Abstract
Osteoporosis (OP) is a systemic bone disease in which bone density and quality decrease and bone fragility increases due to a variety of causes, making it prone to fractures. The development of OP is closely related to oxidative stress. Uric acid (UA) is the end product of purine metabolism in the human body. Extracellular UA has antioxidant properties and is thought to have a protective effect on bone metabolism. However, the process of UA degradation can lead to intracellular oxidative stress, which together with UA-induced inflammatory factors, leads to increased bone destruction. In addition, UA can inhibit vitamin D production, resulting in secondary hyperparathyroidism and further exacerbating UA-associated bone loss. This review summarizes the relationship between serum UA levels and bone mineral density, bone turnover markers, and so on, in the hope of providing new insights into the pathogenesis and treatment of OP.
Keywords: uric acid, osteoporosis, hyperuricemia, gout, bone metabolism
Introduction
Osteoporosis (OP) is a metabolic bone disease characterized by reduced bone mineral density (BMD) and bone mass and destruction of bone microarchitecture, leading to increased bone fragility and increased risk of fracture (1). The development of osteoporosis is associated with a variety of factors, including hormone changes and calcium and vitamin D deficiency, and there is increasing evidence that oxidative stress is also a potential mechanism for age-related bone loss through enhancing osteoclast bone resorption (2, 3, 4).
Uric acid (UA) is the end product of purine nucleotide degradation in the organism (5). Extracellular UA exerts its antioxidant property through scavenging free radicals in human plasma. The antioxidant property of UA is thought to be related to neuroprotective effects and may reduce the risk of age-related cancers (4, 6); moreover, UA in the physiological state is considered to have a protective effect on bone metabolism due to its antioxidant property (7). Hyperuricemia (HUA) is defined as the level of serum uric acid (sUA) >6.0 mg/dL (>360 µmol/L) for females and >7.0 mg/dL (>420 µmol/L) for males (8). Elevated sUA levels may lead to the deposition of urate crystals in the joints, kidneys, and other areas, resulting in joint and kidney damage. The adverse effect of HUA on bone is probably caused by intracellular oxidative stress produced by UA degradation and UA-induced inflammatory factor aggregation, which will slowly damage bones by inhibiting bone formation by osteoblasts while promoting bone resorption by osteoclasts, finally leading to joint deformity and seriously affecting the quality of life (5, 7, 9, 10). Besides, it has been reported that UA can inhibit the expression of 1α-hydroxylase in the proximal tubule, resulting in secondary hyperparathyroidism, and further exacerbating UA-related bone loss and impairing bone reconstruction, thus increasing the risk of fracture (7). The aim of this review is to summarize the relationship between UA and bone metabolism, with the expectation of providing new insights into the pathogenesis and treatment of OP.
Development of osteoporosis
Osteoclast-mediated bone resorption and osteoblast-mediated bone formation are in a dynamic equilibrium under normal conditions, the processes of bone resorption and bone formation are coupled in space and time. When this balance is disturbed, osteoblast function gradually decreases and osteoclast bone resorption is relatively enhanced, resulting in greater bone resorption than bone formation, a decrease in bone mineral and organic matrix, and a tendency for bone volume to decline, making the bone mechanically less strong and susceptible to OP (11).
The development of OP is influenced by many factors, such as sex hormone deficiency, calcium and vitamin D deficiency, elevated parathyroid hormone (PTH) levels, disturbance of inflammatory cytokines induced by inflammatory diseases, etc., resulting in decreased osteoblasts function and increased osteoclasts activity (1, 12). Moreover, recent research implicated that oxidative stress also plays a pivotal role in the pathogenesis of OP. Oxidative stress refers to the excessiveness of free radicals such as reactive oxygen species (ROS). ROS are continuously produced in mitochondria under normal physiological conditions, but excessive accumulation of ROS can damage cell membranes, cytoplasm, and even DNA. Excessive ROS can not only inhibit the differentiation and proliferation of osteoblasts through inhibiting the expression of Runx2 and Osterix (Runx2 and Osterix are key transcriptional regulators of osteoblast differentiation) but also negatively affect their activity, viability, and apoptosis, resulting in a decrease in the number and function of osteoblasts. Furthermore, excess ROS can promote the expression of osteoclastogenic markers such as c-Fos, NFATc1, and TRAP to enhance osteoclastogenesis, finally leading to more bone destruction (2, 13, 14, 15).
