Abstract
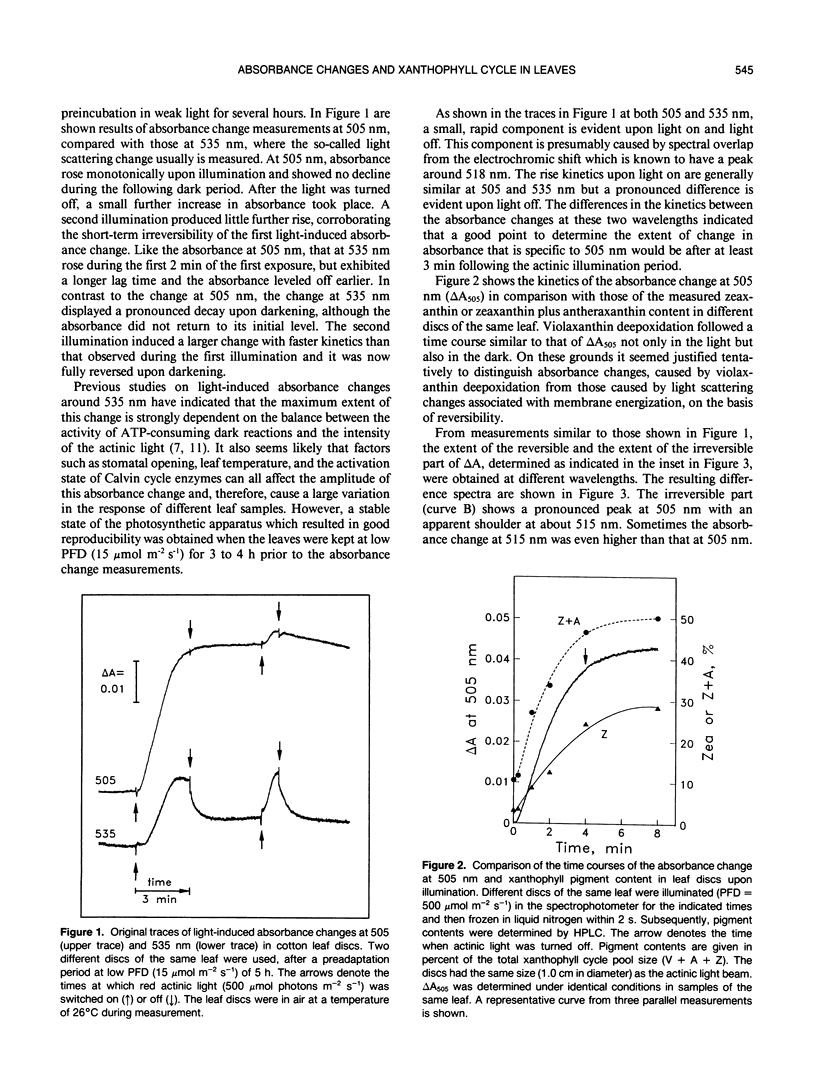
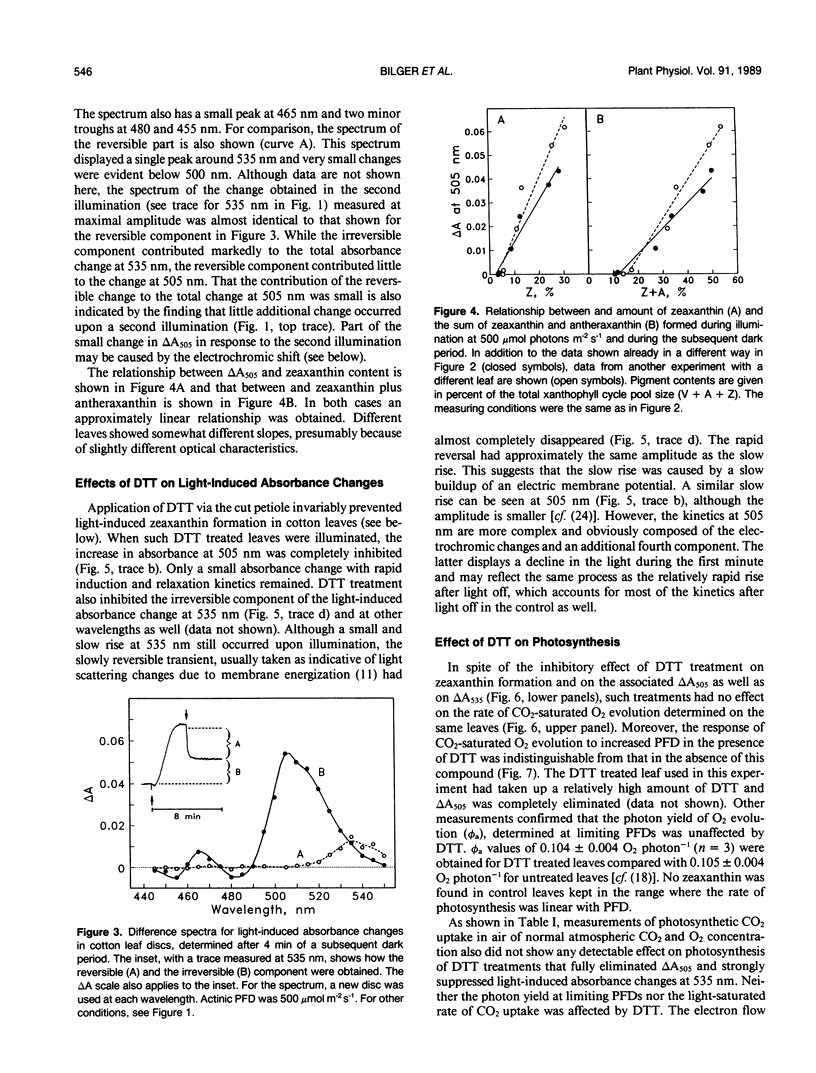
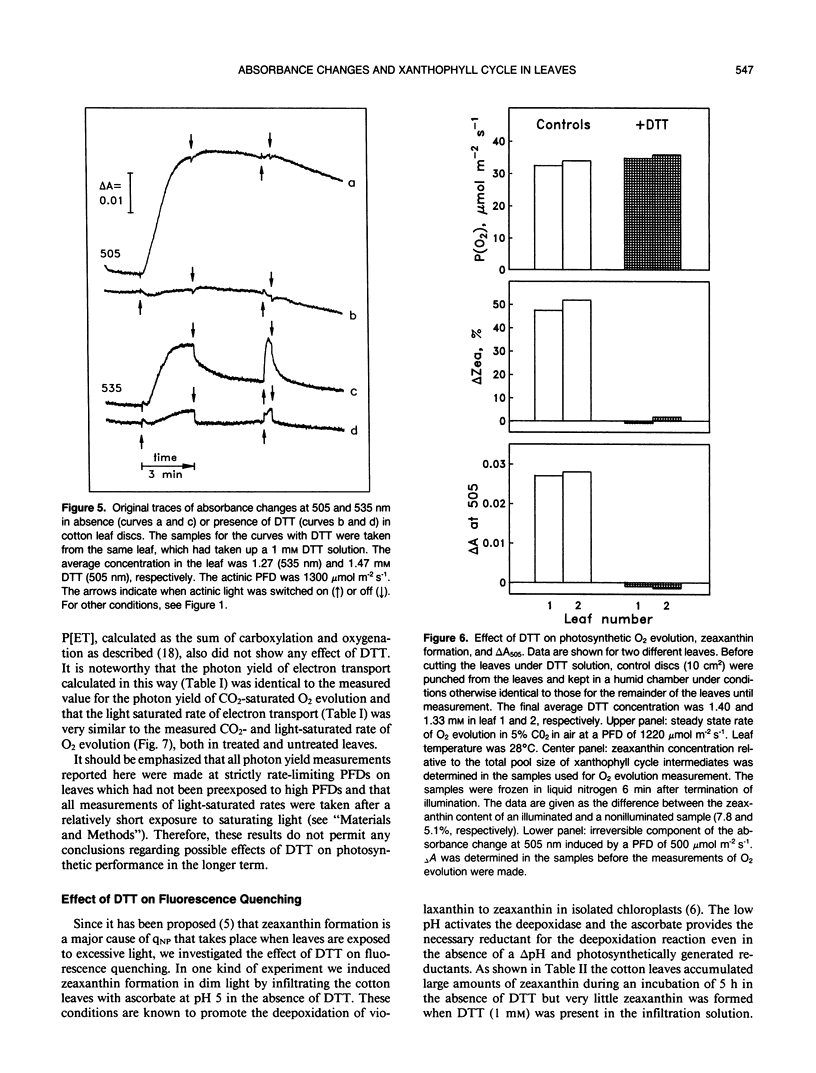
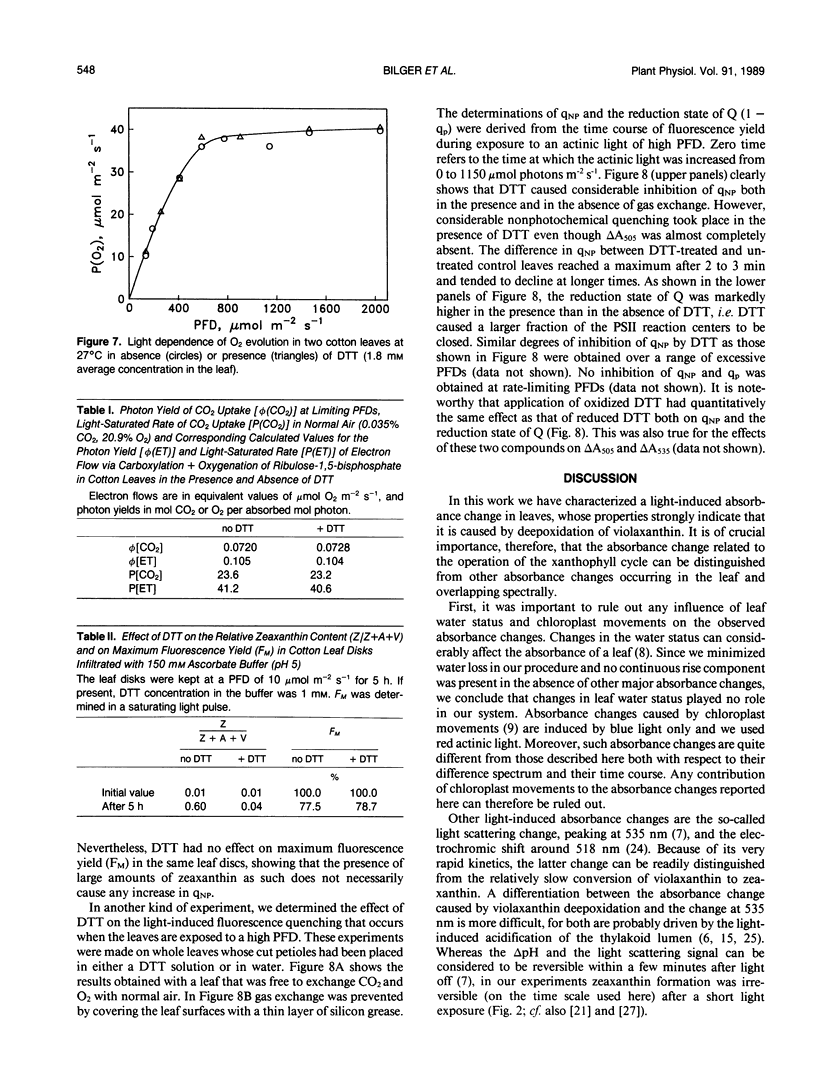
When cotton (Gossypium hirsutum L., cv Acaia SJC-1) leaves kept in weak light were suddenly exposed to strong red actinic light a spectral absorbance change took place having the following prominent characteristics. (a) It was irreversible within the first four minute period after darkening. (b) The difference in leaf absorbance between illuminated and predarkened leaves had a major peak at 505 nanometers, a minor peak at 465 nanometers, a shoulder around 515 nanometers, and minor troughs at 455 and 480 nanometers. (c) On the basis of its spectral and kinetic characteristics this absorbance change can be readily distinguished from the much faster electrochromic shift which has a peak at 515 nanometers, from the slow, so-called light-scattering change which has a broad peak centered around 535 nanometers and is reversed upon darkening, and from absorbance changes associated with light-induced chloroplast rearrangements. (d) The extent and time course of this absorbance change closely matched that of the deepoxidation of violaxanthin to zeaxanthin in the same leaves. (e) Both the absorbance change and the ability to form