Abstract
A 48-year-old woman presented with hyperreninemic hypertension and renal dysfunction and was diagnosed with hydronephrosis-related Page kidney. The pathophysiology was “renal tamponade”, in which the kidney was compressed by the renal pelvis and Gerota’s fascia, resulting in intrarenal microvascular ischemia. Ureteral stent placement promptly improved the hyperreninemic hypertension and renal dysfunction, and additional perirenal fluid drainage gradually improved these conditions. These observations indicated the following three points. First, renal compression-induced renin–angiotensin–aldosterone system upregulation plays an important role in the pathogenesis of Page kidney. Second, physicians should consider perirenal fluid drainage as a therapeutic option in addition to ureteral stenting in patients with hydronephrosis-related Page kidney. Third, bilateral perirenal subcapsular hematomas in this case could be caused by hydronephrosis. Hydronephrosis-induced intrarenal pressure elevation possibly caused chronic perirenal subcapsular hemorrhage at the vulnerable sites of the renal cortex and peeling of the renal capsule from the cortex, resulting in the bilateral massive subcapsular hematomas and Page kidney.
Keywords: Hydronephrosis-related Page kidney, Renal tamponade, Renal compression, Renin–angiotensin–aldosterone system (RAAS)
Introduction
In 1955, Page et al. reported an unusual case in which severe hypertension improved following the surgical removal of both kidneys that had perirenal fluid collection [1]. Based on animal experiments in which hypertension was produced by wrapping a kidney in cellophane [2], it was concluded that changes in intrarenal hemodynamics, rather than the compression of the renal artery, led to hypertension. This unusual phenomenon was named Page kidney and is believed to occur when external compression of the kidney activates the renin–angiotensin–aldosterone system (RAAS) via microvascular ischemia [3–24]. We herein report an unusual case of hydronephrosis-related Page kidney in which “renal tamponade” occurred due to renal compression from the renal pelvis and Gerota’s fascia.
Case report
