Abstract
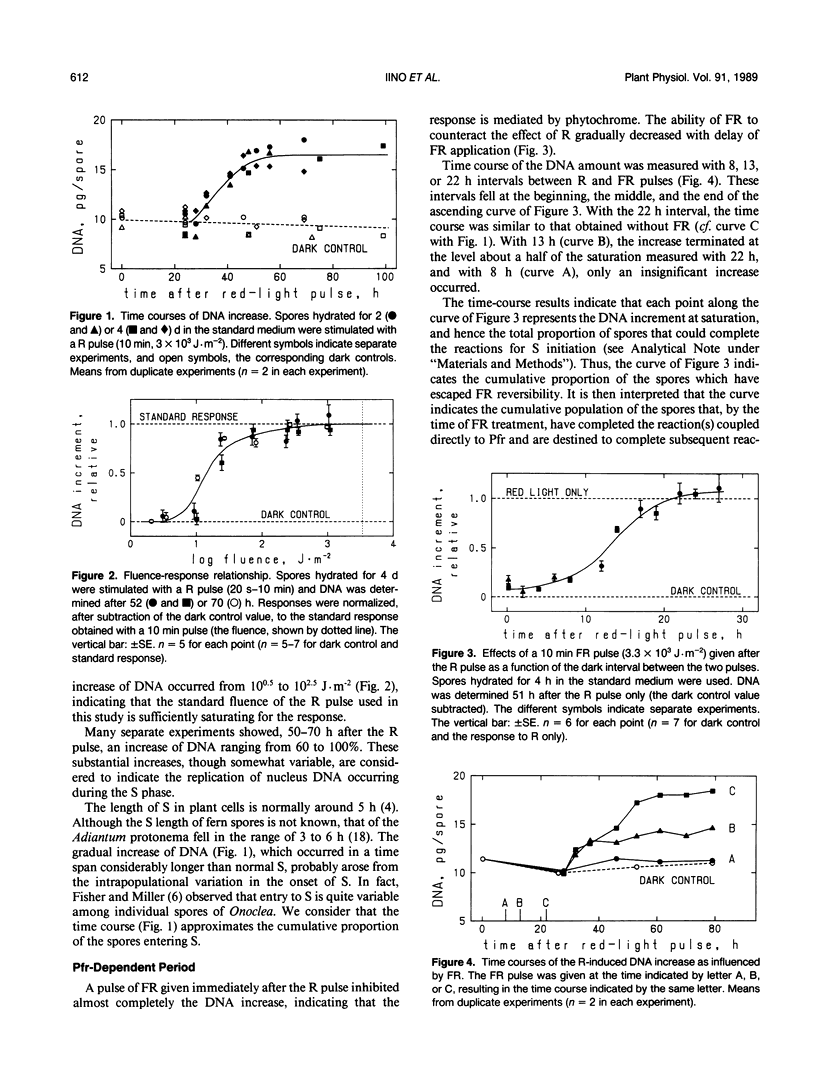
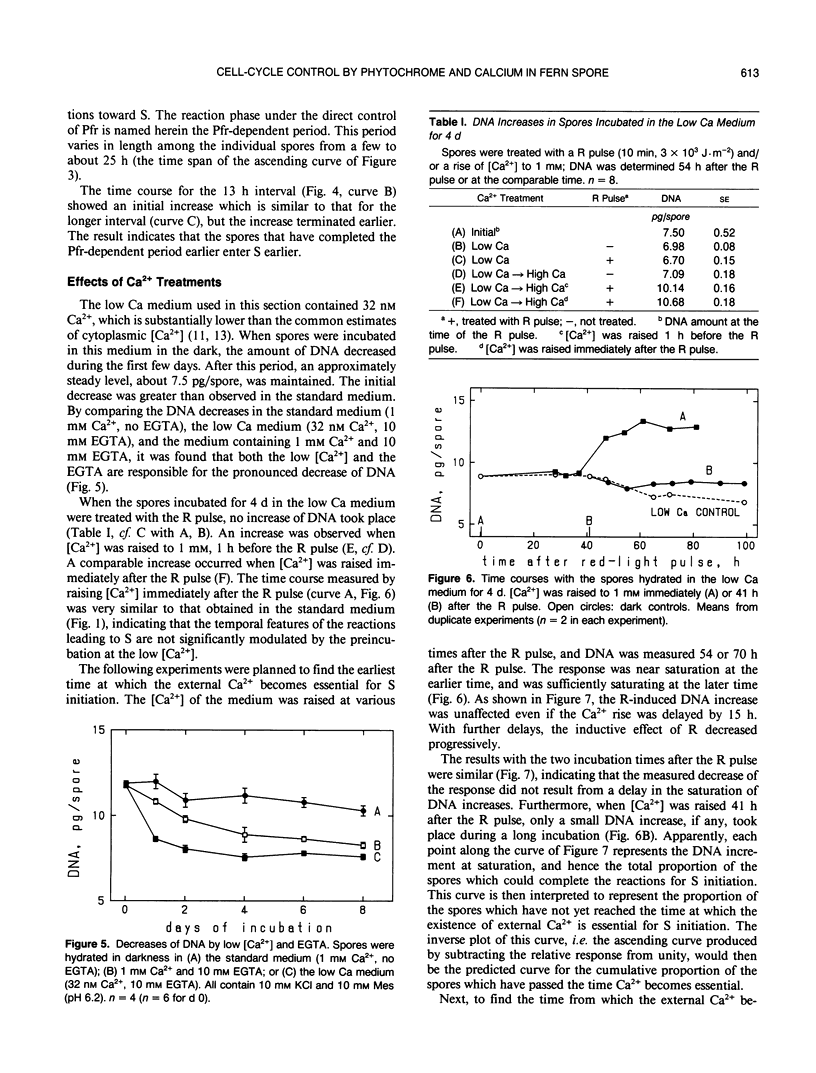
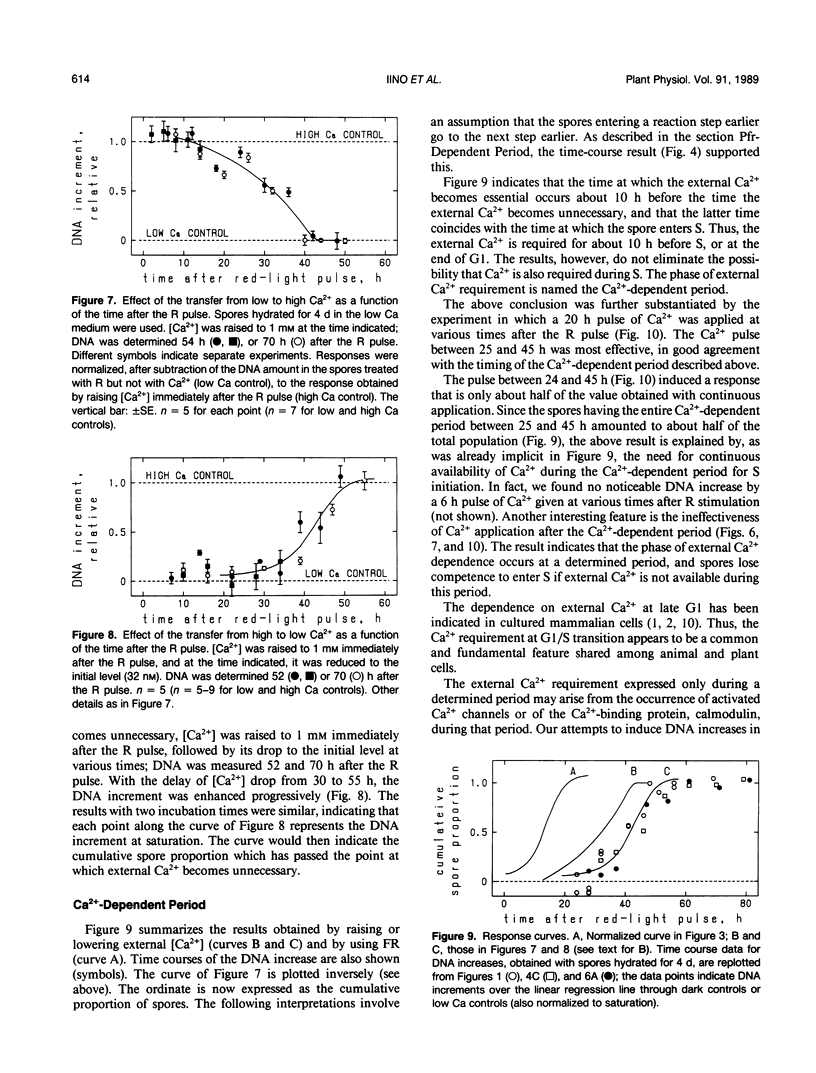
The first cell cycle of Adiantum spores, which is induced by red light (R), was studied with regard to its temporal dependence on Pfr (the active form of phytochrome) and Ca2+. Responses were quantified with increases of the spore content of DNA, thus limiting the investigation to the reactions taking place before the S phase of the cell cycle (i.e. during G0/G1/S transitions). Spores hydrated for more than 2 days in the standard medium (includes 1 millimolar free Ca2+) showed, after stimulation with a saturating R pulse, an increase of DNA beginning at about 25 hours and saturating at about 55 hours. Reversal by far-red light of the inductive effect of R was used to examine the temporal requirement for Pfr. Spores became dependent on the supply of external Ca2+ when incubated in a low Ca medium (32 nanomolar free Ca2+ with 10 millimolar EGTA); this culture condition was used, after observing that the DNA increase occurs similarly if Ca2+ is supplied after the R pulse, to examine