]
- 5.Kondoh S, Sugawara H, Harada N, Matsumoto N, Ohashi H, Sato M, et al. A novel gene is disrupted at a 14q13 breakpoint of t(2;14) in a patient with mirror-image polydactyly of hands and feet. J Hum Genet. 2002;47:136–9. doi: 10.1007/s100380200015. [DOI] [PubMed] [Google Scholar]
- 6.Klopocki E, Kähler C, Foulds N, Shah H, Joseph B, Vogel H, et al. Deletions in PITX1 cause a spectrum of lower-limb malformations including mirror-image polydactyly. Eur J Hum Genet. 2012;20:705–8. doi: 10.1038/ejhg.2011.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalsoom UE, Klopocki E, Wasif N, Tariq M, Khan S, Hecht J, et al. Whole exome sequencing identified a novel zinc-finger gene ZNF141 associated with autosomal recessive postaxial polydactyly type A. J Med Genet. 2013;50:47–53. doi: 10.1136/jmedgenet-2012-101219. [DOI] [PubMed] [Google Scholar]
- 8.Bakar A, Ullah A, Bibi N, Khan H, Rahman AU, Ahmad W, et al. A novel homozygous variant in the GLI1 underlies postaxial polydactyly in a large consanguineous family with intra familial variable phenotypes. Eur J Med Genet. 2022;65:104599. doi: 10.1016/j.ejmg.2022.104599. [DOI] [PubMed] [Google Scholar]
- 9.Umair M, Shah K, Alhaddad B, Haack TB, Graf E, Strom TM, et al. Exome sequencing revealed a splice site variant in the IQCE gene underlying post-axial polydactyly type A restricted to lower limb. Eur J Hum Genet. 2017;25:960–5. doi: 10.1038/ejhg.2017.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ullah I, Kakar N, Schrauwen I, Hussain S, Chakchouk I, Liaqat K, et al. Variants in KIAA0825 underlie autosomal recessive postaxial polydactyly. Hum Genet. 2019;138:593–600. doi: 10.1007/s00439-019-02000-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Umair M, Wasif N, Albalawi AM, Ramzan K, Alfadhel M, Ahmad W, et al. Exome sequencing revealed a novel loss‐of‐function variant in the GLI3 transcriptional activator 2 domain underlies nonsyndromic postaxial polydactyly. Mol Genet Genom Med. 2019;7:e00627. doi: 10.1002/mgg3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schrauwen I, Giese AP, Aziz A, Lafont DT, Chakchouk I, Santos-Cortez RLP, et al. FAM92A underlies nonsyndromic postaxial polydactyly in humans and an abnormal limb and digit skeletal phenotype in mice. J Bone Min Res. 2019;34:375–86. doi: 10.1002/jbmr.3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Umair M, Palander O, Bilal M, Almuzzaini B, Alam Q, Ahmad F, et al. Biallelic variant in DACH1, encoding Dachshund Homolog 1, defines a novel candidate locus for recessive postaxial polydactyly type A. Genomics. 2021;113:2495–502. doi: 10.1016/j.ygeno.2021.05.015. [DOI] [PubMed] [Google Scholar]
- 14.Pusapati GV, Hughes CE, Dorn KV, Zhang D, Sugianto P, Aravind L, et al. EFCAB7 and IQCE regulate hedgehog signaling by tethering the EVC-EVC2 complex to the base of primary cilia. Dev Cell. 2014;28:483–96. doi: 10.1016/j.devcel.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;2009:1754–60. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, et al. The Genome AnalysisToolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20:1297–303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang H, Wang K. Genomic variant annotation and prioritization with ANNOVAR and wANNOVAR. Nat Protoc. 2015;10:1556–66. doi: 10.1038/nprot.2015.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kircher M, Witten DM, Jain P, O’roak BJ, Cooper GM, Shendure J. A general framework for estimating the relative pathogenicity of human genetic variants. Nat Genet. 2014;46:310–5. doi: 10.1038/ng.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karczewski KJ, Francioli LC, Tiao G, Cummings BB, Alföldi J, Wang Q, et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature. 