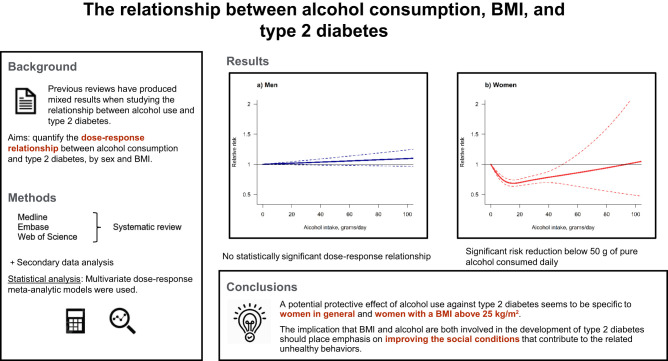
Abstract
Background
Moderate alcohol use may be associated with lower risk of type 2 diabetes mellitus (T2DM). Previous reviews have reached mixed conclusions.
Purpose
To quantify the dose-response relationship between alcohol consumption and T2DM, accounting for differential effects by sex and BMI.
Data Sources
Medline, Embase, Web of Science, and one secondary data source.
Study Selection
Cohort studies on the relationship between alcohol use and T2DM.
Data Extraction
Fifty-five studies, and one secondary data source, were included with a combined sample size of 1,363,355 men and 1,290,628 women, with 89,983 and 57,974 individuals, respectively, diagnosed with T2DM.
Data Synthesis
Multivariate dose-response meta-analytic random-effect models were used. For women, a J-shaped relationship was found with a maximum risk reduction of 31% (relative risk [RR] 0.69, 95% CI 0.64–0.74) at an intake of 16 g of pure alcohol per day compared with lifetime abstainers. The protective association ceased above 49 g per day (RR 0.82, 95% CI 0.68–0.99). For men, no statistically significant relationship was identified. When results were stratified by BMI, the protective association was only found in overweight and obese women.
Limitations
Our analysis relied on aggregate data. We included some articles that determined exposure and cases via self-report, and the studies did not account for temporal variations in alcohol use.
Conclusions
The observed reduced risk seems to be specific to women in general and women with a BMI ≥25 kg/m2. Our findings allow for a more precise prediction of the sex-specific relationship between T2DM and alcohol use, as our results differ from those of previous studies.
Graphical Abstract
Introduction
More than 500 million adults live with diabetes worldwide (1). Type 2 diabetes mellitus (T2DM) accounts for over 90% of all diabetes cases worldwide and is a growing public health concern. It ranked fourth among the noncommunicable diseases in 2019 for disability-adjusted life years and seventh for mortality (2), representing a 45% increase in deaths since 2000. Its incidence has escalated globally, increasing from 203 per 100,000 individuals in 2000 to 260 per 100,000 individuals in 2019 (2), with no declines forecast (3).
T2DM is influenced by multiple individual risk factors, and the increase in its rates has mostly been attributed to changes in the living environment and lifestyle across populations, such as unhealthy diets (e.g., increased intake of refined carbohydrates) and decreased physical activity levels (4). Alcohol consumption has also been associated with T2DM development. The most recent reviews on this topic have identified a nonlinear relationship between alcohol use and the risk of T2DM (5–7). However, they reached different conclusions. Baliunas et al. (5), in 2009, identified a J-shaped dose-response relationship, indicating that with low to moderate consumption there is a protective association of alcohol use on incident T2DM, whereas at higher levels of consumption there is an increased risk of T2DM. In line with Baliunas et al. (5), a review published in 2016 identified a reduced risk of T2DM with alcohol consumption of less than 20 g per day in women and less than 40 g per day in men (6). In contrast, Knott et al. (7) reported that the reduction in risk at low drinking levels seemed to be specific to women.
The association between alcohol use and T2DM may be influenced by potential effect modifiers that must be analyzed to better understand this complex relationship. One such modifier is BMI, a significant individual risk factor for developing T2DM (8). Studies have found no relationship or a positive relationship between alcohol use and BMI in men (9,10) but an inverse relationship in women (11,12), which may partially explain sex differences in the association between alcohol use and T2DM (13). BMI may modify the relationship by exacerbating the effects of excessive alcohol intake on insulin resistance among those with a higher BMI (10), and they may experience amplified proinflammatory effects of alcohol, thereby increasing the risk of T2DM (14). Socioeconomic status (SES) might also play an important role. One meta-analysis found that the lowest-SES groups, as measured by education, occupation, and income, had an increased risk of T2DM compared with the highest-SES groups (15). The relationship with T2DM is not yet fully understood. Individuals with lower SES may face challenges in accessing healthy foods, which, when combined with excessive alcohol intake, may overproportionally increase the risk of T2DM. SES can influence psychosocial factors that interact with alcohol consumption and may impact the risk of T2DM.