Metabolism of uric acid
UA is the end product of purine nucleotide metabolism in the human body. Hypoxanthine and xanthine are the direct precursors of UA, hypoxanthine is oxidized to xanthine by xanthine oxidase (XO), and xanthine is finally oxidized into UA in the human body (6, 7). About 30% of the UA in the organism is excreted by the intestine and 70% by the kidneys. In the kidney, urate is freely filtered by the glomeruli and several urate transporters in the proximal tubule are responsible for regulating the excretion and reabsorption of UA. These urate transporters include those that mediate the passage of urate from the lumen into the cell, such as URAT1 (SLC22A12), OAT4 (SLC22A11), OAT10 (SLC22A13), and GLUT9 (SLC2A9). ABCG2 (ATP-binding cassette subfamily G member 2), ABCC4 (ATP-binding cassette subfamily C member 4), and NPT1 (SLC17A1) are transporters that regulate urate excretion and are responsible for transporting of urate from the proximal tubule cells to the tubular fluid. OAT1 (SLC22A6), OAT2 (SLC22A7), and OAT3 (SLC22A8) are responsible for the transport of urate to the proximal tubular cells, which is known as urate secretion. GLUT9 (SLC2A9) also regulates urate reabsorption. ABCG2 is also closely associated with intestinal urate excretion, and genetic variants in ABCG2 can lead to inadequate extrarenal urate excretion and HUA (5, 10).
The biological significance of urate is complicated, it may have antioxidant properties that maintains blood pressure in people who are on a low-salt diet and reduces the risk of neurological disease (6, 9, 10). However, when endogenous or exogenous factors cause urate in the body to exceed its saturation concentration, urate is deposited in the joints and other parts of the body, forming monosodium urate (MSU) crystals and triggering gout attacks through an inflammatory response, mainly in the form of redness, swelling, heat, pain, and impaired mobility of the affected joints and possibly leading to bone erosion in the affected joints (10, 16).
Serum uric acid and bone mineral density
BMD is commonly measured with dual-energy X-ray absorptiometry (DXA) in clinical practice, which is strongly associated with fracture risk. For every 1 SD reduction in BMD, there is an overall 1.5–2 times increase in fracture risk (11). OP is defined as a BMD T-score of ≤−2.5 at the lumbar spine or femoral neck (17). Several observational studies have reported that sUA levels are positively correlated with higher BMD within a certain range and have a strong protective effect against bone loss and OP, thereby reducing the risk of fragility fractures (18, 19, 20, 21). As mentioned above, oxidative stress is thought to inhibit bone formation while promoting bone resorption. As an important antioxidant in the organism, the protective effect of UA on bone metabolism in the physiological state may originate from its antioxidant effect (4, 7, 20, 22, 23). However, HUA and gout increase the risk of fracture because oxidative stress and inflammatory cytokines increase bone resorption and decrease bone formation (7, 24). We have summarized the association of sUA with BMD in different age groups and genders, as well as with BMD in postmenopausal women in published cross-sectional studies.
Higher sUA levels are positively associated with higher BMD in different age groups
Several cross-sectional studies have shown that higher levels of sUA are positively correlated with higher BMD across age groups. A cross-sectional study in Iranian adolescents (221 girls and 192 boys) aged 9–19 years showed that sUA was associated with BMD and bone mineral content at all sites, with higher BMD in adolescents with higher UA levels (25). In Chinese adolescents aged 12–19 years, elevated sUA levels are beneficial for bone health in adolescents with low sUA levels (24). In Qatari adults with a mean age of 36.4 ± 11.1 years, a cross-sectional study demonstrated that higher sUA levels were associated with higher BMD (18). Another cross-sectional study from India in healthy subjects with a mean age of 47.2 ± 12.2 years (sample size 310; female: 43.5%; male: 56.5%) showed that BMD at all skeletal sites was significantly higher in subjects with UA > 5.4 mg/dL compared to those with UA ≤ 5.4 mg/dL (P < 0.001), and UA was positively correlated with BMD at all skeletal sites(r = 0.211–0.277; P < 0.05) (26). In men over 50 years of age, sUA levels were positively correlated with higher BMD in the lumbar spine (27). A cross-sectional study of 631 Chinese adult male T2DM patients with a mean age of 57.3 years found that elevated sUA levels were protective against OP and bone loss (28). In older men aged 65 years and older, higher sUA levels were associated with higher BMD and lower incidence of fragility fractures at all skeletal sites (29).
Correlation between sUA and BMD by gender
The correlation between sUA levels and BMD varied by gender. In Chinese female adolescents, it has been suggested that the relationship between sUA and total BMD follows an inverted U-shaped curve and that higher sUA levels (with a turning point of 3.9 mg/dL) may impair bone health (24). A cross-sectional study of Chinese men and postmenopausal women (943 men and 4256 postmenopausal women) found that sUA was significantly associated with BMD at the femoral neck and total hip in both men and women, and a significant correlation between sUA and BMD of L1–L4 was observed only in women (23). Another cross-sectional study including 626 men with T2DM and 609 postmenopausal women (mean age: 60.66 ± 11.80 years for men and 66.25 ± 10.22 years for women) in China showed that sUA levels were positively correlated with BMD at the lumbar spine, femoral neck, and total hip in men (P<0.05), and in postmenopausal women, sUA levels were significantly positively correlated with BMD of the lumbar spine, femoral neck, and total hip (P < 0.005) (30). However, the above studies were only observed in the Chinese population and may have limitations.