zeaxanthin were completely blocked in leaves to which dithiothreitol (DTT) had been provided through the cut petlole. DTT treatment also caused strong inhibition of that component of the 535-nanometer absorbance change which is reversed in less than 4 minutes upon darkening and considered to be caused by increased light scattering. Moreover, DTT inhibited a large part of nonphotochemical quenching of chlorophyll fluorescence in the presence of excessive light. However, DTT had no detectable effect on the photon yield of photosynthesis measured under strictly rate-limiting photon flux densities or on the light-saturated photosynthetic capacity, at least in the short term. We conclude that it is possible to monitor light-induced violaxanthin de-epoxidation in green intact leaves by measurement of the absorbance change at 505 nanometers. Determination of absorbance changes in conjunction with measurements of photosynthesis in the presence and absence of DTT provide a system well suited for future studies of meachanisms of dissipation of excessive excitation energy in intact leaves.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Demmig B., Winter K., Krüger A., Czygan F. C. Photoinhibition and zeaxanthin formation in intact leaves : a possible role of the xanthophyll cycle in the dissipation of excess light energy. Plant Physiol. 1987 Jun;84(2):218–224. doi: 10.1104/pp.84.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demmig B., Winter K., Krüger A., Czygan F. C. Zeaxanthin and the Heat Dissipation of Excess Light Energy in Nerium oleander Exposed to a Combination of High Light and Water Stress. Plant Physiol. 1988 May;87(1):17–24. doi: 10.1104/pp.87.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heber U. Conformational changes of chloroplasts induced by illumination of leaves in vivo. Biochim Biophys Acta. 1969 Jun 24;180(2):302–319. doi: 10.1016/0005-2728(69)90116-9. [DOI] [PubMed] [Google Scholar]
- Kitajima M., Butler W. L. Quenching of chlorophyll fluorescence and primary photochemistry in chloroplasts by dibromothymoquinone. Biochim Biophys Acta. 1975 Jan 31;376(1):105–115. doi: 10.1016/0005-2728(75)90209-1. [DOI] [PubMed] [Google Scholar]
- Krause G. H. The high-energy state of the thylakoid system as indicated by chlorophyll fluorescence and chloroplast shrinkage. Biochim Biophys Acta. 1973 Apr 5;292(3):715–728. doi: 10.1016/0005-2728(73)90019-4. [DOI] [PubMed] [Google Scholar]
- LATIMER P., RABINOWITCH E. Selective scattering of light by pigments in vivo. Arch Biochem Biophys. 1959 Oct;84:428–441. doi: 10.1016/0003-9861(59)90605-8. [DOI] [PubMed] [Google Scholar]