Our study aimed to update sex-specific dose-response curves on the risk relationship between alcohol consumption and T2DM and to examine how BMI and SES may influence this association.
Research Design and Methods
Systematic Review
A systematic search was conducted in Medline, Embase, and Web of Science from their inception to 6 July 2022. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses criteria (Supplementary Table 1) was used as a guideline (16). The search was conducted using a combination of keywords and MeSH (Medical Subject Headings) terms (Supplementary Table 2). In addition, the reference list of relevant articles was manually reviewed. No language restrictions were imposed. The references were independently screened by two authors (L.L.-F., C.K., and/or T.C.) by following a two-step approach of 1) title/abstract screenings and 2) full-text screenings. Agreement between the reviewers was quantified using the Cohen κ coefficient. For the title/abstract screening, the agreement was substantial (κ = 0.77) and almost perfect (κ = 0.81). For the full-text screening, the agreement was almost perfect (κ = 0.83) and substantial (κ = 0.63). Conflicts were resolved via team discussion. The study protocol was registered with PROSPERO (CRD42022340247).
The following inclusion criteria were used: case-control or cohort studies that reported on the relationship between alcohol consumption and the risk of T2DM; studies that reported the quantity of alcohol use as the exposure variable with at least two categories compared with a third reference category (e.g., lifetime abstainers or current abstainers), as it has been previously reported that the association between alcohol consumption and T2DM is nonlinear; the outcome was risk of T2DM (diagnosis defined by the American Diabetes Association guidelines [17] or ICD-10 code E11, ICD-8, and ICD-9 code 250), including incident and/or mortality cases; the method of assessing T2DM was either by an objective assessment (e.g., laboratory findings or medical records) or self-report; and studies that reported odds ratios, relative risks (RR), or hazard ratios (HR) and their 95% CI or information allowing us to compute them. Studies were excluded if they were not published as full reports, if they did not have enough data to compute quantitative results, if alcohol consumption could not be converted into grams per day, or if the outcome was type 1, autoimmune, or gestational diabetes, insulin resistance, prediabetes, or specific complications (e.g., diabetic retinopathy or neuropathy).
Data Extraction
The following data were extracted by one author (L.L.-F.), with the verification of half of the studies by three authors (C.K., T.C., or J.R.), using a standardized spreadsheet: title, first author, year of publication, country, study design, name of the cohort, follow-up years, sample size, sex, age, SES, BMI, alcohol consumption categories and measures, period of alcohol consumption, risk estimates, adjustments made, and outcome ascertainment. Half of the studies were randomly reviewed to determine the error rate (<1% error rate acceptable for continuing without revising the remaining). Conflicts were resolved via team discussions.
If the information on alcohol consumption was not reported in grams of pure alcohol per day, we converted it to this metric using the size of a standard drink in the study’s country of origin (18), unless the study provided a specific conversion factor. If alcohol consumption was given in ranges, the midpoint was taken. If the highest category did not have an upper bound, we calculated the midpoint of that category by adding 75% of the width of the previous category’s range to the lower bound of that category. In addition, in studies where crucial information was not available, authors were contacted via e-mail to obtain the data needed for our analysis.
Quality Assessment
The Newcastle-Ottawa Scale (NOS) was used to assess quality in the selected studies (for details and scoring system, see Supplementary File 3) (19). Studies were independently assessed by two authors. In cases of disagreement, the quality assessment was reevaluated. The evidence was rated based on the Grading of Recommendations Assessment, Development, and Evaluation approach.
Secondary Data Source
One secondary data analysis using nationally representative data from the U.S. was conducted for inclusion in this review. Cross-sectional data from the National Health Interview Survey (NHIS), covering the annual survey years from 1997 to 2018 and linked to the mortality data from the U.S. National Death Index (follow-up until 2019), was used. Adult participants aged ≥25 years were included in the analyses. Missing values in covariates were excluded, which accounted for <5% of the total sample. The final sample size was 562,042 (246,004 men and 316,038 women), which included 2,503 T2DM deaths (1,115 men and 1,388 women). Sex- and BMI-specific analyses were conducted using Cox proportional hazard models to calculate the point estimates for the risk of T2DM mortality at various levels of alcohol use. The models were adjusted for sex, education (high school degree or less, some college education but no degree, and college degree or more), race/ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic, and others), marital status, and survey year. Additionally, we accounted for the complex survey design through survey weights, stratum, and primary sampling unit.