the temporal requirement for external Ca2+. It was concluded that the G1 phase of the spore is separated into three subphases: (a) the Pfr-dependent period which immediately follows the R pulse and varies among individual spores from a few to about 25 hours, (b) the Ca2+-dependent period (about 10 hours) which occurs immediately before the S phase, and (c) a gap (15-20 hours) between the two periods. In the Ca2+-dependent period, spores require the presence of extracellular Ca2+. This period occurs only during a determined time after the R pulse, and the competence of spores to enter the S phase is lost sharply if external Ca2+ is not available continuously during this period.
Full text
PDF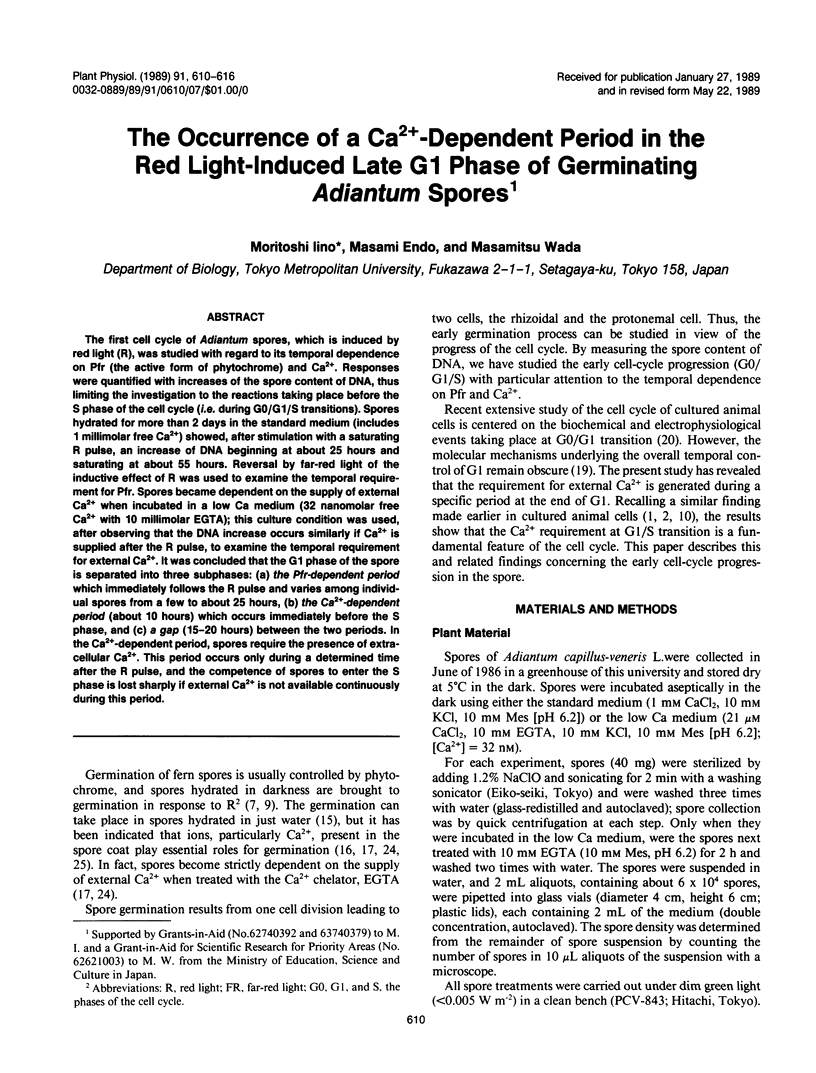


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boynton A. L., Whitfield J. F., Isaacs R. J. The different roles of serum and calcium in the control of proliferation of BALB/c 3T3 mouse cells. In Vitro. 1976 Feb;12(2):120–123. doi: 10.1007/BF02796358. [DOI] [PubMed] [Google Scholar]