A 48-year-old Japanese woman presented with severe hypertension and renal dysfunction. She reported a 3-month history of malaise, anorexia, and bilateral lower back pain. Severe hypertension (194/129 mmHg) was noted at presentation; however, other vital signs and physical examinations were unremarkable. Blood tests revealed renal dysfunction and high renin and aldosterone levels: serum creatinine, 4.01 mg/dL; plasma renin activity, 5.6 ng/mL/h; and aldosterone, 282 pg/mL (Table 1). Chest X-ray, electrocardiogram, and cardiac and abdominal ultrasound did not reveal pulmonary edema, myocardial infarction, cardiac heart failure, or renal artery stenosis. Abdominal computed tomography and ultrasound demonstrated bilateral hydronephrosis and perirenal fluid collection with computed tomography numbers of 10–30 Hounsfield Units (Fig. 1A–B).
Table 1.
Laboratory findings on the first attendance and on perirenal fluid drainage
| On the first attendance | On perirenal fluid drainage | ||||||
|---|---|---|---|---|---|---|---|
| <Blood test> | <Blood test> | <Perirenal fluid component> | |||||
| Blood cell count | Coagulation | Na | 141 mEq/L | Specific gravity | 1.020 | ||
| WBC | 8450/μL | PT-INR | 1.01 | K | 4.5 mEq/L | pH | 8.0 |
| RBC | 3.66×106/μL | APTT | 27.1 s | Cl | 104 mEq/L | Protein | (2+) 3.0 g/dL |
| Hb | 10.3 g/dL | Fibrinogen | 701 mg/dL | Cre | 1.50 mg/dL | Occult blood | (3+) |
| Hct | 33.7 % | Endocrine | BUN | 25.1 mg/dL | RBC | >100/HPF | |
| Plt | 29.4×104/μL | Plasma renin activity | 5.6 ng/mL/h | <Urinalysis> | WBC | 30-49/HPF | |
| Blood chemistry | Aldosterone | 282 pg/mL | Specific gravity | 1.014 | Cast | Negative | |
| CRP | 0.28 mg/dL | ACTH | 12.3 pg/mL | pH | 6.0 | Bacteria | Negative |
| TP | 7.5 g/dL | Cortisol | 11.5 µg/dL | Protein | (2+) 0.127 g/dL | Atypical cell | Negative |
| Alb | 4.0 g/dL | Immunochemistry | Occult blood | (3+) | Na | 140 mEq/L | |
| AST | 10 U/L | ANA | ×40 titer | RBC | >100/HPF | K | 3.9 mEq/L |
| ALT | 6 U/L | MPO-ANCA | Negative | WBC | 30–49/HPF | Cl | 108 mEq/L |
| LDH | 299 U/L | PR3-ANCA | Negative | Cast | Negative | Cre | 1.46 mg/dL |
| T-Bil | 0.5 mg/dL | Anti-GBM antibody | Negative | Na | 107 mEq/L | UN | 24.0 mg/dL |
| BUN | 30.8 mg/dL | <Urinalysis> | K | 27.6 mEq/L | LDH | 679 U/L | |
| Cre | 4.01 mg/dL | Metanephrine | 0.121 mg/gCre | Cl | 108 mEq/L | ||
| Na | 142 mEq/L | Normetanephrine | 0.225 mg/gCre | Cre | 78.9 mg/dL | ||
| K | 5.0 mEq/L | Cre | 57.9 mg/dL | UN | 542 mg/dL | ||
| Cl | 107 mEq/L | ||||||