Statistical Analysis
First, to guarantee comparability across studies, we ensured that all studies quantified the RR related to different levels of alcohol use based on the same reference. We encountered three different scenarios: 1) lifetime abstainers, 2) current abstainers, and 3) other, such as the category of lowest amount of alcohol consumed. Including participants who used to consume alcohol but no longer do may introduce the “sick quitter bias,” as they may have ceased drinking due to health concerns that may be associated with higher risks for T2DM compared with lifetime abstainers (20). We separated lifetime abstainers from former drinkers using a subset of studies, and we used this information to adjust the RR of the studies that originally had a different reference category than lifetime abstainers (for our reasoning, see Supplementary File 4).
Multivariate dose-response meta-analytic models using a restricted maximum likelihood random-effect estimator were used (21). The intercept was modeled to go through zero on the logarithmic scale. Three sets of shapes of the dose-response relationship (linear, quadratic, and restrictive cubic splines) were tested using all studies and were stratified by sex (22). The model that best fits the data was selected based on the Akaike information criterion and Bayesian information criterion. Additionally, dose-response relationships stratified by sex and BMI were fitted. We categorized BMI into healthy weight range (i.e., ≥18 kg/m2 and <25 kg/m2), overweight range (i.e., ≥25 kg/m2 and <30 kg/m2), and obesity range (i.e., ≥30 kg/m2). The underweight range was not considered. There were two studies conducted in Japan that defined overweight as BMI ≥22 kg/m2 and <25 kg/m2 and obesity as BMI ≥25 kg/m2 (see Supplementary Table 5). Some researchers have recommended that different definitions of overweight and obesity should be used in different world regions, because studies on Japanese subjects suggest that they have a higher percentage of body fat at lower BMI levels than Americans or Europeans (23). Accordingly, we incorporated these studies in the overweight or obesity categories stated by the authors in their original publications. To investigate sources of heterogeneity and to study potential interaction effects with the dose of alcohol consumption, we included age as a continuous variable, and sex, NOS score and country of residency (U.S. vs. others and Asian countries vs. others) as categorical variables in meta-regression models. Meta-regression enables us to evaluate multiple variables simultaneously, and our models were used to inform the influence of the aforementioned variables on the risk relationship.
We conducted four sensitivity analyses to assess the robustness of our findings. First, we restricted the analysis to studies that used objective measures to assess the outcome (i.e., laboratory findings, medical records, or registries). Second, since both the American Diabetes Association in 1997 (24) and the World Health Organization in 1998 (25) changed their criteria to diagnose T2DM, we performed a sensitivity analysis using only studies that diagnosed cases based on the new diagnostic criteria (fasting plasma glucose level ≥7.0 mmol/L instead of ≥7.8 mmol/L). In our third sensitivity analysis, we used a one-stage dose-response meta-analysis as an alternative modeling approach and compared it with the results obtained with the methods described above (26). This approach was used as a sensitivity analysis only because it has the limitation that the number of participants and cases per alcohol consumption category is needed to conduct the analyses, and some studies did not provide these data. To investigate the impact of including T2DM mortality as the sole outcome or combining it with incidence, we excluded studies that explicitly indicated the inclusion of mortality cases, along with our secondary data source, in our fourth sensitivity analysis.
All statistical analyses were conducted using meta (27) and metafor (21) packages for the main analysis and dosresmeta (26) for the third sensitivity analysis, in R software, version 4.2.1.
Data Availability
The original contribution presented in the study are included in the article and supplementary material. Further inquiries can be directed to the corresponding author.
