Abstract
Soils samples were obtained from pristine ecosystems in six regions on five continents. Two of the regions were boreal forests, and the other four were Mediterranean ecosystems. Twenty-four soil samples from each of four or five sites in each of the regions were enriched by using 3-chlorobenzoate (3CBA), and 3CBA mineralizers were isolated from most samples. These isolates were analyzed for the ability to mineralize 3CBA, and genotypes were determined with repetitive extragenic palindromic PCR genomic fingerprints and restriction digests of the 16S rRNA genes (amplified ribosomal DNA restriction analysis [ARDRA]). We found that our collection of 150 stable 3CBA-mineralizing isolates included 48 genotypes and 44 ARDRA types, which formed seven distinct clusters. The majority (91%) of the genotypes were unique to the sites from which they were isolated, and each genotype was found only in the region from which it was isolated. A total of 43 of the 44 ARDRA types were found in only one region. A few genotypes were repeatedly found in one region but not in any other continental region, suggesting that they are regionally endemic. A correlation between bacterial genotype and vegetative community was found for the South African samples. These results suggest that the ability to mineralize 3CBA is distributed among very diverse genotypes and that the genotypes are not globally dispersed.
In papers describing the isolation of novel chloroorganic compound degraders, rarely do workers attribute much importance to the geographic location or habitat from which a genotype is derived. Until recently, bacterial taxa were thought to be comprised of a limited number of clones with worldwide distributions. Data on the genetic structure of populations of commensal species, such as Escherichia coli, Haemophilus influenzae, Neisseria meningitidis, and Streptococcus pneumoniae, supported this view (20). More recent studies have shown that the genus Rhizobium, for example, has a population genetic structure that suggests that there has been limited geographic migration of host strains and that there is a high diversity of clones even in a single nodule of a plant host (31). In contrast, the plasmid-borne nodulation genes are shared more broadly, and the distribution of these genes obscures the relative diversity of the genomic hosts (17). The genus Bradyrhizobium and Neisseria gonorrhoeae have proven to be genetically diverse (21). In perhaps the only examination of the genetic structure of populations of free-living bacteria, McArthur et al. (22, 23) showed that members of the species Burkholderia cepacia found in soil are genetically divergent from members of the same species inhabiting nearby stream water. These authors presented some evidence that the degree of habitat variability (temporal variability in physicochemical parameters) correlates with the degree of genetic diversity in this organism (22, 35).
We isolated 610 3-chlorobenzoate (3CBA) degraders from widely separated, relatively pristine ecosystems distributed around the world (11, 32). We chose this phenotype to study questions concerning the biogeography of soil heterotrophs because 3CBA degraders were previously thought to be rare (2, 8, 14–16) and thus a manageable group to study. We hypothesized that either (i) we repeatedly isolated the same genotype from all sites in all regions or (ii) we isolated different strains of the same phenotype. The first hypothesis was derived from the assumption that 3CBA degradation is a recent trait, which evolved in response to selection due to anthropogenically produced xenobiotic compounds, and that recently evolved strains were rapidly distributed worldwide. The second hypothesis was derived from the assumption that the ability to degrade 3CBA is a more ancient and divergent trait and led to additional hypotheses concerning the possible determinants of the patterns of the diversity. We predicted that if the strains were different from each other, then the genotypes of the strains might reflect their geographic origins or the types of vegetation growing at the sites from which they were collected.
In this paper we present data on the genetic diversity and geographic origins of the stable members of our collection, a total of 150 strains, based on the results of repetitive extragenic palindromic (REP) genomic fingerprinting (4, 33) and amplified ribosomal DNA restriction analysis (ARDRA). The former method reveals diversity at approximately the subspecies level of resolution (4), which we call the genotype in this paper, and the latter method identifies the same or related taxa at the genus-to-species level of resolution (24), which we call the ARDRA type. Our collection of 3CBA degraders has a high degree of genetic diversity and a surprising degree of genotypic endemicity, and to some extent the genotype depends on the vegetative community sharing the soil from which a strain was isolated.
MATERIALS AND METHODS
Strain origins.
The methods used to collect soil samples, determine soil characteristics, enrich soil samples with 3CBA, and isolate mineralizers have been described by Fulthorpe et al. (11). Briefly, soil samples were collected from 5 to 30 cm below the soil surface with sterilized soil corers at pristine, undisturbed sites. Bacteria were enriched from 24 soil cores (one enrichment per core, 24 enrichments per site) obtained along 200-m transects at four or five sites located 100 to 850 km apart in six regions (four Mediterranean regions and two boreal forest areas). The regions from which samples were obtained and the maximum distances between sites were as follows: central California, 850 km; central Chile, 291 km; Cape Region, South Africa, 100 km; western Australia, 620 km; northern Saskatchewan, Canada, 120 km; and Russia, 110 km. The geographic coordinates of the sites, the site names, and the types of vegetation at the sites are shown in Table 1.
TABLE 1.
