Abstract
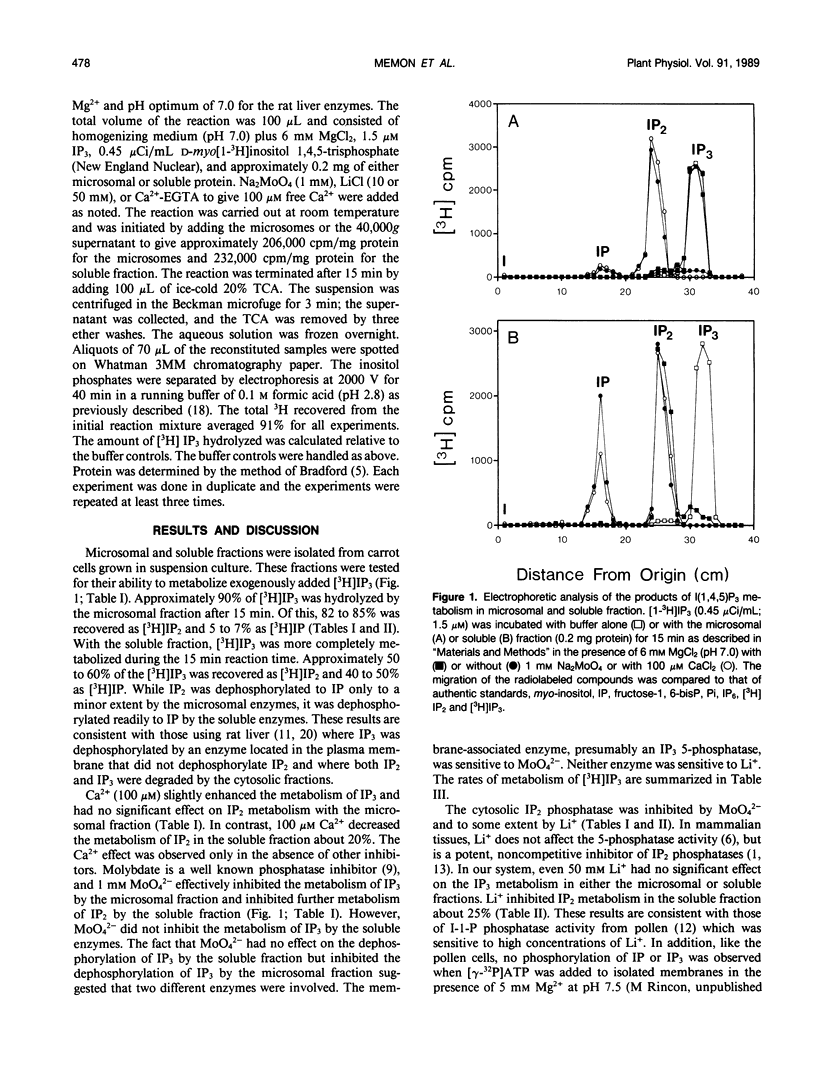
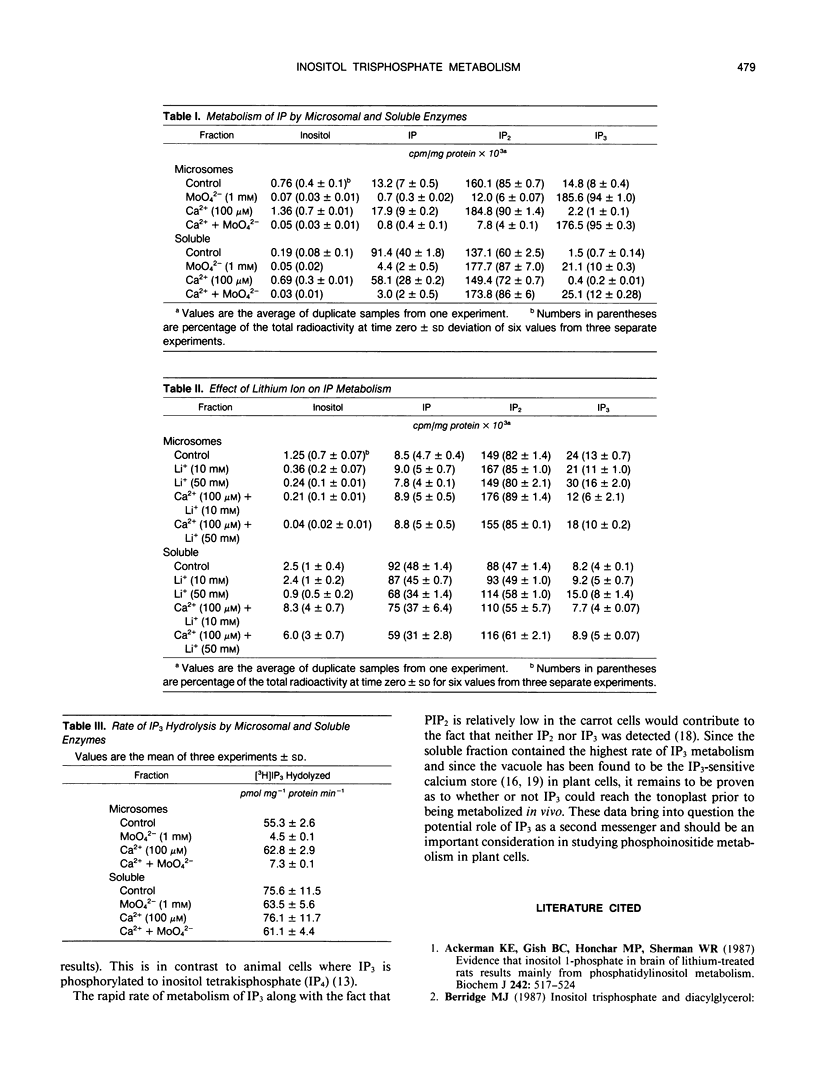
The metabolism of exogenously added d-myo-[1-3H]inositol 1,4,5-trisphosphate (IP3) has been examined in microsomal membrane and soluble fractions of carrot (Daucus carota L.) cells grown in suspension culture. When [3H]IP3 was added to a microsomal membrane fraction, [3H]IP2 was the primary metabolite consisting of approximately 83% of the total recovered [3H] by paper electrophoresis. [3H]IP was only 6% of the [3H] recovered, and 10% of the [3H]IP3 was not further metabolized. In contrast, when [3H]IP3 was added to the soluble fraction, approximately equal amounts of [3H]IP2 and [3H]IP were recovered. Ca2+ (100 micromolar) tended to enhance IP3 dephosphorylation but inhibited the IP2 dephosphorylation in the soluble fraction by about 20%. MoO42− (1 millimolar) inhibited the dephosphorylation of IP3 by the microsomal fraction and the dephosphorylation of IP2 by the soluble fraction. MoO42−, however, did not inhibit the dephosphorylation of IP3 by the soluble fraction. Li+ (10 and 50 millimolar) had no effect on IP3 metabolism in either the soluble or membrane fraction; however, Li+ (50 millimolar) inhibited IP2 dephosphorylation in the soluble fraction about 25%.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackermann K. E., Gish B. G., Honchar M. P., Sherman W. R. Evidence that inositol 1-phosphate in brain of lithium-treated rats results mainly from phosphatidylinositol metabolism. Biochem J. 1987 Mar 1;242(2):517–524. doi: 10.1042/bj2420517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boss W. F., Massel M. O. Polyphosphoinositides are present in plant tissue culture cells. Biochem Biophys Res Commun. 1985 Nov 15;132(3):1018–1023. doi: 10.1016/0006-291x(85)91908-4. [DOI] [PubMed] [Google Scholar]
- Boss W. F., Ruesink A. W. Isolation and Characterization of Concanavalin A-labeled Plasma Membranes of Carrot Protoplasts. Plant Physiol. 1979 Dec;64(6):1005–1011. doi: 10.1104/pp.64.6.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Connolly T. M., Bross T. E., Majerus P. W. Isolation of a phosphomonoesterase from human platelets that specifically hydrolyzes the 5-phosphate of inositol 1,4,5-trisphosphate. J Biol Chem. 1985 Jul 5;260(13):7868–7874. [PubMed] [Google Scholar]