Renal dysfunction with elevated plasma renin activity and serum aldosterone was observed on the first attendance; however, there were no laboratory findings suggestive of thrombotic microangiopathy, pheochromocytoma, coagulopathy, autoimmune disease, perirenal tumor invasion, or infection. On perirenal fluid drainage, examination of the fluid revealed almost the same levels of electrolytes, urea nitrogen, and creatinine as those in the blood and an elevated level of lactate dehydrogenase with many red and white blood cells, suggesting that the fluid was hemolyzed blood rather than urine
ACTH adrenocorticotropic hormone, ANA antinuclear antibody, MPO myeloperoxidase, PR3 proteinase 3, ANCA anti-neutrophil cytoplasmic antibody, GBM glomerular basement membrane
Fig. 1.
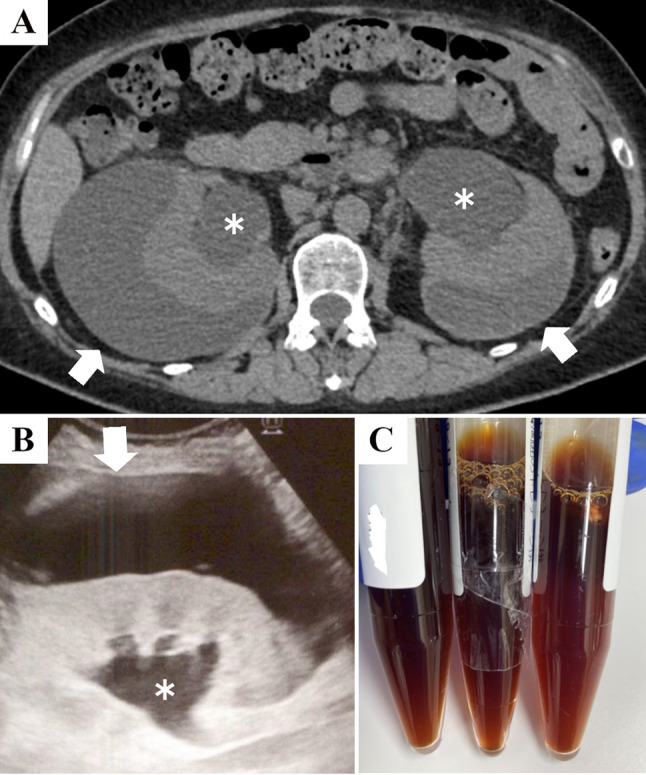
The image findings and appearance of the perirenal fluid. Abdominal computed tomography (A) and ultrasound (B) suggesting hydronephrosis (asterisks) and bilateral perirenal fluid collection (white arrows) at presentation are shown. Upon drainage, the fluid is serous brown (C)
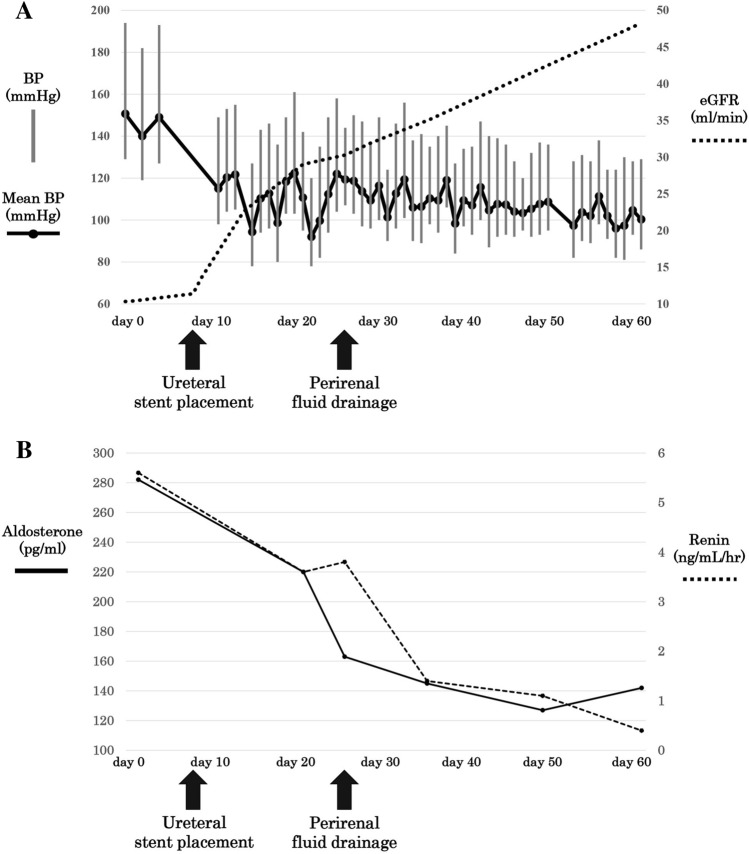
As the patient’s renal function did not improve after an indwelling bladder catheter was inserted, the patient was determined not to have urinary retention. Further studies revealed that the ureter obstruction was caused by new-onset metastatic cervical cancer. Therefore, bilateral ureteral stents were placed 8 days after presentation (day 8). Thereafter, the improvement in the renal function of the patient was proportional to the decrease in blood pressure and plasma renin activity; however, these changes plateaued on days 21–25. A follow-up ultrasound on day 21 revealed remaining perirenal fluid with an improvement in hydronephrosis (Fig. 2); therefore, we suspected that the perirenal fluid contributed to the hyperreninemic state. Ultrasound-guided fluid drainage was performed in the right kidney on day 26, and 30 mL of serous brown fluid was drained (Fig. 1C). Examination of the fluid revealed almost the same levels of electrolytes, urea nitrogen, and creatinine as those in the blood and an elevated level of lactate dehydrogenase with many red and white blood cells, suggesting that the fluid was hemolyzed blood rather than urine. Further, there was no evidence of infection or malignancy in the fluid (Table 1). After the drainage, the patient’s renal function, blood pressure, and plasma renin activity improved gradually, and chemotherapy for metastatic cervical cancer was initiated on day 51. No hydronephrosis or perirenal fluid was noted on follow-up computed tomography on day 83. No antihypertensive medication was used in the patient’s clinical course (Fig. 3).
Fig. 2.