Locations and dominant vegetation types of sampling sitesa
| Climate | Soil type | Region | Site
|
Dominant vegetation | ||
|---|---|---|---|---|---|---|
| Name | Location | Abbreviation | ||||
| Mediterranean | Chromic luvisols | Southwestern Australia | Bridgetown | 34°0′S, 116°15′E | BN | Eucalyptus forest |
| Geraldton | 28°24′S, 114°51′E | GE | Acacia, meadow | |||
| Jarrahdale | 32°23′S, 116°07′E | JD | Dense eucalyptus forest | |||
| Kelleberrin | 31°24′S, 117°46′E | KE | Acacia, shrubs | |||
| Merredin | 31°23′S, 118°41′E | ME | Eucalyptus, acacia woodland | |||
| South Africa | Helshoogte | 33°56′S, 18°54′E | HH | Eucalyptus over renosterveld | ||
| Mooreesburg | 33°4′S, 18°40′E | MB | Renosterveld | |||
| Mamreweg | 33°38′S, 18°28′E | MR | Renosterveld | |||
| Welgevallen | 33°57′S, 18°52′E | WG | Fynbos | |||
| Paarl Mountain | 33°44′S, 18°56′E | PM | Fynbos | |||
| California | Chabot | 37°45′N, 122°10′W | CH | Eucalyptus | ||
| Cloverdale | 40°29′N, 122°29′W | CL | Oak, pine meadow | |||
| Hillgate | 40°10′N, 122°30′W | HG | Oak, pine meadow | |||
| Murrieta | 33°25′N, 119°41′W | MU | Oak meadow, chapparal | |||
| Venice Hills | 36°20′N, 119°41′W | VH | Meadow | |||
| Central Chile | Rio Clarillo | 33°51′S, 70°29′W | RC | Acacia, cryptocaria, lithraea | ||
| La Campana | 32°57′S, 71°05′W | LC | Cryptocaria, acacia, lithraea, chusquea | |||
| Lago Penuelas | 33°20′S, 70°30′W | LP | Acacia, pine | |||
| Fray Jorge | 30°38′S, 71°35′W | FJ | Kageneckia, myrceugenia | |||
| Boreal forest | Albic luvisols | Saskatchewan, Canada | Bittern | 53°55′N, 105°27′W | BT | White spruce, poplar, birch |
| Napatak | 54°52′N, 105°24′W | NP | Jack pine, white spruce | |||
| Porcupine | 52°39′N, 102°23′W | PC | Poplar | |||
| Waitville | 53°41′N, 105°22′W | WV | White spruce, poplar, jack pine, birch | |||
| Waskesieu | 54°0′N, 106°35′W | WK | Jack pine, birch, white spruce | |||
| Russia | RI | 38°0′E, 60°0′N | Spruce, birch | |||
| RII | 38°30′E, 60°30′N | Spruce, birch | ||||
| RIII | 39°0′E, 60°30′N | Pine, spruce, birch | ||||
| RIV | 39°0′E, 61°0′N | Pine, spruce, birch | ||||
Data is reproduced with the permission of the publisher from reference 11.
Media and strain preparation.
A total of 610 isolates which originally could release carbon dioxide from ring-labeled [14C]3CBA were obtained, and two subsets were analyzed by REP-PCR. One subset included all of the strains isolated from the Saskatchewan and California sites. The other subset included all of the strains from all regions that could degrade 80% or more of 1 mM 3CBA, as determined by high-performance liquid chromatography (HPLC) (see below). The media used in the enrichment procedures have been described by Fulthorpe et al. (11). In HPLC tests and prior to genomic extraction, strains were grown in the presence of 1 mM 3CBA in a minimal medium (medium A) (36) supplemented with 5 mg of yeast extract (Difco) per liter. Strains were maintained in 20% glycerol at −84°C or on agar plates having the chemical composition described above supplemented with 1 mM 3CBA. To check culture purity, strains were streaked onto R2A medium (BBL). If contamination was found, strains were retested for growth on 3CBA-containing agar. Genomic DNA was extracted from cells grown on medium containing 1 mM 3CBA to the stationary phase by the cetyltrimethylammonium bromide extraction method (1).
REP genotyping.
Genotypes of isolates were determined by using the PCR and primers derived from previously described REP sequences (4, 33). The target DNA was obtained from 1 μl of a glycerol-preserved culture. The fingerprint patterns obtained were later confirmed by using total genomic DNA.
Amplification and restriction of ribosomal DNA.
Primers rD1 and fD1 as described by Weisburg et al. (34) were used to amplify 1,500 bp of 16S rRNA genes from each strain by the PCR performed with Taq (Pharmacia) and buffer. The amplification reaction mixtures contained 100 μl, and 10-μl portions of these reaction mixtures were used as templates for six separate restriction digestions with the following tetrameric restriction enzymes: TaqI (digestion at 70°C), RsaI, HaeIII, MspI, CfoI, and AluI. The fragments were separated on a 3% agarose gel in TBE by using a 100-bp ladder as a marker.
3CBA degradation.
The strains used in this study were originally tested for the ability to degrade 3CBA and to release chloride (11). The original tests were performed with a Hewlett-Packard series 1050 liquid chromatograph equipped with a type C18 column and a multiple-wavelength UV detector set to 218, 230, and 280 nm, and 70% methanol–30% 0.1% phosphoric acid was used as the solvent. Under these conditions 3CBA was detected at 3.2 min, and metabolites were transiently detected at 1.6, 1.9, and 2.2 min. All of the strains were retested by growing each strain in 5-ml portions of mineral medium A containing 1 mM 3CBA in test tubes without shaking. The culture medium was analyzed after 7 days of growth with a Waters HPLC system equipped with two model 501 pumps, a gradient controller, a type C18 Bondapak column, and a model 9600 diode array detector, and a 60:40 mixture of 1% phosphoric acid and acetonitrile was used as the running buffer. Under these conditions, 3CBA was detected at 7.1 min, and metabolites were transiently detected at 2.4, 3.7, and 4.3 min in some of the cultures.
Taxonomic characterization of isolates.
Subsets of the collection were used for species identification by fatty acid methyl ester analysis (MIDI Inc., Newark, Del.) and with Biolog GN microplates (Biolog Inc., Hayward, Calif.) and API 20 NFT test kits (bioMérieux Vitek, St. Louis, Mo.).
Enzyme assays.
Two strains were chosen at random for enzyme assays. Cells were grown for 15 to 18 h in liquid medium containing 1.5 mM 3CBA. The cultures were then harvested by centrifugation and washed twice with 50 mM Tris-HCl buffer (pH 8.0) containing 0.1 mM dithiothreitol. After the cells were disrupted by sonication for 2 min at 0°C, cell extracts were separated from whole cells and cell debris by centrifugation at 16,000 × g for 1 h at 5°C.