The role of sUA on BMD in postmenopausal women
Postmenopausal women often show a higher prevalence of OP due to factors such as estrogen deficiency, and studies have shown a positive correlation between sUA and BMD in premenopausal and postmenopausal women (31, 32). A cross-sectional study of 390 healthy postmenopausal Chinese women aged 47–89 years showed significant differences in sUA levels between the normal BMD group and the OP group. After adjusting for age, lumbar spine BMD was positively correlated with sUA in postmenopausal women (r = 0.212). Lumbar spine BMD in postmenopausal women was linearly related to sUA levels in the normal physiological range (33). A retrospective study in Italy included 124 premenopausal women (mean age 35 ± 10 years) and 234 postmenopausal women (mean age 55 ± 4 years), the analysis showed that sUA was significantly positively associated with total hip BMD (r = 0.165, P < 0.05) and greater trochanteric BMD (r = 0.151, P < 0.05) in estrogen-deficient women (34). Table 1 concluded the studies published on PubMed in the last 5 years about the beneficial effects of sUA on BMD.
Table 1.
Studies about the relationship between sUA and bone published on PubMed in the last 5 years.
| No. and Ref. | Nation | Study design | Study population | Conclusion |
|---|---|---|---|---|
| 1 (24) | China | Cross-sectional study | Adolescents aged 12–19 years |
|
| 2 (25) | Iran | Cross-sectional study | 413 adolescents aged 9–19 years (22 women and 192 men) | Higher BMD is correlated with higher UA levels. |
| 3 (18) | Qataris | Cross-sectional study | 2981 healthy adults (36.4 ± 11.1 years old) | High levels of sUA are significantly associated with increased BMD (P<0.001). |
| 4 (34) | Italy | Retrospective study | 124 premenopausal women (35 ± 10 years old) 234 postmenopausal women (55 ± 4 years old) |
In estrogen-deficient women, sUA is significantly and positively associated with total hip BMD (P<0.05) and greater trochanter BMD (P<0.05). |
| 5 (26) | India | Cross-sectional study | 310 health checkups (women: 43.5%; men: 56.5%) (47.2 ± 12.2 years old) | UA is positively correlated with BMD at all skeletal sites (P<0.05). |
| 6 (35) | South Korea | Cross-sectional study | 6588 healthy males (48.2 ± 10.7 years old) | For every 1 mg/dL increase in sUA, lumbar BMD increased by 0.0054 g/cm2 (P = 0.004). |
| 7 (27) | China | Cross-sectional study | 385 males >50 years of age | sUA levels are positively correlated with higher BMD and T values in the lumbar spine. |
| 8 (28) | China | Cross-sectional study | 631 men with T2DM (57.3 ± 12.0 years old) | sUA is positively correlated with BMD in the lumbar spine (P<0.001), femoral neck (P = 0.002), and total hip (P = 0.001). |
| 9 (19) | China | Cross-sectional study | 3465 people >60 years of age |
|
| 10 (23) | China | Cross-sectional study | 943 males and 4256 postmenopausal females (63.7 ± 4.2 years old) | sUA levels are positively associated with femoral neck BMD, and total hip BMD. |
| 11 (33) | China | Cross-sectional study | 390 healthy postmenopausal women aged 47–89 years | Lumbar BMD is positively correlated with sUA. |
| 12 (30) | China | Cross-sectional study | 626 males with T2DM (60.66 ± 11.80 years old) | sUA levels are positively correlated with BMD in the lumbar spine, femoral neck, and total hip (P< 0.05). |
sUA and bone turnover markers
Several cross-sectional studies have proposed that sUA levels are positively associated with higher BMD, and that sUA has a similar positive effect with BTMs. Studies have indicated that sUA levels are negatively correlated with N-terminal procollagen of type I collagen (PINP) and osteocalcin (OC) in postmenopausal females (23, 32). PINP and OC are markers of bone formation that reflect osteoblast activity. Bone formation and resorption are tightly coupled during the bone turnover process, and sUA is negatively correlated with bone formation markers, suggesting that sUA can reduce bone turnover and thus decrease bone loss (23). In male patients with type 2 diabetes mellitus (T2DM), sUA is negatively associated with OC, PINP, and type I collagen carboxy-terminal peptide (CTX) (CTX is a marker of bone resorption reflecting osteoclast activity), suggesting that sUA has an inhibitory effect on the bone turnover process in male patients with T2DM (28). In postmenopausal women with T2DM, sUA is also negatively associated with serum CTX (30). Bone alkaline phosphatase (BALP) is also one of the most commonly used markers of bone formation in clinical practice, and it has been suggested that high levels of sUA can lead to a decrease in BALP levels (36).