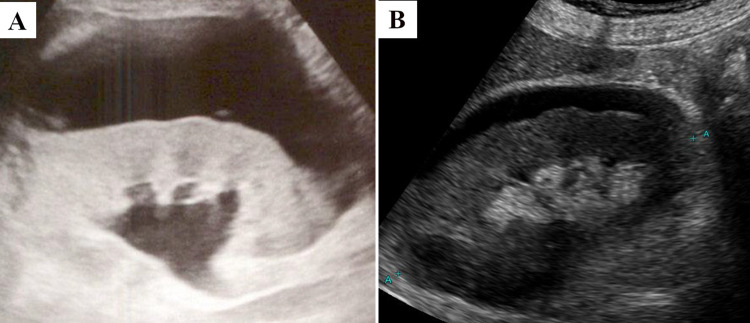
Comparison between the initial and follow-up ultrasound. Compared with the initial ultrasound at presentation (A), a follow-up ultrasound on day 21 (B) showed a reduction in perirenal fluid and an improvement in hydronephrosis
Fig. 3.
The clinical course. The scheme (A) shows the patient’s renal function and BP. Plasma renin activity and serum aldosterone are also shown (B). After a ureteral stent placement on day 8, the patient’s renal function improved in proportion with a decrease in BP and plasma renin activity; however, the changes in these parameters plateaued on days 21–25. After perirenal fluid drainage on day 26, the patient’s renal function, BP, and plasma renin activity improved without the use of antihypertensive medications. Abbreviations: BP blood pressure, eGFR estimated glomerular filtration rate
The differential diagnosis of hyperreninemic hypertension is shown in Table 2 [25]. The usual causes of hyperreninemic hypertension were excluded based on the patient’s clinical course, history, and examination. First, malignant hypertension was unlikely since there were no laboratory findings suggestive of thrombotic microangiopathy including hemolytic anemia, thrombocytopenia, or schistocyte. Further, it was atypical of patients with malignant hypertension to not develop cardiac failure or hypertensive encephalopathy if they were not taking any antihypertensive medication. Second, clinical examination did not reveal renal artery stenosis, renin-secreting tumors, pheochromocytoma, hypertensive encephalopathy, pulmonary edema, or cardiac or aortic diseases. Third, the patient had no history of trauma, drug use, or operation.
Table 2.
Causes of hyperreninemic hypertension
| Disorders with high renin |
| Malignant hypertension |
| Other medium to high renin states |
| Renal trauma |
| Renal parenchymal hypertension due to other diseases than the above |
| Renovascular hypertension |
| Renin-secreting tumors |
| Pheochromocytoma |
| Drugs: cocaine abuse, clonidine or methyl DOPA withdrawal |
| Probable medium to high renin states: PRA >0.65 ng/mL/h |
| Hypertensive encephalopathy |
| Cerebral hemorrhage or stroke |
| Pulmonary edema |
| Acute myocardial infarction or unstable angina |
| Dissecting aortic aneurysm |
| Perioperative hypertension |
The table is a modified citation from the reference Blumenfeld et al. [25]
DOPA 3,4-Dihydroxyphenylalanine, PRA plasma renin activity
Therefore, an unusual case of renal parenchymal hypertension possibly occurred. Minimally invasive procedures were effective in relieving renal compression. Thus, we concluded that the pathophysiology was “renal tamponade,” in which the kidney was compressed by the renal pelvis and Gerota’s fascia, leading to intrarenal microvascular ischemia. Indeed, renal compression-induced hyperreninemic hypertension could occur both in hydronephrosis [26, 27] and Page kidney [4–22]. Hydronephrosis primarily contributed to the pathophysiology in this case since the blood pressure improved promptly after bilateral ureteral stenting. On the other hand, Page kidney seemed to be a secondary cause of the hyperreninemic state since renal function, blood pressure, and plasma renin activity plateaued at approximately 2 weeks after the ureteral stent placement, and these parameters improved after additional perirenal fluid drainage.