Chlorocatechol 1,2-dioxygenase activity was measured spectrophotometrically at room temperature by monitoring changes in absorbance at 260 nm (6). Each reaction mixture contained 50 mM Tris-HCl buffer (pH 8.0), 0.1 mM catechol or 0.1 mM substituted catechols, and 1.3 mM EDTA. The reaction was started by adding cell extract. The muconate cycloisomerase activity was measured by monitoring the decrease in absorbance at 260 nm in a reaction mixture containing 50 mM Tris-HCl buffer (pH 8.0), 1.5 mM manganese chloride, and 0.1 mM muconates or chloromuconates. One unit of enzyme activity was defined as the amount of enzyme that catalyzed the formation of 1 μmol of product per min. The molar absorption coefficients used were those reported by Dorn and Knackmuss (7). Chlorocatechols were obtained from Helix Biotechnologies (Vancouver, British Columbia, Canada), and chloromuconates were derived from chlorocatechols via oxidation catalyzed by a purified preparation of chlorocatechol dioxygenase obtained from M. Schlomann (University of Stuttgart).
Hybridization experiments.
Sixteen strains from California sites (three strains from four of the sites and four strains from the fifth site) were chosen early in the study for use in DNA hybridization experiments. Total genomic DNAs from cultures actively growing on 3CBA were obtained and digested with EcoRI, and the fragments were separated by electrophoresis through a 1% agarose gel and Southern blotted onto nylon membrane filters. These membrane filters were hybridized under low-stringency conditions (10) with DNA probes that were representative of the two known pathways of 3CBA degradation. The probe for ortho cleavage of 3CBA was a 1.1-kb fragment obtained from SalI-BglII digestion of pDC100 carrying the chlorocatechol dioxygenase gene (clcA) cloned from Pseudomonas putida(pAC127) (3). The probe for the meta cleavage pathway was a 5.2-kb fragment derived from NotI-HindIII-digested pBRCN6 carrying the cba genes of Tn5271 (26). The control DNAs on the gels included DNAs of pDC100 and JMP134 and a clone of tfdC (28), all of which code for the ortho pathway (as described by Fulthorpe et al. [10]), and DNAs of pBRCN6 and two strains carrying pBRC60, which code for the meta pathway.
RESULTS
Strain identification.
We attempted to identify the strains by fatty acid methyl ester analysis and with API 20NE strips and Biolog GN plates. In none of these tests were reliable identifications obtained; i.e., matching strains were not in any of the three databases used. With the API 20NE strips the closest species match with any of the strains was B. cepacia (12 strains tested). The similarity indices obtained in the fatty acid methyl ester analysis were less than 0.3, and the best matches were with B. cepacia or Burkholderia gladioli in the majority of cases (49 strains tested). The Biolog GN plate analysis resulted in levels of similarity to the closest-matching species (B. cepacia and Ralstonia eutrophus) of less than 0.5 (12 strains tested).
Strain diversity of California and Saskatchewan isolates.
REP-PCR fingerprinting of all of the 3CBA mineralizers from the California and Saskatchewan sites revealed a tremendous diversity of genotypes in this collection of strains. In order to compare the large number of isolates, the size of each band in the fingerprints was determined by comparison to the nearest ladder (migration distances were converted to base pair values by using a best-fit polynomial analysis performed with Sigmaplot version 5 for Windows). The band sizes were converted to a matrix of band-sharing coefficients by using a custom-made BASIC program (available from R.R.F.). This matrix was used to perform a cluster analysis (nearest neighbor joining; SYSTAT), and the resulting dendrogram provided a guide for comparisons of the REP patterns on new gels. The strains deemed most similar were run again on the same gel, and after this it was clear if the patterns were identical. Patterns in which 80% or more of the bands were the same on the same gel were considered patterns of the same genotype.
We obtained 92 isolates representing 81 genotypes from the California sites and 119 isolates representing 98 genotypes from the Saskatchewan sites. In many cases we obtained more than one genotype from a single sample (that is, from a single enrichment). There was only one site at which the same genotype was repeatedly isolated from different samples along the transect, and this site was in the eucalyptus forest in California (Chabot site). In Saskatchewan, a few genotypes were found repeatedly, but only two genotypes were found at more than one site. None of the genotypes found in California were found in Saskatchewan and vice versa.
Genotype and ARDRA type diversity of strains from all six regions.
A more detailed analysis was performed with 150 strains selected from all six regions; the strains used were chosen because they degraded 3CBA rapidly and completely when they were retested. In this group we found a total of 48 genotypes (Fig. 1). These genotypes were assigned unique codes based on the site or region in which they were found. A single representative of each of these unique genotypes was used for ARDRA typing. All of the genotypes produced the same cut pattern when they were restricted with RsaI and MspI, indicating that there was some level of similarity. However, when the rRNA genes were digested with AluI, TaqI, HaeIII, and CfoI, we detected a total of 44 ARDRA types, although some were quite similar and differed in only one fragment size for one enzyme. In order to visualize the relationships among the isolates, each genotype was scored for the presence or absence of all fragment sizes for each enzyme used. Theoretical cuts of B. cepacia (from GenBank accession no. X87275 and L28675) and R. eutrophus (from GenBank accession no. M32021) sequences were included. A matrix of Jaccard similarity coefficients was generated with a custom BASIC program. This matrix was subjected to a neighbor-joining cluster analysis by using the Wards method in Statistica (version 5.1; Statsoft, Inc., Tulsa, Okla.). The resulting dendrogram is shown in Fig. 2. The groups suggested by this analysis were used to define the ARDRA types shown in Table 2.