Results
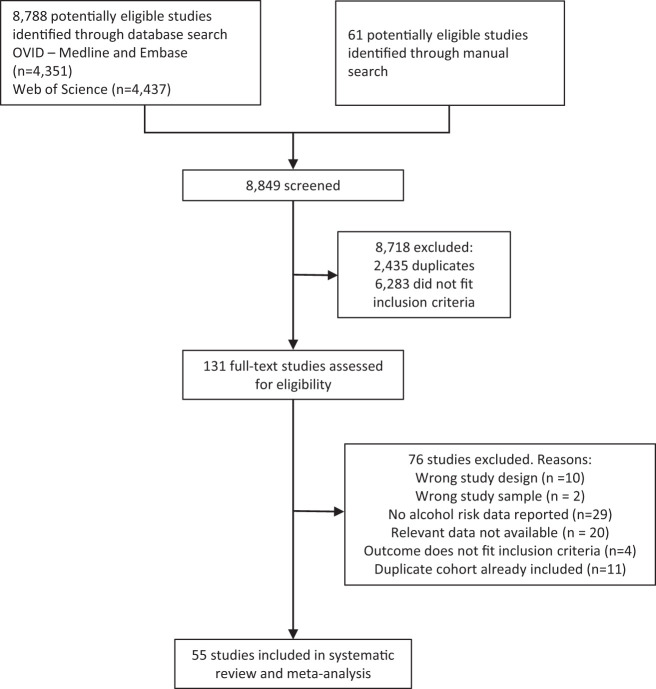
A total of 55 articles fulfilled our inclusion criteria (Fig. 1). The characteristics of all studies included in our analysis, including the secondary data source, are summarized in Supplementary Table 5. There were collectively 1,363,355 men and 1,290,628 women and a total of 89,983 and 57,974 diagnoses of T2DM for men and women, respectively. All selected studies were cohort studies. The majority of studies were conducted in the U.S. (27%), followed by Japan (20%), South Korea (7%), and Australia, China, and Finland (all three with 5%). A total of 15 studies (27%) were rated as being high-quality studies based on NOS criteria, 30 studies (55%) were rated as being of moderate quality, and 10 (18%) were rated as being of low quality. A total of eight studies and our secondary data source provided risk estimates stratified by sex and BMI, and only one study (28) provided risk estimates stratified by occupation as an SES indicator.
Figure 1.
Flowchart for study selection.
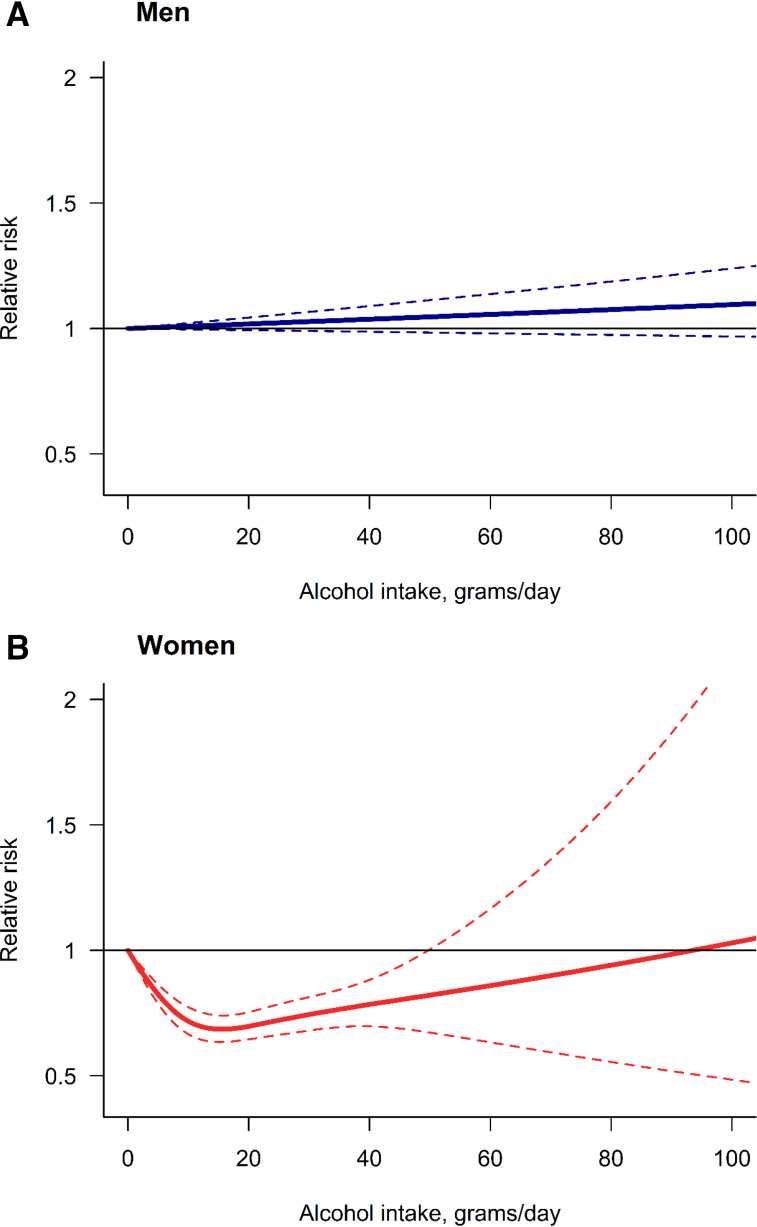
A total of 13 studies provided risk estimates for both sexes combined, while 40 studies provided risk estimates for men and 29 studies provided risk estimates for women. For men, we did not find an indication of a dose-response relationship between alcohol use and T2DM (Fig. 2A; for model selection, see Supplementary File 6). While the linear model fit the data best, there was no increase or reduction of the risk at any level of alcohol consumption compared with lifetime abstainers. For women, the restrictive cubic spline showed the best fit, describing a J-shaped dose-response relationship (Fig. 2B and Supplementary File 6). We identified a minimum reduction of 31% in the risk of T2DM (RR 0.69, 95% CI 0.64–0.74) at 16 g of pure alcohol per day compared with lifetime abstainers. Above 49 g per day (RR 0.82, 95% CI 0.68–0.99), the protective association ceased. Table 1 presents some examples of RR for men and women. The results for both sexes combined can be found in Supplementary File 7. For this analysis, a J-shaped dose-response relationship was identified.