The relationship between sUA and BMD is still controversial
Although many cross-sectional studies have shown that higher levels of sUA in physiological states are positively associated with higher BMD, it has also been shown that a positive association between sUA and BMD is currently observed primarily in Asian populations but not in the US population (37). A randomized controlled trial of inosine supplementation in postmenopausal women in New Zealand suggested that there is no direct biological effect of urate on BTMs (37). Animal studies showed that there was no difference in BMD between HUA rats and control rats with normal sUA (38). Similarly, the results of Mendelian randomization analysis did not support a causal relationship between UA levels, gout, and BMD at the lumbar spine or femoral neck (39). Additionally, sUA concentrations are influenced by many other variables, such as physical condition, diet, lifestyle, and diuretic and hormone use, which also affect BMD and fracture risk, so it is unclear whether the positive correlation between sUA and BMD is attributable to other confounding factors (40).
Hyperuricemia and gout affect bone remodeling
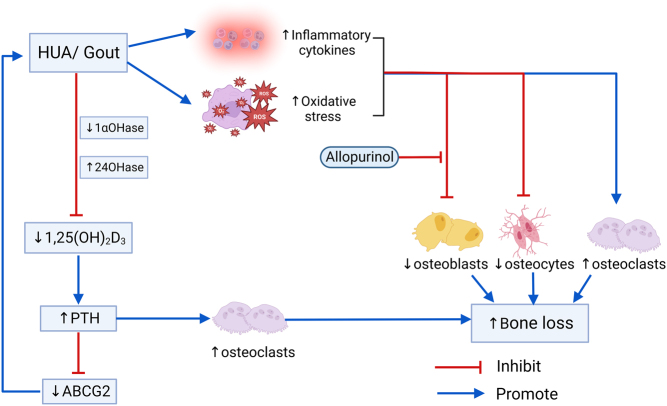
Although the relationship between sUA levels and BMD remains confusing, there is an agreement on the adverse effects of HUA and gout on bone. A prospective cohort study with 22 years of follow-up demonstrated that a history of gout in women was related to an increased risk of hip fracture (41). HUA and gout can adversely affect bone by stimulating bone resorption by osteoclasts and inhibiting bone formation by osteoblasts (7, 20, 42, 43). HUA and gout increase the risk of fracture, which may be attributed to oxidative stress, the release, and the accumulation of inflammatory cytokines. In addition, vitamin D deficiency and secondary hyperparathyroidism may further increase the risk of bone resorption and bone loss in UA-induced OP (7) (Fig. 1).
Figure 1.
Influence of HUA/gout on bone loss. Note: ABCG2 (ATP-binding cassette subfamily G member 2) is an important urate transporter, which belongs to the ABC (ATP-binding cassette) transporter superfamily and can transport various substrates across the membrane and is expressed in epithelial cells of the intestine, kidney, etc.
Osteoclasts
Osteoclasts are multinucleated cells derived from hematopoietic stem cells whose primary function is to resorb mineralized bone and contribute to the maintenance of normal bone mass during physiological bone remodeling. Osteoclasts’ differentiation and activation are regulated by signaling pathways mediated by RANKL, which can be induced by 1-25 vitamin D, PTH, and interlukin-6 (IL-6). RANKL binds to RANK to promote osteoblast differentiation and maturation. OPG plays an inhibitory role in osteoclast differentiation and activation by interfering with the binding of RANKL to RANK. During the acute episode of gout, a number of pro-inflammatory cytokines such as IL-1, tumor necrosis factor alpha (TNF-α), IL-6 and IL-8 are released. IL-1, together with the RANKL signaling receptor activator, promotes the proliferation and differentiation of osteoclast progenitors and is an important activator of bone resorption. IL-6 increases the expression of RANKL in osteoblasts by increasing osteoclast bone resorption and plays an active role in osteoclast differentiation. TNF-α stimulates osteoclast differentiation and activity by promoting RANKL expression in macrophages, bone marrow stromal cells and macrophage colony-stimulating factor (M-CSF) in stromal cells (7, 12). To sum up, in HUA or gout, increased osteoclast proliferation, differentiation, and activity ultimately lead to bone erosion and destruction (44).