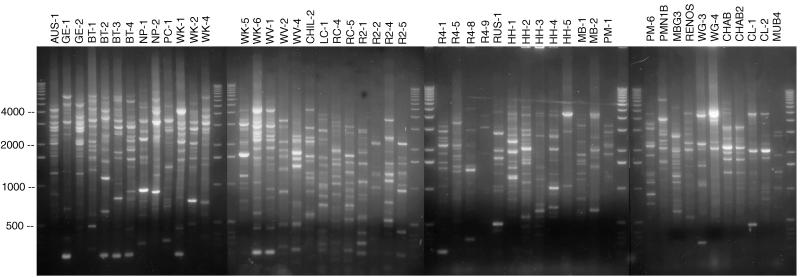
FIG. 1.
Composite photograph of 1.2% agarose gels showing the REP fingerprints of the unique genotypes. The genotypes are indicated at the top (each genotype designation includes the site or region and, in most cases, a number). From left to right the strains were isolated from sites in Australia, Saskatchewan, Chile, Russia, South Africa, and California. RC-2 is not included. Three of the genotypes, PMN1B, MBG3, and MUB4, lost the ability to degrade 3CBA. The left lane and the right lane of each gel are 1-kb marker lanes. Numbers at the left are marker fragment sizes in base pairs.
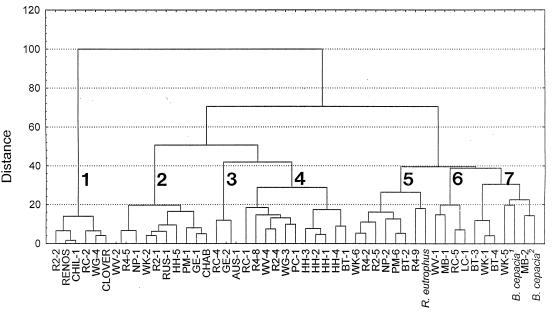
FIG. 2.
Dendrogram showing the degrees of similarity of the ARDRA patterns of the genotypes. The analysis was performed by using the Wards method of neighbor joining of Jaccard similarity coefficients calculated from the presence or absence of restriction digestion fragments in each of the strains. R. eutrophus (GenBank accession no. M32021), B. cepacia1 (GenBank accession no. X87275), and B. cepacia2 (GenBank accession no. L28675) were included in the analysis.
TABLE 2.
Geographic distribution of genotypes
| Region | REP pattern (genotype) | No. of sitesa | No. of strainsb | ARDRA cluster |
|---|---|---|---|---|
| Chile | CHIL-1 | 3 | 8 | 1b |
| RC-2 | 1 | 1 | 1a | |
| RC-4 | 1 | 1 | 3b | |
| RC-1 | 1 | 1 | 4k | |
| RC-5 | 1 | 1 | 6c | |
| LC-1 | 1 | 1 | 6d | |
| California | CHAB | 1 | 12 | 2i |
| CHAB2 | 1 | 1 | 2i | |
| CL-1 | 1 | 1 | 1c | |
| CL-2 | 1 | 1 | 1c | |
| South Africa | RENOS | 3 | 33 | 1d |
| WG-4 | 1 | 1 | 1e | |
| PM-1 | 1 | 14 | 2a | |
| HH-5 | 1 | 3 | 2b | |
| HH-1 | 1 | 2 | 4a | |
| HH-2 | 1 | 1 | 4b | |
| HH-3 | 1 | 1 | 4c | |
| HH-4 | 1 | 3 | 4d | |
| WG-3 | 1 | 1 | 4e | |
| PM-6 | 1 | 1 | 5f | |
| MB-1 | 1 | 1 | 6a | |
| MB-2 | 1 | 1 | 7a | |
| Australia | GE-1 | 1 | 1 | 2h |
| AUS-1 | 2 | 2 | 3a | |
| GE-2 | 1 | 1 | 3a | |
| Canada | WV-2 | 1 | 2 | 2e |
| WK-2 | 2 | 4 | 2f | |
| NP-1 | 1 | 1 | 2g | |
| BT-1 | 1 | 2 | 4f | |
| WV-4 | 1 | 1 | 4g | |
| PC-1 | 1 | 2 | 4h | |
| WK-6 | 1 | 1 | 5d | |
| BT-2 | 1 | 5 | 5e | |
| NP-2 | 1 | 1 | 5f | |
| WV-1 | 1 | 1 | 6b | |
| BT-3 | 1 | 1 | 7b | |
| BT-4 | 1 | 1 | 7c | |
| WK-1 | 1 | 4 | 7d | |
| WK-5 | 1 | 1 | 7e | |
| Russia | R2-2 | 1 | 2 | 1f |
| RUS-1 | 3 | 13 | 2c | |
| R2-1 | 1 | 1 | 2d | |
| R4-5 | 1 | 3 | 2e | |
| R2-4 | 1 | 1 | 4i | |
| R4-8 | 1 | 1 | 4j | |
| R2-5 | 1 | 2 | 5a | |
| R4-9 | 1 | 1 | 5b | |
| R4-2 | 1 | 4 | 5c |
Number of sites where the genotype was found.
In most cases each isolate came from a separate soil sample in a transect, and the sampling sites were at least 5 m apart.
For four of the six regions sampled, we found one genotype that was present at more than one site. We found widely distributed genotypes at sites in Chile (REP pattern CHIL-1), Russia (RUS-1), South Africa (RENOS), and Australia (AUS-1), but none of these regionally distributed genotypes was found outside its apparent native region. Hence, these genotypes appear to be endemic to their regions. However, all of the regionally distributed strains were rare, except for those from South Africa. The majority of the genotypes were unique to the sites from which the samples were obtained.