Discussion
Page kidney is believed to occur when external compression of the kidney activates RAAS via microvascular ischemia; however, few studies have analyzed the perirenal fluid components or changes in RAAS activity during treatment for Page kidney. In this case, changes in RAAS activity during the treatment and the efficacy of minimally invasive procedures were evaluated. First, minimally invasive procedures relieved the renal compression, leading to the downregulation of the RAAS and the improvement in renal function and blood pressure. These observations support the hypothesis that renal compression-induced RAAS upregulation has an important role in the pathogenesis of Page kidney. Second, renal function, blood pressure, and plasma renin activity plateaued approximately 2 weeks after the ureteral stent placement, and these parameters improved after additional perirenal fluid drainage. If hyperreninemic hypertension and renal dysfunction are insufficiently ameliorated via ureteral stenting, physicians should consider performing perirenal fluid drainage.
In this case, bilateral perirenal subcapsular hematomas could have been caused by hydronephrosis, and not merely by coincidence. There are two grounds for this hypothesis: ultrasonographic changes during treatment and no applicable cause of perirenal fluid collection. First, compared with the initial ultrasound, a follow-up ultrasound presented a reduction in the subcapsular hematoma approximately 2 weeks after ureteral stenting (Fig. 2). This suggests that an increase in hydronephrosis-induced intrarenal pressure contributed to the subcapsular hematoma formation. Second, there was no applicable cause for perirenal fluid collection in this case. Generally, the causes of perirenal fluid collection include trauma [3–10]; iatrogenic causes such as renal biopsy [11–15] and kidney transplantation [16–19]; perirenal tumors [20]; urinomas [21–24]; and renal lymphangiectasias [28]. However, such etiologies were excluded based on the clinical history and examination of this patient. Further, laboratory findings showed no evidence of coagulopathy, autoimmune disease, or perirenal tumor invasion or infection (Table 1).
Therefore, this case implies the possibility of a novel etiology of Page kidney; hydronephrosis-induced intrarenal pressure elevation caused chronic perirenal subcapsular hemorrhage at the vulnerable sites of the renal cortex and peeling of the renal capsule from the cortex, resulting in bilateral massive subcapsular hematomas and Page kidney. The draining fluid had elevated lactate dehydrogenase levels and contained several red and white blood cells. Further, the electrolyte, urea nitrogen, and creatinine levels in the fluid were similar to those in blood, not urine (Table 1). These findings indicate hemolysis with obsolete perirenal hemorrhage, but not drainage-induced acute hemorrhage. In a mouse model of hydronephrosis, renal compression-induced mesenchymal ischemia and hypoxia resulted in inflammation and fibrosis of the renal cortex [29]. Similarly, in this case, hydronephrosis-induced renal compression could have caused renal cortical damage and tissue vulnerability. As a result, subcapsular hemorrhage could have occurred at the vulnerable sites of the renal cortex due to chronic intrarenal pressure elevation, leading to the bilateral massive subcapsular hematomas and Page kidney.
In summary, the patient in this report had hydronephrosis-related Page kidney. The pathophysiology was “renal tamponade” in which the kidney was compressed by the renal pelvis and Gerota’s fascia due to hydronephrosis and perirenal fluid, resulting in intrarenal microvascular ischemia causing hyperreninemic hypertension and renal dysfunction. Ureteral stent placement promptly improved hyperreninemic hypertension and renal dysfunction, and additional perirenal fluid drainage gradually improved these conditions. These observations indicated the following three points. First, renal compression-induced renin–angiotensin–aldosterone system upregulation plays an important role in the pathogenesis of Page kidney. Second, physicians should consider perirenal fluid drainage as a therapeutic option in addition to ureteral stenting in patients with hydronephrosis-related Page kidney. Third, bilateral perirenal subcapsular hematomas in this case could be caused by hydronephrosis. Hydronephrosis-induced intrarenal pressure elevation possibly caused chronic perirenal subcapsular hemorrhage at the vulnerable sites of the renal cortex and peeling of the renal capsule from the cortex, resulting in the bilateral massive subcapsular hematomas and Page kidney.
Acknowledgements
We thank Kazuma Hiramatsu (Department of Urology, Kyoto City Hospital, Kyoto, Japan) and Yuji Takahashi (Department of Obstetrics and Gynecology, Kyoto City Hospital, Kyoto, Japan) for their assistance and Editage (www.editage.com) for English language editing.
Declarations
Conflict of interest
The authors have declared that no conflict of interest exists.