Osteoblasts
Osteoblasts are the main functional cells of bone formation, responsible for the synthesis, secretion, and mineralization of bone matrix (44). In the HUA rats experiment, it was shown that the levels of Wnt3a, a key regulator of osteoblastogenesis, were significantly reduced, and the levels of other factors critical for osteoblastogenesis, including Cbfa1 (Runx2, a key transcriptional regulator of osteoblast differentiation, plays an important role in osteoblast maturation and homeostasis in vivo (45)), Sp7 (Osterix, an essential transcription factor for osteoblast differentiation and bone mineralization (46)), Ibsp (bone salivary protein), and Bglap (OC) were also significantly decreased in HUA rats, while the use of allopurinol increased the expression levels of the above osteoblastogeneic factors. In vitro experiments, Western blot results showed that the intensity and relative density of Wnt3a protein bands were significantly reduced in osteoblasts cultured with MSU crystals, whereas Wnt3a protein expression was restored in osteoblasts cultured with a combination of MSU crystals and allopurinol. Furthermore, this experiment also claimed that HUA decreased osteoblast activity and increased apoptosis, whereas allopurinol treatment reduced apoptosis of osteoblasts to some extent in the HUA group of rats (7, 20, 43, 47). In conclusion, HUA or MSU crystals inhibit osteoblast differentiation, proliferation and activity while promoting osteoclast apoptosis, which may contribute to decreased bone mass and increased risk of OP in patients with HUA or gout.
Osteocytes
Osteocytes are the most important cellular component of the human skeleton. In the adult skeleton, osteocytes account for 90–95% of the total number of cells and are about 20 times the number of osteoblasts. Studies have demonstrated that MSU crystals can directly inhibit osteocytes viability, and indirectly promote osteocytes expression of pro-inflammatory mediators and bone remodeling factors through interacting with macrophages, thereby promoting bone resorption and inflammation, ultimately leading to disorders of bone remodeling (42).
Serum uric acid and vitamin D
Studies have shown that sUA is associated with vitamin D metabolism, which may also be one of the potential mechanisms by which sUA affects bone metabolism. In HUA rats, sUA inhibits 1,25-(OH)2D3 production by inhibiting the expression of 1α-hydroxylase (1α-OHase) in the proximal tubule through activation of nuclear factor κ-B. This study also proposes that the expression of 24-hydroxylase (24-OHase) for the degradation of 25-OH-D or 1,25-(OH)2D is enhanced in these HUA rats. These two effects lead to elevated levels of PTH, which ultimately leads to secondary hyperparathyroidism (7). Data analysis revealed that HUA was significantly associated with vitamin D insufficiency in postmenopausal Han Chinese women, which is consistent with the experimental findings in HUA rats (48). Elevated levels of PTH further downregulate the urate transporter ABCG2 (an excretory transporter protein for sUA in the kidney and intestine (8)), which further increases sUA levels (7, 49, 50, 51). Accordingly, HUA-induced vitamin D deficiency and hyperparathyroidism would further exacerbate the bone loss and impaired bone remodeling associated with UA and significantly increase the risk of fracture (7).
Conclusion
Although many cross-sectional studies have shown that higher levels of sUA in physiological states are positively associated with BMD, the causal relationship between sUA and bone metabolism remains unclear and controversial.
In HUA or gout, high levels of sUA increase bone resorption and inhibit bone formation, thereby increasing the risk of fracture. This is consistent and clear in the available literature.
sUA can inhibit the production of 1,25-(OH)2D3, leading to elevated PTH levels and resulting in secondary hyperparathyroidism. HUA-induced vitamin D deficiency and hyperparathyroidism may further exacerbate UA-associated bone loss and impaired bone reconstruction and significantly increase fracture risk.
Outlook
HUA and gout lead to bone loss and OP by inhibiting bone formation, promoting bone destruction, and inhibiting the production of active vitamin D. This provides a theoretical basis for active UA-lowering therapy in clinical patients with HUA or gout, but the viewpoint that UA levels have a protective effect on bone within a certain range is controversial and needs to be proven in further studies. In OP patients with combined HUA or gout in the clinic, is UA-lowering therapy beneficial for OP remission? Assuming that higher sUA levels at physiological concentrations do have a protective effect on bone metabolism, what is the appropriate range of sUA levels? These questions need to be addressed in further studies.
Declaration of interest
All authors have no conflicts of interest.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Author contribution statement
Baoyu Zhang provided the conception and design of the manuscript; Rong Xu and Difei Lian drafted the manuscript; Yan Xie and Lin Mu collected the related literature; Yali Wu and Zhilei Chen reviewed the manuscript critically.