Most of the diversity in the collection revealed by REP-PCR was also apparent at the ARDRA level of resolution, although six enzymes were needed. In only four cases were strains with different genotypes found to have exactly the same ARDRA type; these cases were the CL-1 and CL-2 strains from the Cloverdale site, the CHAB and CHAB2 strains from the Chabot site, the AUS-1 and GE-2 strains from the Australian sites, and the WV-2 and R4-5 strains from Saskatchewan and Russia (the only example in which the same ARDRA type was found in more than one region). The strains fell roughly into seven major clusters that were separated by approximately 40% of the maximum distance in Fig. 2, as are R. eutrophus and B. cepacia (Fig. 2). These clusters do not correspond to particular geographic areas or ecosystem types. It is interesting that all of the widely distributed strains are members of clusters 1 through 3. The RENOS strain, which was found at several sites in South Africa, is very closely related to the widely distributed CHIL-1 strain from Chile. RUS-1, which was found at three of four Russian sites, PM-1, which was repeatedly isolated from the South African fynbos site, and the CHAB strains, which were the only strains found at the Chabot site, are also closely related as determined by this method. AUS-1 and GE-2 from Australia seem to form a group that includes only one other strain, the RC-4 strain from Chile. The only other Australian genotype, GE-1, was found in cluster 2, a group containing relatively widely distributed strains. Clusters 4 through 7 are comprised solely of genotypes that were unique to their sites.
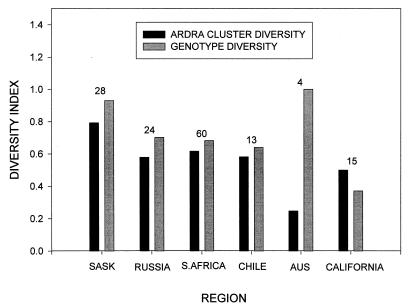
Diversity indices were calculated for each of the regions by using REP genotypes and ARDRA clusters as taxonomic units. The indices were corrected for samples sizes (30) and indicate that the highest levels of diversity were found in Saskatchewan, followed by South Africa and Russia (Fig. 3).
FIG. 3.
Diversity of 3CBA degraders found in each region calculated as ARDRA type cluster diversity (from the number of individuals in each ARDRA type cluster) and genotype diversity (from the number of individuals per REP genotype). Diversity was calculated by using n(1 − Σxi2)/(n − 1), where xi is the frequency of the ith taxon and n is the total number of individuals. The numbers of individuals in the regions are shown above the bars. The term n/(n − 1) corrects for sample size (30). Abbreviations: SASK, Saskatchewan; S.AFRICA, South Africa; AUS, Australia.
Habitat fidelity.
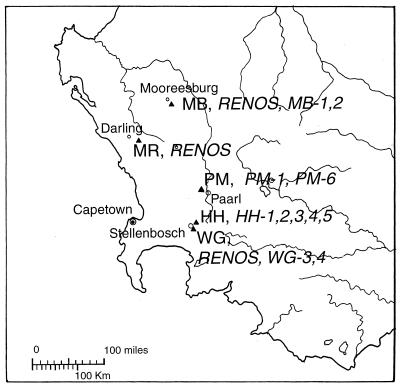
The South African strains in this collection were the source of an interesting observation, the observation that the bacterial genotypes isolated from South African soils were strongly associated with the vegetation of the communities. In this region we obtained samples from three sites with renosterveld ecosystems that were characterized by a predominance of Restia bushes and ericaceous shrubs, one site that was dominated by members of the family Proteaceae (fynbos vegetation), and one site that was dominated by Eucalyptus species overgrowing a former renosterveld site (Fig. 4). Despite the fact that the renosterveld sites were the most widely separated of the five sites examined, they were populated by identical or extremely closely related genotypes, which were designated RENOS (Table 2). The fynbos site was populated by another genotype, PM-1, which belonged to a different ARDRA group. The eucalyptus site yielded five different genotypes, four of which were closely related to each other (HH-1 through HH-4). In all, we isolated 33 RENOS genotypes and only four other genotypes from the renosterveld sites but 29 different genotypes and no RENOS genotypes from the other two sites. On the basis of these findings we tested the association between the RENOS genotypes and the renosterveld vegetation by using a contingency table and the G statistic. We found that the G value was 65.98, which is well above the chi square value of 10.83 (P = 0.001; v = 1), and this value suggests that the probability that this association was due to chance is less than 0.1%.
FIG. 4.
Map of the Cape Region of South Africa showing the locations of the sampling sites and the designations of the genotypes found at each site. Note the widespread distribution of genotype RENOS, found at sites WG, MB, and MR, all of which are vegetated by renosterveld scrub.
Hybridization of strains with known 3CBA catabolic genes.
None of the 16 strains that we probed, all of which actively degraded 3CBA prior to DNA extraction, hybridized to either clcA (ortho pathway) or cba (meta pathway) genes. This was despite the fact that positive controls gave strong bands and the lambda ladder gave faint signals, indicating that the hybridization was carried out under very low-stringency conditions. The clcA probe derived from pDC100 hybridized well to a tfdC clone and the tfdC in the genomic preparation of JMP134 (28) (2,4-dichlorophenoxyacetic acid pathway; 60% nucleotide sequence identity to the probe [12]).
Enzymatic activities.
Two strains were used to examine chlorocatechol dioxygenase and chloromuconate isomerase activities. Each strain appeared to contain both enzymes, but the substrate ranges were different (Table 3).
TABLE 3.