- Boynton A. L., Whitfield J. F., Isaacs R. J., Tremblay R. The control of human WI-38 cell proliferation by extracellular calcium and its elimination by SV-40 virus-induced proliferative transformation. J Cell Physiol. 1977 Aug;92(2):241–247. doi: 10.1002/jcp.1040920212. [DOI] [PubMed] [Google Scholar]
- Brunk C. F., Jones K. C., James T. W. Assay for nanogram quantities of DNA in cellular homogenates. Anal Biochem. 1979 Jan 15;92(2):497–500. doi: 10.1016/0003-2697(79)90690-0. [DOI] [PubMed] [Google Scholar]
- Chafouleas J. G., Lagacé L., Bolton W. E., Boyd A. E., 3rd, Means A. R. Changes in calmodulin and its mRNA accompany reentry of quiescent (G0) cells into the cell cycle. Cell. 1984 Jan;36(1):73–81. doi: 10.1016/0092-8674(84)90075-8. [DOI] [PubMed] [Google Scholar]
- Hazelton B., Mitchell B., Tupper J. Calcium, magnesium, and growth control in the WI-38 human fibroblast cell. J Cell Biol. 1979 Nov;83(2 Pt 1):487–498. doi: 10.1083/jcb.83.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labarca C., Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980 Mar 1;102(2):344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Miller J. H., Vogelmann T. C., Bassel A. R. Promotion of fern rhizoid elongation by metal ions and the function of the spore coat as an ion reservoir. Plant Physiol. 1983 Apr;71(4):828–834. doi: 10.1104/pp.71.4.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott D. M. Cell reproduction. Int Rev Cytol. 1987;100:93–128. doi: 10.1016/s0074-7696(08)61699-x. [DOI] [PubMed] [Google Scholar]
- Rozengurt E. Early signals in the mitogenic response. Science. 1986 Oct 10;234(4773):161–166. doi: 10.1126/science.3018928. [DOI] [PubMed] [Google Scholar]
- Scheuerlein R., Wayne R., Roux S. J. Calcium requirement of phytochrome-mediated fern-spore germination: no direct phytochrome-calcium interaction in the phytochrome-initiated transduction chain. Planta. 1989;178:25–30. [PubMed] [Google Scholar]
- Smith J. A., Martin L. Do cells cycle? Proc Natl Acad Sci U S A. 1973 Apr;70(4):1263–1267. doi: 10.1073/pnas.70.4.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne R., Hepler P. K. Red Light Stimulates an Increase in Intracellular Calcium in the Spores of Onoclea sensibilis. Plant Physiol. 1985 Jan;77(1):8–11. doi: 10.1104/pp.77.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]



