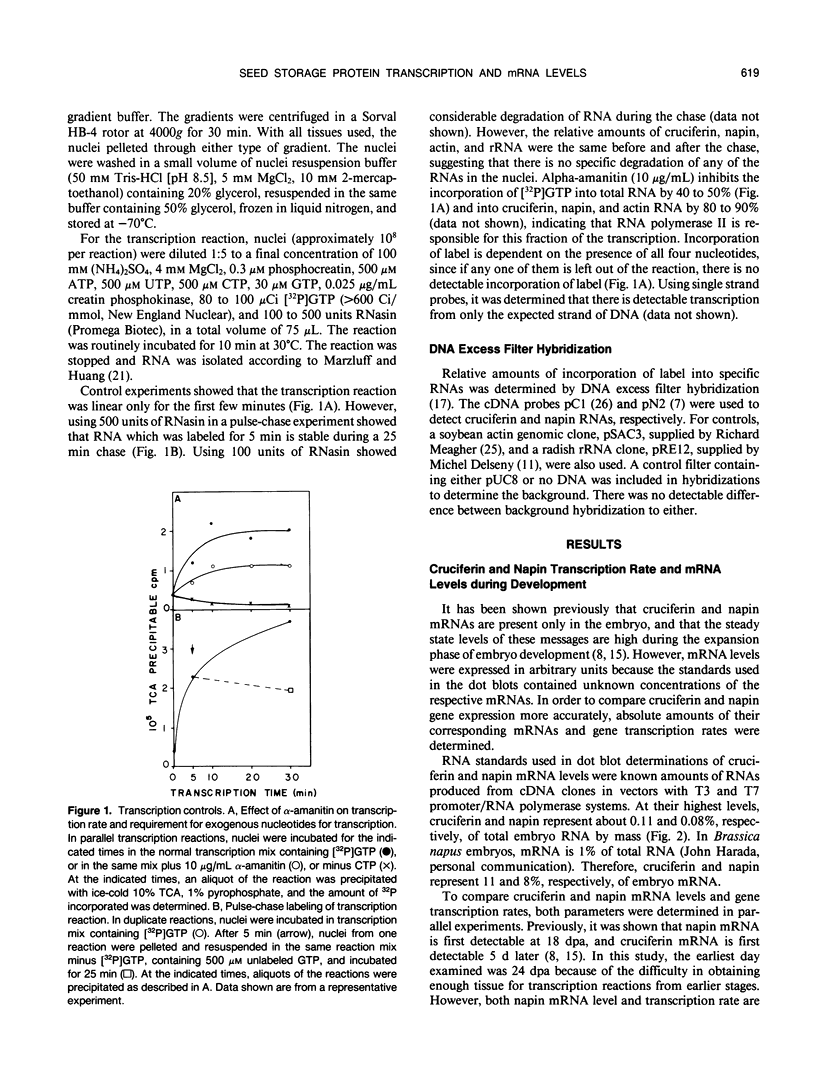
Abstract
Transcription rates and mRNA levels for Brassica napus seed storage protein families, cruciferin and napin, have been determined in embryos developing in the seed, as well as in embryos cultured with and without abscisic acid. Cruciferin and napin mRNAs are high during the cell expansion phase of embryo development, representing as much as 11 and 8%, respectively, of the total embryo mRNA. During the same time cruciferin and napin gene transcription rates, as measured in isolated nuclei, are also high. The data indicate that cruciferin mRNA is more stable than napin mRNA because while the napin transcription rate is higher than the cruciferin transcription rate, the cruciferin mRNA accumulates to higher levels. However, late in embryo development, both cruciferin and napin mRNAs seem to be less stable than earlier because comparable transcription rates result in lower mRNA levels. When embryos are cultured in the presence of abscisic acid, the levels of cruciferin and napin mRNAs are two- to threefold higher than in embryos cultured on basal medium. The transcription rates show a similar increase in the presence of abscisic acid, suggesting that abscisic acid is responsible for the increased mRNA level at least in part through an increase in the transcription rate of the two genes.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bray E. A., Beachy R. N. Regulation by ABA of beta-Conglycinin Expression in Cultured Developing Soybean Cotyledons. Plant Physiol. 1985 Nov;79(3):746–750. doi: 10.1104/pp.79.3.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell J., Chrispeels M. J. Transcriptional and Posttranscriptional Control of Phaseolin and Phytohemagglutinin Gene Expression in Developing Cotyledons of Phaseolus vulgaris. Plant Physiol. 1986 May;81(1):50–54. doi: 10.1104/pp.81.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouch M. L., Tenbarge K. M., Simon A. E., Ferl R. cDNA clones for Brassica napus seed storage proteins: evidence from nucleotide sequence analysis that both subunits of napin are cleaved from a precursor polypeptide. J Mol Appl Genet. 1983;2(3):273–283. [PubMed] [Google Scholar]
- Dure L., Galau G. A. Developmental Biochemistry of Cottonseed Embryogenesis and Germination : XIII. REGULATION OF BIOSYNTHESIS OF PRINCIPAL STORAGE PROTEINS. Plant Physiol. 1981 Jul;68(1):187–194. doi: 10.1104/pp.68.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R. R., Tenbarge K. M., Shumway J. E., Crouch M. L. Role of ABA in Maturation of Rapeseed Embryos. Plant Physiol. 1985 Jul;78(3):630–636. doi: 10.1104/pp.78.3.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galau G. A., Legocki A. B., Greenway S. C., Dure L. S., 3rd Cotton messenger RNA sequences exist in both polyadenylated and nonpolyadenylated forms. J Biol Chem. 1981 Mar 10;256(5):2551–2560. [PubMed] [Google Scholar]
- Hendrickson S. L., Wu J. S., Johnson L. F. Cell cycle regulation of dihydrofolate reductase mRNA metabolism in mouse fibroblasts. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5140–5144. doi: 10.1073/pnas.77.9.5140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthe D. S., Quatrano R. S. Transcription in Isolated Wheat Nuclei: I. ISOLATION OF NUCLEI AND ELIMINATION OF ENDOGENOUS RIBONUCLEASE ACTIVITY. Plant Physiol. 1980 Feb;65(2):305–308. doi: 10.1104/pp.65.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah D. M., Hightower R. C., Meagher R. B. Genes encoding actin in higher plants: intron positions are highly conserved but the coding sequences are not. J Mol Appl Genet. 1983;2(1):111–126. [PubMed] [Google Scholar]
- Walling L., Drews G. N., Goldberg R. B. Transcriptional and post-transcriptional regulation of soybean seed protein mRNA levels. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2123–2127. doi: 10.1073/pnas.83.7.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson J. D., Quatrano R. S., Cuming A. C. Em polypeptide and its messenger RNA levels are modulated by abscisic acid during embryogenesis in wheat. Eur J Biochem. 1985 Oct 15;152(2):501–507. doi: 10.1111/j.1432-1033.1985.tb09224.x. [DOI] [PubMed] [Google Scholar]