References
- 1.Reid IR & Billington EO. Drug therapy for osteoporosis in older adults. Lancet 20223991080–1092. ( 10.1016/S0140-6736(2102646-5) [DOI] [PubMed] [Google Scholar]
- 2.Kimball JS Johnson JP & Carlson DA. Oxidative stress and osteoporosis. Journal of Bone and Joint Surgery. 20211031451–1461. ( 10.2106/JBJS.20.00989) [DOI] [PubMed] [Google Scholar]
- 3.Lu J Zhang Y Liang J Diao J Liu P & Zhao H. Role of exosomal microRNAs and their crosstalk with oxidative stress in the pathogenesis of osteoporosis. Oxidative Medicine and Cellular Longevity 202120216301433. ( 10.1155/2021/6301433) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaushal N Vohora D Jalali RK & Jha S. Review of the literature examining the association of serum uric acid with osteoporosis and mechanistic insights into its effect on bone metabolism. Endocrine, Metabolic and Immune Disorders Drug Targets 201919259–273. ( 10.2174/1871530318666181102115106) [DOI] [PubMed] [Google Scholar]
- 5.Dalbeth N Gosling AL Gaffo A & Abhishek A. Gout. Lancet 20213971843–1855. ( 10.1016/S0140-6736(2100569-9) [DOI] [PubMed] [Google Scholar]
- 6.Estiverne C Mandal AK & Mount DB. Molecular pathophysiology of uric acid homeostasis. Seminars in Nephrology 202040535–549. ( 10.1016/j.semnephrol.2020.12.006) [DOI] [PubMed] [Google Scholar]
- 7.Lin KM, Lu CL, Hung KC, Wu PC, Pan CF, Wu CJ, Syu RS, Chen JS, Hsiao PJ, Lu KC, & Nutrients K-CLJ. The paradoxical role of uric acid in osteoporosis. Nutrients 201911. ( 10.3390/nu11092111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eckenstaler R & Benndorf RA. The role of ABCG2 in the pathogenesis of Primary Hyperuricemia and Gout-An update. International Journal of Molecular Sciences 202122. ( 10.3390/ijms22136678) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dalbeth N Merriman TR & Stamp LK. Gout. Lancet 20163882039–2052. ( 10.1016/S0140-6736(1600346-9) [DOI] [PubMed] [Google Scholar]
- 10.Dalbeth N Choi HK Joosten LAB Khanna PP Matsuo H Perez-Ruiz F & Stamp LK. Gout. Nature Reviews. Disease Primers 2019569. ( 10.1038/s41572-019-0115-y) [DOI] [PubMed] [Google Scholar]
- 11.Compston JE, McClung MR & Leslie WD. Osteoporosis. Lancet 2019393364–376. ( 10.1016/S0140-6736(1832112-3) [DOI] [PubMed] [Google Scholar]
- 12.Kitaura H Marahleh A Ohori F Noguchi T Shen WR Qi J Nara Y Pramusita A Kinjo R & Mizoguchi I. Osteocyte-related cytokines regulate osteoclast formation and bone resorption. International Journal of Molecular Sciences 202021. ( 10.3390/ijms21145169) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geng Q Gao H Yang R Guo K & Miao D. Pyrroloquinoline quinone prevents estrogen deficiency-induced osteoporosis by inhibiting oxidative stress and osteocyte senescence. International Journal of Biological Sciences 20191558–68. ( 10.7150/ijbs.25783) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang C Li H Li J Hu J Yang K & Tao L. Oxidative stress: a common pathological state in a high-risk population for osteoporosis. Biomedicine and Pharmacotherapy 2023163114834. ( 10.1016/j.biopha.2023.114834) [DOI] [PubMed] [Google Scholar]
- 15.Iantomasi T Romagnoli C Palmini G Donati S Falsetti I Miglietta F Aurilia C Marini F Giusti F & Brandi ML. Oxidative stress and inflammation in osteoporosis: molecular mechanisms involved and the relationship with microRNAs. International Journal of Molecular Sciences 202324. ( 10.3390/ijms24043772) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keenan RT. The biology of urate. Seminars in Arthritis and Rheumatism 202050S2–S10. ( 10.1016/j.semarthrit.2020.04.007) [DOI] [PubMed] [Google Scholar]
- 17.Cappola AR Auchus RJ Fuleihan GE-H Handelsman DJ Kalyani RR McClung M Stuenkel CA Thorner MO & Verbalis JG. Hormones and aging: an Endocrine Society scientific statement. Endocrinology 20231081835–1874. ( 10.1210/clinem/dgad225) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ibrahim WN Younes N Shi Z & Abu-Madi MA. Serum uric acid level is positively associated with higher bone mineral density at multiple skeletal sites among healthy Qataris. Frontiers in Endocrinology 202112653685. ( 10.3389/fendo.2021.653685) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen F Wang Y Guo Y Wang J Yang A Lv Q Liu Y Ma G Liu Y & Wang D. Specific higher levels of serum uric acid might have a protective effect on bone mineral density within a Chinese population over 60 years old: a cross-sectional study from northeast China. Clinical Interventions in Aging 2019141065–1073. ( 10.2147/CIA.S186500) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Veronese N, Carraro S, Bano G, Trevisan C, Solmi M, Luchini C, Manzato E, Caccialanza R, Sergi G, Nicetto D, et al. Hyperuricemia protects against low bone mineral density, osteoporosis and fractures: a systematic review and meta-analysis. European Journal of Clinical Investigation 201646920–930. ( 10.1111/eci.12677) [DOI] [PubMed] [Google Scholar]