- Drøbak B. K., Ferguson I. B., Dawson A. P., Irvine R. F. Inositol-containing lipids in suspension-cultured plant cells: an isotopic study. Plant Physiol. 1988 May;87(1):217–222. doi: 10.1104/pp.87.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettlinger C., Lehle L. Auxin induces rapid changes in phosphatidylinositol metabolites. Nature. 1988 Jan 14;331(6152):176–178. doi: 10.1038/331176a0. [DOI] [PubMed] [Google Scholar]
- Gallagher S. R., Leonard R. T. Effect of vanadate, molybdate, and azide on membrane-associated ATPase and soluble phosphatase activities of corn roots. Plant Physiol. 1982 Nov;70(5):1335–1340. doi: 10.1104/pp.70.5.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph S. K., Williams R. J. Subcellular localization and some properties of the enzymes hydrolysing inositol polyphosphates in rat liver. FEBS Lett. 1985 Jan 28;180(2):150–154. doi: 10.1016/0014-5793(85)81061-9. [DOI] [PubMed] [Google Scholar]
- Loewus M. W., Loewus F. A. myo-Inositol-1-Phosphatase from the Pollen of Lilium longiflorum Thunb. Plant Physiol. 1982 Sep;70(3):765–770. doi: 10.1104/pp.70.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majerus P. W., Connolly T. M., Bansal V. S., Inhorn R. C., Ross T. S., Lips D. L. Inositol phosphates: synthesis and degradation. J Biol Chem. 1988 Mar 5;263(7):3051–3054. [PubMed] [Google Scholar]
- Melin P. M., Sommarin M., Sandelius A. S., Jergil B. Identification of Ca2+-stimulated polyphosphoinositide phospholipase C in isolated plant plasma membranes. FEBS Lett. 1987 Oct 19;223(1):87–91. doi: 10.1016/0014-5793(87)80515-x. [DOI] [PubMed] [Google Scholar]
- Morse M. J., Crain R. C., Satter R. L. Light-stimulated inositolphospholipid turnover in Samanea saman leaf pulvini. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7075–7078. doi: 10.1073/pnas.84.20.7075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rincón M., Chen Q., Boss W. F. Characterization of Inositol Phosphates in Carrot (Daucus carota L.) Cells. Plant Physiol. 1989 Jan;89(1):126–132. doi: 10.1104/pp.89.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumaker K. S., Sze H. Inositol 1,4,5-trisphosphate releases Ca2+ from vacuolar membrane vesicles of oat roots. J Biol Chem. 1987 Mar 25;262(9):3944–3946. [PubMed] [Google Scholar]
- Seyfred M. A., Farrell L. E., Wells W. W. Characterization of D-myo-inositol 1,4,5-trisphosphate phosphatase in rat liver plasma membranes. J Biol Chem. 1984 Nov 10;259(21):13204–13208. [PubMed] [Google Scholar]



