Abstract
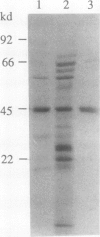
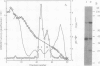
The isoflavonoid phytoalexin pisatin is synthesized by Pisum sativum in response to microbial infection and certain other forms of stress. An enzyme which synthesizes pisatin by methylating the 3-hydroxyl of (+)6a-hydroxymaackiain (HMK) was extracted from CuCl2-stressed pea seedlings. The enzyme was enriched 370-fold by (NH4)2SO4 precipitation, DEAE chromatography, chromatofocusing, and hydrophobic interaction chromatography (HIC), to a specific activity of 8.2 microkatals per gram protein. Enzyme activity profiles from chromatofocusing and HIC columns suggested the presence of two isozymes, of pl 5.2 and 4.9. Nondenaturing gel filtration of the HIC-purified enzyme gave a single peak of activity at the same elution volume as BSA (66 kilodaltons); the active fractions showed two proteins upon SDS-PAGE, of Mr 66,000 and 43,000. The smaller protein was most abundant in chromatographic fractions containing peak enzyme activity throughout purification. In a partially purified preparation, this 43 kilodalton protein was the only one photoaffinity labelled by [3H]S-adenosyl-l-methionine. The purified enzyme preferred the (+) over the (−) stereoisomer of HMK and other pterocarpans; overall, (+)HMK was the best substrate. Km values were 2.3 micromolar for (+)HMK and 35 micromolar for S-adenosyl-l-methionine. The methyltransferase had a pH optimum of 7.9 and no apparent divalent cation requirement.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Dreyfuss G., Adam S. A., Choi Y. D. Physical change in cytoplasmic messenger ribonucleoproteins in cells treated with inhibitors of mRNA transcription. Mol Cell Biol. 1984 Mar;4(3):415–423. doi: 10.1128/mcb.4.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebel J., Hahlbrock K., Grisebach H. Purification and properties of an o-dihydricphenol meta-O-methyltransferase from cell suspension cultures of parsley and its relation to flavonoid biosynthesis. Biochim Biophys Acta. 1972 May 12;268(2):313–326. doi: 10.1016/0005-2744(72)90326-9. [DOI] [PubMed] [Google Scholar]
- Hagmann M. L., Heller W., Grisebach H. Induction of phytoalexin synthesis in soybean. Stereospecific 3,9-dihydroxypterocarpan 6a-hydroxylase from elicitor-induced soybean cell cultures. Eur J Biochem. 1984 Jul 2;142(1):127–131. doi: 10.1111/j.1432-1033.1984.tb08259.x. [DOI] [PubMed] [Google Scholar]
- Hermann C., Legrand M., Geoffroy P., Fritig B. Enzymatic synthesis of lignin: purification to homogeneity of the three O-methyltransferases of tobacco and production of specific antibodies. Arch Biochem Biophys. 1987 Mar;253(2):367–376. doi: 10.1016/0003-9861(87)90190-1. [DOI] [PubMed] [Google Scholar]
- Hurst J. H., Billingsley M. L., Lovenberg W. Photoaffinity labelling of methyltransferase enzymes with S-adenosylmethionine: effects of methyl acceptor substrates. Biochem Biophys Res Commun. 1984 Jul 31;122(2):499–508. doi: 10.1016/s0006-291x(84)80061-3. [DOI] [PubMed] [Google Scholar]
- Khouri H. E., Tahara S., Ibrahim R. K. Partial purification, characterization, and kinetic analysis of isoflavone 5-O-methyltransferase from yellow lupin roots. Arch Biochem Biophys. 1988 May 1;262(2):592–598. doi: 10.1016/0003-9861(88)90410-9. [DOI] [PubMed] [Google Scholar]
- Kochs G., Grisebach H. Enzymic synthesis of isoflavones. Eur J Biochem. 1986 Mar 3;155(2):311–318. doi: 10.1111/j.1432-1033.1986.tb09492.x. [DOI] [PubMed] [Google Scholar]
- Laskey R. A. The use of intensifying screens or organic scintillators for visualizing radioactive molecules resolved by gel electrophoresis. Methods Enzymol. 1980;65(1):363–371. doi: 10.1016/s0076-6879(80)65047-2. [DOI] [PubMed] [Google Scholar]
- Lawton M. A., Lamb C. J. Transcriptional activation of plant defense genes by fungal elicitor, wounding, and infection. Mol Cell Biol. 1987 Jan;7(1):335–341. doi: 10.1128/mcb.7.1.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweigard J. A., Matthews D. E., Vanetten H. D. Synthesis of the phytoalexin pisatin by a methyltransferase from pea. Plant Physiol. 1986 Jan;80(1):277–279. doi: 10.1104/pp.80.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weltring K. M., Turgeon B. G., Yoder O. C., VanEtten H. D. Isolation of a phytoalexin-detoxification gene from the plant pathogenic fungus Nectria haematococca by detecting its expression in Aspergillus nidulans. Gene. 1988 Sep 7;68(2):335–344. doi: 10.1016/0378-1119(88)90036-4. [DOI] [PubMed] [Google Scholar]
- Wengenmayer H., Ebel J., Grisebach H. Purification and properties of a S-adenosylmethionine: isoflavone 4'-O-methyltransferase from cell suspension cultures of Cicer arietinum L. Eur J Biochem. 1974 Dec 16;50(1):135–143. doi: 10.1111/j.1432-1033.1974.tb03881.x. [DOI] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]