Figure 2.
Dose-response meta-analysis between alcohol intake in average grams per day and risk of type 2 diabetes for men (A) and women (B).
Table 1.
RR for T2DM by sex for individuals with some level of alcohol use compared with lifetime abstainers
| Grams of pure alcohol per day | Men RR (95% CI) | Women RR (95% CI) |
|---|---|---|
| 20 | 1.02 (0.994–1.04) | 0.70 (0.65–0.75) |
| 40 | 1.04 (0.987–1.09) | 0.78 (0.70–0.88) |
| 60 | 1.06 (0.981–1.14) | 0.86 (0.63–1.17) |
| 80 | 1.08 (0.975–1.19) | 0.94 (0.56–1.59) |
| 100 | 1.10 (0.968–1.24) | 1.03 (0.48–2.19) |
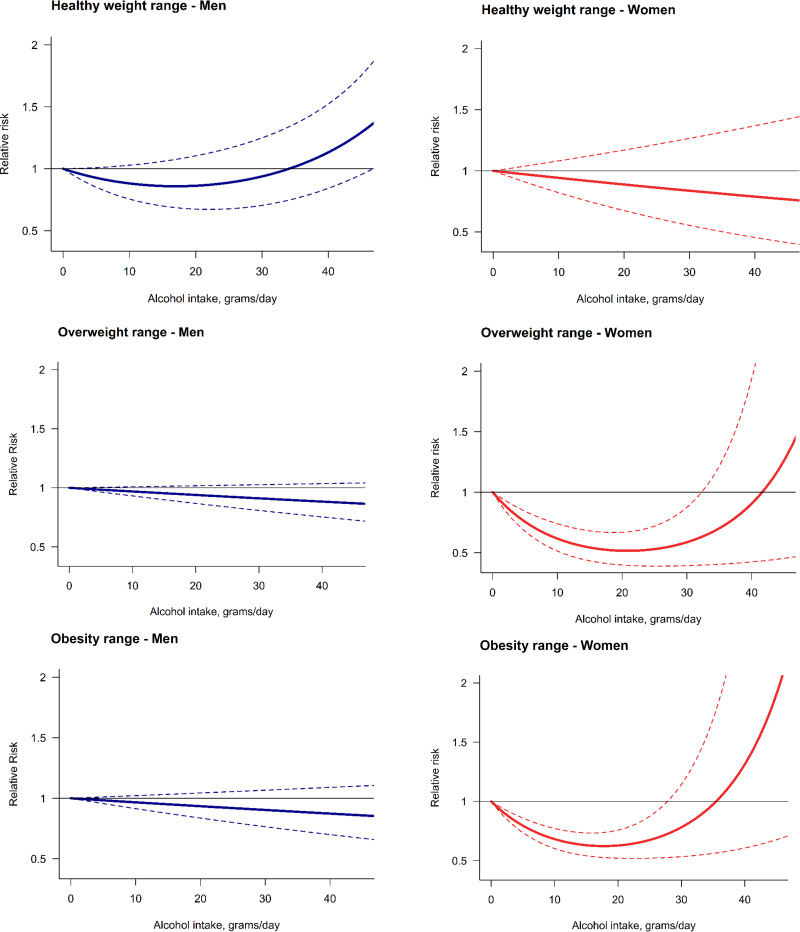
When we stratified by sex and BMI, in men we found no significant risk relationship in any of the three BMI categories (Fig. 3). There was no significant risk relationship for women with a healthy weight; however, we identified a J-shaped relationship present in women with BMI ≥25 kg/m2 (Fig. 3). We identified a peak reduction of 48% in the risk of T2DM (RR 0.52; 95% CI 0.40–0.67) at 21 g per day compared with lifetime abstainers in women with overweight and a peak reduction of 38% (RR 0.62; 95% CI 0.53–0.74) at 18 g per day compared with lifetime abstainers in women who had obesity. The protective association of alcohol use on the risk of T2DM in women with overweight and obesity stopped being significantly different from one at 33 g per day (RR 0.65; 95% CI 0.40–1.05) and 28 g per day (RR 0.73; 95% CI 0.53–1.01), respectively. Only one study reported the risk estimates by SES (28), so there were insufficient data to consider this potential modifier in our analysis. Nonetheless, their results suggest that the protective effect of moderate alcohol consumption was confined to individuals with low occupational positions after adjusting for psychosocial factors (i.e., low job control, defined by freedom to decide their work and possibility of development).