- 21.Muka T & Jonge EAL Muka T de Jonge EA Kiefte-de Jong JC Uitterlinden AG Hofman A Dehghan A Zillikens MC Franco OH & Rivadeneira F. The Influence of Serum Uric Acid on Bone Mineral Density, Hip Geometry, and Fracture Risk: The Rotterdam Study. Journal of Clinical Endocrinology and Metabolism 20161011113–1122. ( 10.1210/jc.2015-2446) [DOI] [PubMed] [Google Scholar]
- 22.Zong Q Hu Y Zhang Q Zhang X Huang J & Wang T. Associations of hyperuricemia, gout, and UA-lowering therapy with the risk of fractures: a meta-analysis of observational studies. Joint Bone Spine 201986419–427. ( 10.1016/j.jbspin.2019.03.003) [DOI] [PubMed] [Google Scholar]
- 23.Yan DD Wang J Hou XH Bao YQ Zhang ZL Hu C & Jia WP. Association of serum uric acid levels with osteoporosis and bone turnover markers in a Chinese population. Acta Pharmacologica Sinica 201839626–632. ( 10.1038/aps.2017.165) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pan K Yao X Liu M & Zhu Z. Association of serum uric acid status with bone mineral density in adolescents aged 12–19 years. Frontiers in Medicine 20207255. ( 10.3389/fmed.2020.00255) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karimi F Dabbaghmanesh MH & Omrani GR. Association between serum uric acid and bone health in adolescents. Osteoporosis International 2019302057–2064. ( 10.1007/s00198-019-05072-w) [DOI] [PubMed] [Google Scholar]
- 26.Kaushal N Vohora D Jalali RK & Jha S. Raised serum uric acid is associated with higher bone mineral density in a cross-sectional study of a healthy Indian population. Therapeutics and Clinical Risk Management 20181475–82. ( 10.2147/TCRM.S147696) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiao J Chen W Feng X Liu W Zhang Z He L & Ye Z. Serum uric acid is associated with lumbar spine bone mineral density in healthy Chinese males older than 50 years. Clinical Interventions in Aging 201712445–452. ( 10.2147/CIA.S130690) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu Y Xiang S Jiang X Wang L Wang K & Hua F. Relationship of bone status with serum uric acid and bilirubin in men with Type 2 diabetes: a cross-sectional study. Medical Science Monitor 202127e930410. ( 10.12659/MSM.930410) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nabipour I Sambrook PN Blyth FM Janu MR Waite LM Naganathan V Handelsman DJ Couteur DGL Cumming RG & Seibel MJ. Serum uric acid is associated with bone health in older men: a cross-sectional population-based study. Journal of Bone and Mineral Research 201126955–964. ( 10.1002/jbmr.286) [DOI] [PubMed] [Google Scholar]
- 30.Xu M, Su J, Hao J, Zhong N, Zhang Z, Cui R, Li F, Sheng C, Zhang G, Sheng H, et al. Positive association between serum uric acid and bone mineral density in Chinese type 2 diabetes mellitus stratified by gender and BMI. Journal of Bone and Mineral Metabolism 201836609–619. ( 10.1007/s00774-017-0877-9) [DOI] [PubMed] [Google Scholar]
- 31.Makovey J Macara M Chen JS Hayward CS March L Seibel MJ & Sambrook PN. Serum uric acid plays a protective role for bone loss in peri- and postmenopausal women: a longitudinal study. Bone 201352400–406. ( 10.1016/j.bone.2012.10.025) [DOI] [PubMed] [Google Scholar]
- 32.Ahn SH Lee SH Kim BJ Lim KH Bae SJ Kim EH Kim HK Choe JW Koh JM & Kim GS. Higher serum uric acid is associated with higher bone mass, lower bone turnover, and lower prevalence of vertebral fracture in healthy postmenopausal women. Osteoporosis International 2013242961–2970. ( 10.1007/s00198-013-2377-7) [DOI] [PubMed] [Google Scholar]
- 33.Han W Bai X Wang N Han L Sun X & Chen X. Association between lumbar bone mineral density and serum uric acid in postmenopausal women: a cross-sectional study of healthy Chinese population. Archives of Osteoporosis 20171250. ( 10.1007/s11657-017-0345-0) [DOI] [PubMed] [Google Scholar]
- 34.Bonaccorsi G Trentini A Greco P Tisato V Gemmati D Bianchi N Giganti M Rossini M Guglielmi G & Cervellati C. Changes in adipose tissue distribution and association between uric acid and bone health during menopause transition. International Journal of Molecular Sciences 201920. ( 10.3390/ijms20246321) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hwang J Hwang JH Ryu S & Ahn JK. Higher serum uric acid is associated with higher lumbar spine bone mineral density in male health-screening examinees: a cross-sectional study. Journal of Bone and Mineral Metabolism 201937142–151. ( 10.1007/s00774-018-0905-4) [DOI] [PubMed] [Google Scholar]