Ethical approval
Not applicable.
Informed consent
The patient provided written informed consent for the case presentation.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Engel WJ, Page IH. Hypertension due to renal compression resulting from subcapsular hematoma. J Urol. 1955;73:735–739. doi: 10.1016/S0022-5347(17)67466-4. [DOI] [PubMed] [Google Scholar]
- 2.Page IH. The production of persistent arterial hypertension by cellophane perinephritis. JAMA. 1939;113:2046–2048. [Google Scholar]
- 3.Massumi RA, Andrade A, Kramer N. Arterial hypertension in traumatic subcapsular perirenal hematoma (Page kidney). Evidence for renal ischemia. Am J Med. 1969;46:635–639. doi: 10.1016/0002-9343(69)90082-5. [DOI] [PubMed] [Google Scholar]
- 4.Sufrin G. The Page kidney: a correctable form of arterial hypertension. J Urol. 1975;113:450–454. doi: 10.1016/S0022-5347(17)59499-9. [DOI] [PubMed] [Google Scholar]
- 5.Mullins MF, Nilson JP, Ross G. Unilateral, “Page kidney” hypertension in man: studies of the renin-angiotensin-aldosterone system before and after nephrectomy. JAMA. 1975;231:42–45. doi: 10.1001/jama.1975.03240130024019. [DOI] [PubMed] [Google Scholar]
- 6.Spark RF, Berg S. Renal trauma and hypertension: the role of renin. Arch Intern Med. 1976;136:1097–1100. doi: 10.1001/archinte.1976.03630100015006. [DOI] [PubMed] [Google Scholar]
- 7.Freed TA, Tavel FR. Diagnosis and surgical treatment of page kidney: selected aspects. Urology. 1976;7:330–333. doi: 10.1016/0090-4295(76)90474-X. [DOI] [PubMed] [Google Scholar]
- 8.Scott PL, Yune HY, Weinberger MH. Page kidney: an unusual cause of hypertension. Radiology. 1976;119:547–548. doi: 10.1148/119.3.547. [DOI] [PubMed] [Google Scholar]
- 9.Dopson SJ, Jayakumar S, Velez JCQ. Page kidney as a rare cause of hypertension: case report and review of the literature. Am J Kidney Dis. 2009;54:334–339. doi: 10.1053/j.ajkd.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 10.Babel N, Sakpal SV, Chamberlain RS. The Page kidney phenomenon secondary to a traumatic fall. Eur J Emerg Med. 2010;17:24–26. doi: 10.1097/MEJ.0b013e32832ce8ba. [DOI] [PubMed] [Google Scholar]
- 11.Hellebusch AA, Simmons JL, Holland N. Renal ischemia and hypertension from a constrictive perirenal hematoma. JAMA. 1970;214:757–759. doi: 10.1001/jama.1970.03180040059017. [DOI] [PubMed] [Google Scholar]
- 12.Lingårdh G, Schönebeck J. Acute hypertension and perirenal haematoma—a new aspect of Page’s syndrome. Br J Urol. 1975;47:359–361. doi: 10.1111/j.1464-410X.1975.tb03984.x. [DOI] [PubMed] [Google Scholar]
- 13.McCune TR, Stone WJ, Breyer JA. Page kidney: case report and review of the literature. Am J Kidney Dis. 1991;18:593–599. doi: 10.1016/S0272-6386(12)80656-1. [DOI] [PubMed] [Google Scholar]
- 14.Chung J, Caumartin Y, Warren J, Luke PP. Acute Page kidney following renal allograft biopsy: a complication requiring early recognition and treatment. Am J Transplant. 2008;8:1323–1328. doi: 10.1111/j.1600-6143.2008.02215.x. [DOI] [PubMed] [Google Scholar]
- 15.Heffernan E, Zwirewich C, Harris A, Nguan C. Page kidney after renal allograft biopsy: sonographic findings. J Clin Ultrasound. 2009;37:226–229. doi: 10.1002/jcu.20465. [DOI] [PubMed] [Google Scholar]