- Sokolove P. M., Marsho T. V. Ascorbate-independent carotenoid de-epoxidation in intact spinach chloroplasts. Biochim Biophys Acta. 1976 May 14;430(2):321–326. doi: 10.1016/0005-2728(76)90088-8. [DOI] [PubMed] [Google Scholar]
- Thorne S. W., Horvath G., Kahn A., Boardman N. K. Light-dependent absorption and selective scattering changes at 518 nm in chloroplast thylakoid membranes. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3858–3862. doi: 10.1073/pnas.72.10.3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt H. T. Energy conversion in the functional membrane of photosynthesis. Analysis by light pulse and electric pulse methods. The central role of the electric field. Biochim Biophys Acta. 1979 Mar 14;505(3-4):355–427. doi: 10.1016/0304-4173(79)90008-9. [DOI] [PubMed] [Google Scholar]
- Yamamoto H. Y., Kamite L. The effects of dithiothreitol on violaxanthin de-epoxidation and absorbance changes in the 500-nm region. Biochim Biophys Acta. 1972 Jun 23;267(3):538–543. doi: 10.1016/0005-2728(72)90182-x. [DOI] [PubMed] [Google Scholar]
- Yamamoto H. Y., Kamite L., Wang Y. Y. An Ascorbate-induced Absorbance Change in Chloroplasts from Violaxanthin De-epoxidation. Plant Physiol. 1972 Feb;49(2):224–228. doi: 10.1104/pp.49.2.224. [DOI] [PMC free article] [PubMed] [Google Scholar]