2020;581:434–43. doi: 10.1038/s41586-020-2308-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kowalski MH, Qian H, Hou Z, Rosen JD, Tapia AL, Shan Y, et al. Use of >100,000 NHLBI Trans-Omics for PrecisionMedicine (TOPMed) Consortium whole genome sequences improves imputation quality anddetection of rare variant associations in admixed African and Hispanic/Latino populations. PLOS Genet. 2019;15:e1008500. doi: 10.1371/journal.pgen.1008500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scott EM, Halees A, Itan Y, Spencer EG, He Y, Azab MA, et al. Characterization of Greater Middle Eastern genetic variation for enhanced disease gene discovery. Nat Genet. 2016;48:1071–6. doi: 10.1038/ng.3592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lyles CR, Lunn MR, Obedin-Maliver J, Bibbins-Domingo K. The new era of precision population health: insights for the All of Us Research Program and beyond. J Transl Med. 2018;16:1–4. doi: 10.1186/s12967-018-1585-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khan H, Ahmed S, Nawaz S, Ahmad W, Rafiq MA. Greig cephalopolysyndactyly syndrome: phenotypic variability associated with variants in two different domains of GLI3. Klin Padiatr. 2021;233:53–8. doi: 10.1055/a-1223-2489. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Z, Xin D, Wang P, Zhou L, Hu L, Kong X, et al. Noisy splicing, more than expression regulation, explains why some exons are subject to nonsense-mediated mRNA decay. BMC Biol. 2009;7:1–3. doi: 10.1186/1741-7007-7-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dorn KV, Hughes CE, Rohatgi R. A Smoothened-Evc2 complex transduces the Hedgehog signal at primary cilia. Dev Cell. 2012;23:823–35. doi: 10.1016/j.devcel.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gigante ED, Caspary T. Signaling in the primary cilium through the lens of the Hedgehog pathway. Wiley Interdiscip Rev: Dev Biol. 2020;9:e377. doi: 10.1002/wdev.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Speir ML, Bhaduri A, Markov NS, Moreno P, Nowakowski TJ, Papatheodorou I, et al. UCSC Cell Browser: visualize your single-cell data. Bioinformatics. 2021;37:4578–80. doi: 10.1093/bioinformatics/btab503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muñoz-Fuentes V, Cacheiro P, Meehan TF, Aguilar-Pimentel JA, Brown SD, Flenniken AM, et al. The International Mouse Phenotyping Consortium (IMPC): a functional catalogue of the mammalian genome that informs conservation. Conserv Genet. 2018;19:995–1005. doi: 10.1007/s10592-018-1072-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Breslow DK, Hoogendoorn S, Kopp AR, Morgens DW, Vu BK, Kennedy MC, et al. A CRISPR-based screen for Hedgehog signaling provides insights into ciliary function and ciliopathies. Nat Genet. 2018;50:460–71. doi: 10.1038/s41588-018-0054-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Umair M, Seidel H, Ahmed I, Ullah A, Haack TB, Alhaddad B, et al. Ellis–van Creveld syndrome and profound deafness resulted by sequence variants in the EVC/EVC2 and TMC1 genes. J Genet. 2017;96:1005–14. doi: 10.1007/s12041-017-0868-6. [DOI] [PubMed] [Google Scholar]
- 31.Hussain S, Nawaz S, Khan H, Acharya A, Schrauwen I, Ahmad W, et al. A splice site variant in TCTN3 underlies an atypical form of orofaciodigital syndrome IV. Ann Hum Genet. 2022;86:291–6. doi: 10.1111/ahg.12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shamseldin HE, Shaheen R, Ewida N, Bubshait DK, Alkuraya H, Almardawi E, et al. The morbid genome of ciliopathies: an update. Genet Med. 2020;22:1051–60. doi: 10.1038/s41436-020-0761-1. [DOI] [PubMed] [Google Scholar]
- 33.Nguyen TQ, Doan NM, Trinh HT, Mizuguchi M. Novel mutation in EFCAB7 alters expression and interaction of Ellis–van Creveld ciliary proteins. Congenit Anom. 2019;59:49–50. doi: 10.1111/cga.12291. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The identified variants have been submitted to the ClinVar database [Accession numbers: SCV003804323 and SCV003804374].