- 16.Cromie WJ, Jordan MH, Leapman SB. Pseudorejection: the Page kidney phenomenon in renal allografts. J Urol. 1976;116:658–659. doi: 10.1016/S0022-5347(17)58953-3. [DOI] [PubMed] [Google Scholar]
- 17.Yussim A, Shmuely D, Levy J, Servadio C, Shapira Z. Page kidney phenomenon in kidney allograft following peritransplant lymphocele. Urology. 1988;31:512–514. doi: 10.1016/0090-4295(88)90219-1. [DOI] [PubMed] [Google Scholar]
- 18.Caldés S, Fernández A, Rivera M, et al. A Page kidney case report with diastolic flow reversion in Doppler ultrasonography. Transplantation. 2009;87:303–304. doi: 10.1097/TP.0b013e3181938a8f. [DOI] [PubMed] [Google Scholar]
- 19.Butt FK, Seawright AH, Kokko KE, Hawxby AM. An unusual presentation of a Page kidney 24 days after transplantation: case report. Transplant Proc. 2010;42:4291–4294. doi: 10.1016/j.transproceed.2010.09.042. [DOI] [PubMed] [Google Scholar]
- 20.Nakano S, Kigoshi T, Uchida K, Morimoto S, Tsugawa R, Matsunou H. Hypertension and unilateral renal ischemia (Page kidney) due to compression of a retroperitoneal paraganglioma. Am J Nephrol. 1996;16:91–94. doi: 10.1159/000168976. [DOI] [PubMed] [Google Scholar]
- 21.Matlaga BR, Veys JA, Jung F, Hutcheson JC. Subcapsular urinoma: an unusual form of page kidney in a high school wrestler. J Urol. 2002;168:672. doi: 10.1016/S0022-5347(05)64719-2. [DOI] [PubMed] [Google Scholar]
- 22.Patel MR, Mooppan MM, Kim H. Subcapsular urinoma: unusual form of “page kidney” in newborn. Urology. 1984;23:585–587. doi: 10.1016/0090-4295(84)90076-1. [DOI] [PubMed] [Google Scholar]
- 23.Efe O, McGrath MM, Hu FY, et al. Urinoma from surgical cyst rupture and page kidney phenomenon in a kidney transplant recipient. Kidney Med. 2021;3:307–308. doi: 10.1016/j.xkme.2020.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Min A, Ditkofsky N. Page kidneys. N Engl J Med. 2022;386:673. doi: 10.1056/NEJMicm2114692. [DOI] [PubMed] [Google Scholar]
- 25.Blumenfeld JD, Laragh JH. Management of hypertensive crises: the scientific basis for treatment decisions. Am J Hypertens. 2001;14:1154–1167. doi: 10.1016/S0895-7061(01)02245-2. [DOI] [PubMed] [Google Scholar]
- 26.Riehle RA, Vaughan ED. Renin participation in hypertension associated with unilateral hydronephrosis. J Urol. 1981;126:243–246. doi: 10.1016/S0022-5347(17)54461-4. [DOI] [PubMed] [Google Scholar]
- 27.Weidmann P, Piccoli CB, Hirsch D, Reubi FC, Massry SG. Curable hypertension with unilateral hydronephrosis. Studies on the role of circulating renin. Ann Intern Med. 1977;87:437–440. doi: 10.7326/0003-4819-87-4-437. [DOI] [PubMed] [Google Scholar]
- 28.Umapathy S, Alavandar E, Renganathan R, Thambidurai S, Arunachalam VK. Renal lymphangiectasia: an unusual mimicker of cystic renal disease—a case series and literature review. Cureus. 2020;12:e10849. doi: 10.7759/cureus.10849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ucero AC, Gonçalves S, Martin AB, Santamaría B, Ramos AM, Berzal S, et al. Obstructive renal injury: from fluid mechanics to molecular cell biology. Open Access J Urol. 2010;2:41–55. doi: 10.2147/rru.s6597. [DOI] [PMC free article] [PubMed] [Google Scholar]