Figure 3.
Dose-response meta-analysis between alcohol intake in average grams per day and risk of type 2 diabetes by sex and BMI.
Statistically significant differences between men and women were identified (for model results, see Supplementary Table 8). There was a statistically significant interaction in studies based in the U.S. compared with studies based in other countries, with a more protective association identified. A statistically significant interaction in studies based in Asian countries, with a higher risk, was also identified. Age was found to have a significant inverse association, with a higher mean age in the sample presenting a lower risk. Low-quality studies presented a higher risk than moderate- and high-quality studies. These results were also observed in sex-stratified models.
In our first sensitivity analysis, when we restricted the analysis to studies that diagnosed T2DM with an objective measurement, a total of 19 and 11 studies, in addition to our secondary analysis, reported risk estimates for men and women, respectively (Supplementary File 9). The shape of the curve did not vary, with men presenting no significant increase or decrease in the risk and women showing a peak reduction of 28% (RR 0.72; 95% CI 0.62–0.84) in the risk of T2DM at 16 g of pure alcohol per day compared with lifetime abstention, based on a J-shaped dose-response relationship. In our second sensitivity analysis, we identified a total of 27 studies for men and 17 studies for women, in addition to our secondary analysis, which used the new T2DM diagnosis criteria (Supplementary File 10). In men, there was no significant increase or decrease in the risk. In women, a J-shaped dose-response was identified. A significant peak reduction was found at 16 g per day (RR 0.71, 95% CI 0.64–0.78). Our results from the main models were confirmed using the alternative one-stage dose-response meta-analysis (Supplementary File 11). Finally, the direction and shape of the sex-specific curves were similar to those for our main results when we excluded studies that included mortality cases (Supplementary File 12).
Conclusions
Our study suggests that low and moderate alcohol use (below 50 g per day) is associated with a reduced risk of T2DM for women, while no statistically significant dose-response relationship was found for men. Moreover, the protective association seems to be specific to women with BMI ≥25 kg/m2, suggesting that the potential protective effect of alcohol on T2DM risk is dependent on both the level of consumption and the individual’s sex and BMI. Despite the potential impact of SES as an effect modifier, we were only able to identify one study that analyzed this factor.
The results are in line with the previous meta-analysis published by Knott et al. (7), which also identified a protective effect of low and moderate alcohol use for women. In contrast, Li et al. (6) found a lower risk of T2DM with low and moderate alcohol use in both sexes. Compared with these two most recently published meta-analyses, our systematic review was able to identify 20 additional studies not included by Knott et al. (7) and 34 additional studies not included by Li et al. (6). Therefore, our study is the most comprehensive, high-quality review on the relationship between alcohol use and T2DM currently available. We conducted a comprehensive assessment of the included studies to ensure conceptual homogeneity. All studies were cohort studies, and our outcome of interest was consistently defined and measured across them. A random effect model, a risk-of-bias assessment, and a series of sensitivity analyses were performed to address heterogeneity among the results. By using lifetime abstention as the reference group, our analysis accounts for the sick quitter effect (20), which could overestimate the protective association of moderate drinking and underestimate the risk for higher levels of alcohol use. Finally, our study is the first to build dose-response relationships stratified by BMI. These results were based on a limited number of studies reporting sex- and BMI-specific risk estimates.
The reduced risk for low to moderate alcohol consumption identified in women may be partially attributed to the increased insulin sensitivity associated with alcohol use (29,30). However, the literature on this topic is inconsistent, and the underlying mechanism is not entirely clear. Schrieks et al. (31) found that moderate alcohol consumption (less than 40 g per day) decreased fasting insulin concentrations and HbA1c levels (an indicator of blood glucose levels). The authors also suggested that the effects of moderate alcohol consumption on insulin sensitivity are specific to women. Moderate alcohol consumption has been associated with modest increases in HDL cholesterol levels (14), and studies have found that it might enhance the lipid profile and raise plasma levels of adiponectin in postmenopausal women, potentially contributing to the improvement of glycemic and inflammatory markers (32).