Catechol pathway enzyme activities of cell extracts of two strains growing on 1.5 mM 3CBAa
| Genotype | Enzyme | Substrate | Activity (μmol/mg of protein · min−1) | % Activity |
|---|---|---|---|---|
| WV-4b | Catechol dioxygenase | Catechol | 300 | 100 |
| 3-Chlorocatechol | 49 | 16 | ||
| 4-Chlorocatechol | 192 | 64 | ||
| 3,5-Dichlorocatechol | 191 | 78 | ||
| 3-Methylcatechol | 220 | 232 | ||
| 4-Methylcatechol | 697 | 100 | ||
| Muconate cycloisomerase | Muconate | 181 | 100 | |
| 2-Chloromuconate | 11 | 6 | ||
| 3-Chloromuconate | 37 | 21 | ||
| PM-1c | Catechol dioxygenase | Catechol | 352 | 100 |
| 3-Chlorocatechol | 5 | 2 | ||
| 4-Chlorocatechol | 91 | 26 | ||
| 3,5-Dichlorocatechol | 2 | 0.6 | ||
| 3-Methylcatechol | 104 | 30 | ||
| 4-Methylcatechol | 245 | 70 | ||
| Muconate cycloisomerase | Muconate | 142 | 100 | |
| 2-Chloromuconate | 2 | 17 | ||
| 3-Chloromuconate | 51 | 352 |
See text for details.
Strain WV-7-301.
Strain PM-P-301.
DISCUSSION
This study revealed two new main findings. The first is that diverse bacteria isolated from uncontaminated ecosystems have the ability to mineralize a chloroaromatic compound that, to our knowledge, is not naturally produced. The second is that geography and vegetation seem to determine to some extent which 3CBA degraders are present in the soils that we analyzed.
The mineralization of 3CBA has been studied as a model for the evolution of novel genes involved in the catabolism of xenobiotic compounds. We found that a number of different genotypes that were isolated in relatively pristine areas have the ability to mineralize 3CBA. Despite the diversity at the genotype level, the isolates appear to fall into one taxonomic group, as judged by their similar responses to the taxonomic tests and the numbers of similar ARDRA bands (25). This was recently confirmed by the results of partial 16S rRNA sequence analyses which showed that the strains are most similar to the Alcaligenes-Burkholderia assemblage of the beta subgroup of the class Proteobacteria (18). The finding that there is a high level of genotype diversity within a family suggests that either (i) the ability to mineralize 3CBA is relatively old or (ii) the horizontal transfer of novel genes between geographically distant but related strains is common. We are confident that the genotypes isolated in this study were originally present in the soils which we sampled and not the product of laboratory evolution during enrichment. As shown by Fulthorpe et al. (11), the ability to mineralize 3CBA was found in all of the soils that we sampled (672 separate enrichments), and it was present within 1 week after 3CBA was added. It is unlikely that novel evolutionary events would be so reproducible.
3CBA is currently known to be degraded by the following two pathways: via ortho cleavage of a catechol intermediate (28) and via meta cleavage of a protocatechuate intermediate (26). We could not detect the presence of the known genetic determinants isolated from other 3CBA degraders in a subset of our collection, suggesting that widespread horizontal transfer of known genes is not responsible for 3CBA mineralization, at least in the part of this group analyzed. However, the results of the enzyme assays suggest that at least two of the strains use an ortho cleavage pathway. There are three known chlorocatechol dioxygenases that have been fully sequenced (tcbC, tfdC, and clcA), and these enzymes are sufficiently different from each other that Schlomann concluded that the evolution of this enzyme has been ongoing for the last 70 to 90 million years (29). We successfully amplified putative chlorocatechol dioxygenase gene fragments from the strains in our collection by using redundant PCR primers that target the conserved area of the genes mentioned above. The nucleotide sequences of these fragments differ from the clcA nucleotide sequence by more than 40% and are in fact more similar to the tfdC nucleotide sequence (18, 19). The presence of other variants of these enzymes in the diverse collection of strains isolated from pristine areas supports the idea that this chloroaromatic pathway has an ancient origin.
We once thought that all chlorinated organic compounds had an anthropogenic origin, but the growing list of naturally occurring chloroorganic compounds has forced us to change this view (13). In particular, our understanding of the selective pressures acting on chloroaromatic compound degraders has been drastically changed by recent reports of high levels (up to 75 ppm) of chlorinated anisaldehydes and anisic acids produced by wood decay fungi in natural systems (5). However, these compounds do not seem to be responsible for the selection of the 3CBA degraders. When our isolates were tested on 3-chloroanisic acid, none of the genotypes isolated in this study could mineralize this compound (9). Further research on the substrate ranges of these isolates and on the chloroaromatic compound contents of natural systems is merited.
At the outset of this study we predicted that the diversity of the isofunctional bacteria isolated from a particular community would reflect their geographic origin, or the type of vegetation growing at the site from which they were isolated. These predictions were derived from the hypotheses that bacterial diversification is ongoing and that current free-living soil bacteria are not globally mixed. The sizes of the samples obtained from three of the regions were too small to draw meaningful conclusions about correlations with diversity; however, it is interesting that the Saskatchewan samples exhibited greater diversity than the South African samples and that the Russian samples exhibited diversity equivalent to that of the South African samples. The boreal forests are dominated by very few tree species and a few understory plants, while the Cape Region of South Africa is an area with extremely high vegetative diversity. The soils were originally chosen for this study because they were as similar as possible given the climatic differences. In both regions the soils are luvisols whose pH values range from 5.5 to 6.1, and the organic carbon contents of both types of soils range from 0.4 to 5% (11). The boreal forest soils were generally wetter (water content, 23.5% ± 7%) than the South African soils (9.7% ± 3.5%) at the time of sampling, although the moisture contents were identical during the enrichment steps. This may have contributed to the lower initial diversity of viable organisms. However, the most striking difference between the soils of these two areas is the amount of organic matter that overlies them. In the boreal forest systems the soils were covered by 10 to 20 cm of feather mosses, and below that there was a thick humic layer which was removed prior to sampling. In South Africa, the soils were all but bare, perhaps having small patches of organic litter or moss. The boreal forest soils might contain a greater diversity of standing organic matter, and this might be more important to bacterial community structure than the diversity of the live plant standing crop is. However, another explanation might be that the bacteria residing in the mineral soils of boreal forests are much more spatially isolated, and greater diversification is possible. In the exposed soils of the renosterveld vegetation, mixing of soil communities via wind, rain, and animal movement must be much more common.