- 36.Wu ZQ Chen XT Xu YY Tian MJ Chen HY Zhou GP & Oncotarget HG. High uric acid (UA) downregulates bone alkaline phosphatase (BALP) expression through inhibition of its promoter activity. Oncotarget 2017885670–85679. ( 10.18632/oncotarget.21110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li X Li L Yang L Yang J & Lu H. No association between serum uric acid and lumbar spine bone mineral density in US adult males: a cross sectional study. Scientific Reports 20211115588. ( 10.1038/s41598-021-95207-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang D Bobulescu IA Maalouf NM Adams-Huet B Poindexter J Park S Wei F Chen C Moe OW & Sakhaee K. Relationship between serum uric acid and bone mineral density in the general population and in rats with experimental hyperuricemia. Journal of Bone and Mineral Research 201530992–999. ( 10.1002/jbmr.2430) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee YH & Song GG. Uric acid level, gout and bone mineral density: a Mendelian randomization study. European Journal of Clinical Investigation 201949e13156. ( 10.1111/eci.13156) [DOI] [PubMed] [Google Scholar]
- 40.Dalbeth N Topless R Flynn T Cadzow M Bolland MJ & Merriman TR. Mendelian randomization analysis to examine for a causal effect of urate on bone mineral density. Journal of Bone and Mineral Research 2015309, 85–99. ( 10.1002/jbmr.2434) [DOI] [PubMed] [Google Scholar]
- 41.Paik JM Kim SC Feskanich D Choi HK Solomon DH & Curhan GC. Gout and Risk of Fracture in Women: a Prospective Cohort Study. Arthritis and Rheumatology 201769422–428. ( 10.1002/art.39852) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chhana A Pool B Callon KE Tay ML Musson D Naot D McCarthy G McGlashan S Cornish J & Dalbeth N. Monosodium urate crystals reduce osteocyte viability and indirectly promote a shift in osteocyte function towards a proinflammatory and proresorptive state. Arthritis Research and Therapy 201820208. ( 10.1186/s13075-018-1704-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yan B Liu D Zhu J & Pang X. The effects of hyperuricemia on the differentiation and proliferation of osteoblasts and vascular smooth muscle cells are implicated in the elevated risk of osteopenia and vascular calcification in gout: an in vivo and in vitro analysis. Journal of Cellular Biochemistry 201912019660–19672. ( 10.1002/jcb.29272) [DOI] [PubMed] [Google Scholar]
- 44.Chhana A & Dalbeth N. Structural joint damage in gout. Rheumatic Disease Clinics of North America 201440291–309. ( 10.1016/j.rdc.2014.01.006) [DOI] [PubMed] [Google Scholar]
- 45.Ziros PG Basdra EK & Papavassiliou AG. Runx2: of bone and stretch. International Journal of Biochemistry and Cell Biology 2008401659–1663. ( 10.1016/j.biocel.2007.05.024) [DOI] [PubMed] [Google Scholar]
- 46.Liu Q Li M Wang S Xiao Z Xiong Y & Wang G. Recent advances of osterix transcription factor in osteoblast differentiation and bone formation. Frontiers in Cell and Developmental Biology 20208601224. ( 10.3389/fcell.2020.601224) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Orriss IR Arnett TR George J & Witham MD. Allopurinol and oxypurinol promote osteoblast differentiation and increase bone formation. Experimental Cell Research 2016342166–174. ( 10.1016/j.yexcr.2016.03.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peng H Li H Li C Chao X Zhang Q & Zhang Y. Association between vitamin D insufficiency and elevated serum uric acid among middle-aged and elderly Chinese Han women. PLoS One 20138e61159. ( 10.1371/journal.pone.0061159) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen W Roncal-Jimenez C Lanaspa M Gerard S Chonchol M Johnson RJ & Jalal D. Uric acid suppresses 1 alpha hydroxylase in vitro and in vivo. Metabolism: Clinical and Experimental 201463150–160. ( 10.1016/j.metabol.2013.09.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chin KY Nirwana SI & Ngah WZW. Significant association between parathyroid hormone and uric acid level in men. Clinical Interventions in Aging 2015101377–1380. ( 10.2147/CIA.S90233) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hui JY Choi JWJ Mount DB Zhu Y Zhang Y & Choi HK. The independent association between parathyroid hormone levels and hyperuricemia: a national population study. Arthritis Research and Therapy 201214R56. ( 10.1186/ar3769) [DOI] [PMC free article] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a