The specific protective association observed in individuals who are in the range of overweight and obesity seems biologically plausible, because obesity-induced insulin resistance might be attenuated by the enhanced insulin sensitivity produced by moderate alcohol use, as observed in animal models (33). Additionally, the protective association in women found in our study may also be explained by the sex differences in the risk relationship between alcohol consumption and BMI (12). An inverse association between alcohol consumption and BMI in women was seen (11,12), but a null or positive association in men has been found (9,10). Two possible explanations have been proposed for such findings (12). First, from the perspective of energy intake, male drinkers tended to add alcohol to their dietary intake, while female drinkers tended to substitute alcohol for other energy sources (particularly carbohydrates) without increasing their total energy intake. Second, differences in alcohol metabolism have also been observed in previous studies, with energy expenditure substantially increased in women yet only moderately changed in men after drinking alcohol (34,35). As a result, alcohol consumption would lead to an increase of energy balance in men yet a decrease in energy balance in women, which may be the reason for an inverse association between alcohol consumption and BMI in women only (12). However, our results showed that alcohol was not protective in women with healthy weight, but it was protective in women who already had an increased risk for T2DM because of their weight. Thus, concluding that alcohol use per se has a protective association in terms of T2DM development in women could be misleading. Instead, supporting the population to maintain a healthy weight should remain a priority preventive measure.
This study has several limitations. First, our analysis relies on aggregate data, which is based on various statistical models, distinct historical periods, and heterogeneous populations from the underlying published studies. Therefore, our study does not include individual participant data, and as a result, the sex- and BMI-specific analyses rely on the available studies that provided such disaggregated information. Second, we included articles that ascertained their cases via self-report. Nevertheless, we observed only a minimal variation when we selected studies that used only objective measurement to identify T2DM cases. Third, alcohol consumption was measured based on self-report, which may be subjective and may underestimate the true alcohol use, potentially leading to biases (36). However, self-reported measures have been shown to be valid overall (37). Fourth, almost all of our sampled studies did not account for temporal variations in alcohol use. Relying on a single assessment may introduce exposure misclassification and may not fully capture the dynamic nature of alcohol consumption and its impact on T2DM. Additionally, our analysis focused on average daily alcohol consumption, and we were not able to account for the potential impact of heavy episodic drinking. To our knowledge, there is not enough epidemiological research available to incorporate this question into our dose-response analyses.
Despite these limitations, our results allow for a more precise and accurate prediction of sex-specific risk curves in the relationship between alcohol use and T2DM. Our findings underscore the importance of considering both sex and BMI when examining the association between alcohol use and the risk of T2DM. This review highlights the complexity of individual risk interactions and emphasizes the importance of an approach based on the structural determinants of health. The implication that alcohol and BMI are both involved in the development of T2DM should place emphasis on improving the social conditions that contribute to the related unhealthy behaviors, such as heavy alcohol consumption, poor diet, and lack of physical activity. While low to moderate alcohol use may be associated with beneficial outcomes in specific populations, and our study identified a reduced T2DM risk in women, our results should not be interpreted as a call to encourage alcohol consumption at any level. The potential benefits of alcohol on insulin sensitivity must be weighed against the potential risks. The harm caused by alcohol use is proportional to the level of consumption, and it is crucial to emphasize that there is no safe level of consumption (38,39). In light of alcohol’s detrimental effects, it is even more important to understand why alcohol can lower the risk for some diseases and whether these protective effects are specific to certain populations. We encourage future comparative risk assessments, such as the Global Burden of Disease Study, to use our risk relationships, as our updated results differ from their model assumptions, where a negative effect for men at high levels of alcohol use is currently used (40). Finally, future research should focus on investigating the potential effects and interactions of SES on this association, given the research gap identified on this topic and its potential role as an effect modifier in the complex relationship between alcohol use and T2DM.
This article contains supplementary material online at https://doi.org/10.2337/figshare.24058689.
Article Information
Acknowledgments. We thank Astrid Otto for referencing and copyediting the text.