Our South African samples did reveal a clear link between habitat and 3CBA degrader genotype. The mixing argument described above is not likely to explain the similarity of the bacteria of the renosterveld sites, since both the fynbos site and the eucalyptus site had the same bare, exposed soils yet harbored quite different genotypes than the renosterveld sites, one of which was relatively nearby. It is more likely that the organic inputs from the renosterveld vegetation selected for the RENOS genotype.
The ARDRA data revealed that no ARDRA type was found in more than one region, except for one Saskatchewan strain and one Russian strain, two strains from very similar environments. However, there was little correlation between geographic origin and ARDRA type in the cluster analysis. This is not particularly surprising given the coarse level of resolution provided by restriction fragment length polymorphism comparisons of the 16S rRNA genes. The seven clusters that correspond roughly to genera in our collection would have diverged hundreds of millions of years ago (i.e., around the time that continental land masses were joined) (27). Therefore, widespread representation of genera was expected. What is clear is that within the seven clusters identified diversification is ongoing and producing strains that are not widely distributed. At a finer level of genetic resolution, as revealed by the REP analysis, soil bacteria in fact do seem to have limited distributions, and these distributions may be strongly affected by the vegetation growing in the soil.
ACKNOWLEDGMENTS
This research was supported by grant BIR9120006 from the National Science Foundation and was part of the Joint Research Project on Microbial Evolution with the Research and Development Corporation of Japan (JRDC). Additional work was supported by a Natural Sciences and Engineering Research Council of Canada grant to R.R.F.
We thank Michelle Leander and Robert Sroufe for technical assistance, John Dunbar for advice on REP PCR troubleshooting, and Cindy Nakatsu for providing pBRCN6 for the hybridization studies.
REFERENCES
- 1.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. New York, N.Y: John Wiley and Sons; 1987. [Google Scholar]
- 2.Brunsbach F R, Reineke W. Degradation of chlorobenzoates in soil slurry by special organisms. Appl Microbiol Biotechnol. 1993;39:117–122. doi: 10.1007/BF00902751. [DOI] [PubMed] [Google Scholar]
- 3.Chatterjee D K, Chakrabarty A M. Restriction mapping of a chlorobenzoate degradative plasmid and molecular cloning of the degradative genes. Gene. 1984;27:173–181. doi: 10.1016/0378-1119(84)90138-0. [DOI] [PubMed] [Google Scholar]
- 4.de Bruijn F J. Use of repetitive (repetitive extragenic palindromic and enterobacterial repetitive intergeneric consensus) sequences and the polymerase chain reaction to fingerprint the genomes of Rhizobium meliloti isolates and other soil bacteria. Appl Environ Microbiol. 1992;58:2180–2187. doi: 10.1128/aem.58.7.2180-2187.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Jong E, Field J A, Spinnler H-E, Wijnberg J B P A, de Bont J A M. Significant biogenesis of chlorinated aromatics by fungi in natural environments. Appl Environ Microbiol. 1994;60:264–270. doi: 10.1128/aem.60.1.264-270.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dorn E, Knackmuss H-J. Chemical structure and biodegradability of halogenated aromatic compounds. Two catechol 1,2-dioxygenases from a 3-chlorobenzoate grown pseudomonad. Biochem J. 1978;174:73–84. doi: 10.1042/bj1740073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dorn E, Knackmuss H-J. Chemical structure and biodegradability of halogenated aromatic compounds. Substituent effects on 1,2-dioxygenation of catechol. Biochem J. 1978;174:85–94. doi: 10.1042/bj1740085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Focht D D, Shelton D. Growth kinetics of Pseudomonas alcaligenes C-0 relative to inoculation and 3-chlorobenzoate metabolism in soil. Appl Environ Microbiol. 1987;53:1846–1849. doi: 10.1128/aem.53.8.1846-1849.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fulthorpe R R. 46th Annual Canadian Society of Microbiology Meeting. Ottawa, Ontario, Canada: Canadian Society of Microbiologists; 1996. Degradation of chlorinated pollutants in pristine soils, abstr. EMp27. [Google Scholar]
- 10.Fulthorpe R R, McGowan C, Maltseva O V, Holben W E, Tiedje J M. 2,4-Dichlorophenoxyacetic acid-degrading bacteria contain mosaics of catabolic genes. Appl Environ Microbiol. 1995;61:3274–3281. doi: 10.1128/aem.61.9.3274-3281.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fulthorpe R R, Rhodes A N, Tiedje J M. Pristine soils mineralize 3-chlorobenzoate and 2,4-dichlorophenoxyacetate via different microbial populations. Appl Environ Microbiol. 1996;62:1159–1166. doi: 10.1128/aem.62.4.1159-1166.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghosal D, You I S. Nucleotide homology and organization of chlorocatechol oxidation genes of plasmids pJP4 and pAC27. Mol Gen Genet. 1988;211:113–120. doi: 10.1007/BF00338401. [DOI] [PubMed] [Google Scholar]
- 13.Gribble G W. Naturally occurring organohalogen compounds—a survey. J Nat Prod. 1992;55:1353–1395. doi: 10.1021/acs.jnatprod.3c00803. [DOI] [PubMed] [Google Scholar]
- 14.Hernandez B S, Arensdorf J J, Focht D D. Catabolic characteristics of biphenyl-utilizing isolates which cometabolize PCBs. Biodegradation. 1995;6:75–82. [Google Scholar]
- 15.Hickey W J, Focht D D. Degradation of mono-, di-, and trihalogenated benzoic acids by Pseudomonas aeruginosa JB2. Appl Environ Microbiol. 1990;56:3842–3850. doi: 10.1128/aem.56.12.3842-3850.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hickey W J, Searles D B, Focht D D. Enhanced mineralization of polychlorinated biphenyls in soil inoculated with chlorobenzoate-degrading bacteria. Appl Environ Microbiol. 1993;59:1194–1200. doi: 10.1128/aem.59.4.1194-1200.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaijalainen S, Lindstrom K. Restriction fragment length polymorphism analysis of Rhizobium galegae strains. J Bacteriol. 1989;171:5561–5566. doi: 10.1128/jb.171.10.5561-5566.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leander M V, Fulthorpe R R. 47th Annual Canadian Society of Microbiology Meeting. Ottawa, Ontario, Canada: Canadian Society of Microbiologists; 1997. Diversity of chlorocatechol dioxygenases isolated from pristine soil chlorobenzoate degraders, abstr. EMp10. [Google Scholar]
- 19.Leander, M. V., T. Vallaeys, and R. R. Fulthorpe. Amplification of chlorocatechol dioxygenase genes from beta and alpha proteobacteria. Can. J. Microbiol., in press. [DOI] [PubMed]
- 20.Maynard-Smith J, Dowson C G. Localized sex in bacteria. Nature. 1991;349:29–31. doi: 10.1038/349029a0. [DOI] [PubMed] [Google Scholar]
- 21.Maynard-Smith J, Smith N H, O’Rourke M, Spratt B. How clonal are bacteria? Proc Natl Acad Sci USA. 1993;90:4384–4388. doi: 10.1073/pnas.90.10.4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McArthur J V, Kovacic D A, Smith M H. Genetic diversity in natural populations of a soil bacterium across a landscape gradient. Proc Natl Acad Sci USA. 1988;85:9621–9624. doi: 10.1073/pnas.85.24.9621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McArthur J V, Lefrf L G, Smith M H. Genetic diversity of bacteria along a stream continuum. J N Am Benth Soc. 1992;11:269–277. [Google Scholar]
- 24.Moyer C L, Dobbs F C, Karl D M. Estimation of diversity and community structure through restriction fragment length polymorphism distribution analysis of bacterial 16S rRNA genes from a microbial mat at an active, hydrothermal vent system, Loihi Seamount, Hawaii. Appl Environ Microbiol. 1994;60:871–879. doi: 10.1128/aem.60.3.871-879.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moyer C L, Tiedje J M, Dobbs F C, Karl D M. A computer-simulated restriction fragment length polymorphism analysis of bacterial small-subunit rRNA genes: efficacy of selected tetrameric restriction enzymes for studies of microbial diversity in nature. Appl Environ Microbiol. 1996;62:2501–2507. doi: 10.1128/aem.62.7.2501-2507.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakatsu C H, Wyndham R C. Cloning and expression of the transposable chlorobenzoate-3,4-dioxygenase genes of Alcaligenes sp. strain BR60. Appl Environ Microbiol. 1993;59:3625–3633. doi: 10.1128/aem.59.11.3625-3633.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ochman H, Wilson A C. Evolution in bacteria, evidence for universal substitution rates in cellular genomes. J Mol Evol. 1987;26:74–86. doi: 10.1007/BF02111283. [DOI] [PubMed] [Google Scholar]
- 28.Perkins E J, Gordon M P, Caceres O, Lurquin P F. Organization and sequence analysis of the 2,4-dichlorophenol hydroxylase and dichlorocatechol oxidative operons of plasmid pJP4. J Bacteriol. 1990;172:2351–2359. doi: 10.1128/jb.172.5.2351-2359.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schlomann M. Evolution of chlorocatechol catabolic pathways. Biodegradation. 1994;5:301–321. doi: 10.1007/BF00696467. [DOI] [PubMed] [Google Scholar]
- 30.Selander R K, Caugant D A, Ochman H, Musser J M, Gilmour M N, Whittam T X. Methods of multilocus enzyme electrophoresis for bacterial population genetics and systematics. Appl Environ Microbiol. 1986;51:873–884. doi: 10.1128/aem.51.5.873-884.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Souza V, Nguyen T T, Hudson R R, Pinero D, Lenski R E. Hierarchical analysis of linkage disequilibrium in Rhizobium populations: evidence for sex? Proc Natl Acad Sci USA. 1992;89:8389–8393. doi: 10.1073/pnas.89.17.8389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tiedje J M, Fulthorpe R, Kamagata Y, McGown C, Rhodes A, Takami H. What is the global pattern of chloroaromatic degrading microbial populations? In: Horikoshi K, Fukuda M, Kudo T, editors. Microbial diversity and genetics of biodegradation. Tokyo, Japan: Japan Scientific Societies Press; 1997. pp. 65–74. [Google Scholar]
- 33.Versalovic J, Koeuth T, Lupski J R. Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Res. 1991;19:6823–6831. doi: 10.1093/nar/19.24.6823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weisburg W G, Barns S M, Pelletier D A, Lane D J. 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol. 1991;173:697–703. doi: 10.1128/jb.173.2.697-703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wise M G, McArthur J V, Wheat C, Shimkets L J. Temporal variation in genetic diversity and structure of a lotic population of Burkholderia (Pseudomonas) cepacia. Appl Environ Microbiol. 1996;62:1558–1562. doi: 10.1128/aem.62.5.1558-1562.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wyndham R C. Evolved aniline catabolism in Acinetobacter calcoaceticus during continuous culture of river water. Appl Environ Microbiol. 1986;51:781–789. doi: 10.1128/aem.51.4.781-789.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]