Funding. Research reported in this publication was supported by the National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health under award number R01AA028009.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. Conceptualization, L.L.-F. and C.P.; methodology, L.L.-F, J.R., and C.P.; data curation, L.L.-F., T.C., and C.K.; formal analysis, L.L.-F., T.C., C.K., and C.P.; funding acquisition, C.P.; investigation, L.L.-F., S.B., C.B., T.C., C.K., A.M.L., J.M.L., Y.Z., and C.P.; project administration, C.P.; resources, J.R. and C.P.; software, L.L.-F. and C.P.; supervision, J.R. and C.P.; validation, L.L.-F. and C.P.; visualization, L.L.-F.; writing—original draft, L.L.-F., T.C., C.K., and C.P.; and writing—review and editing, all authors. All authors have read and agreed to the published version of the manuscript. L.L.-F. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding Statement
Research reported in this publication was supported by the National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health under award number R01AA028009.
Footnotes
Systematic review and meta-analysis protocol reg. no. CRD42022340247, PROSPERO https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=340247
References
- 1. International Diabetes Federation . IDF Diabetes Atlas, 10th edition. Brussels, Belgium, International Diabetes Federation, 2021. Accessed 8 November 2022. Available from https://www.diabetesatlas.org [Google Scholar]
- 2. Institute for Health Metrics and Evaluation (IHME) . GBD Results, 2022. Accessed 29 July 2022. Available from https://vizhub.healthdata.org/gbd-results/
- 3. Khan MAB, Hashim MJ, King JK, Govender RD, Mustafa H, Al Kaabi J. Epidemiology of type 2 diabetes—global burden of disease and forecasted trends. J Epidemiol Glob Health 2020;10:107–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tinajero MG, Malik VS. An update on the epidemiology of type 2 diabetes: a global perspective. Endocrinol Metab Clin North Am 2021;50:337–355 [DOI] [PubMed] [Google Scholar]
- 5. Baliunas DO, Taylor BJ, Irving H, et al. Alcohol as a risk factor for type 2 diabetes: a systematic review and meta-analysis. Diabetes Care 2009;32:2123–2132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li XH, Yu FF, Zhou YH, He J. Association between alcohol consumption and the risk of incident type 2 diabetes: a systematic review and dose-response meta-analysis. Am J Clin Nutr 2016;103:818–829 [DOI] [PubMed] [Google Scholar]
- 7. Knott C, Bell S, Britton A. Alcohol consumption and the risk of type 2 diabetes: a systematic review and dose-response meta-analysis of more than 1.9 million individuals from 38 observational studies. Diabetes Care 2015;38:1804–1812 [DOI] [PubMed] [Google Scholar]
- 8. Tajik S, Mirzababaei A, Ghaedi E, Kord-Varkaneh H, Mirzaei K. Risk of type 2 diabetes in metabolically healthy people in different categories of body mass index: an updated network meta-analysis of prospective cohort studies. J Cardiovasc Thorac Res 2019;11:254–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Colditz GA, Giovannucci E, Rimm EB, et al. Alcohol intake in relation to diet and obesity in women and men. Am J Clin Nutr 1991;54:49–55 [DOI] [PubMed] [Google Scholar]
- 10. Lahti-Koski M, Pietinen P, Heliövaara M, Vartiainen E. Associations of body mass index and obesity with physical activity, food choices, alcohol intake, and smoking in the 1982-1997 FINRISK studies. Am J Clin Nutr 2002;75:809–817 [DOI] [PubMed] [Google Scholar]
- 11. Ruf T, Nagel G, Altenburg HP, Miller AB, Thorand B. Food and nutrient intake, anthropometric measurements and smoking according to alcohol consumption in the EPIC Heidelberg study. Ann Nutr Metab 2005;49:16–25 [DOI] [PubMed] [Google Scholar]
- 12. Wang L, Lee IM, Manson JE, Buring JE, Sesso HD. Alcohol consumption, weight gain, and risk of becoming overweight in middle-aged and older women. Arch Intern Med 2010;170:453–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Beulens JW, van der Schouw YT, Bergmann MM, et al.; InterAct Consortium . Alcohol consumption and risk of type 2 diabetes in European men and women: influence of beverage type and body size The EPIC-InterAct study. J Intern Med 2012;272:358–370 [DOI] [PubMed] [Google Scholar]
- 14. Beulens JW, van der Schouw YT, Moons KG, Boshuizen HC, van der A DL, Groenwold RH. Estimating the mediating effect of different biomarkers on the relation of alcohol consumption with the risk of type 2 diabetes. Ann Epidemiol 2013;23:193–197 [DOI] [PubMed] [Google Scholar]
- 15. Agardh E, Allebeck P, Hallqvist J, Moradi T, Sidorchuk A. Type 2 diabetes incidence and socio-economic position: a systematic review and meta-analysis. Int J Epidemiol 2011;40:804–818 [DOI] [PubMed] [Google Scholar]
- 16. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. American Diabetes Association Professional Practice Committee . 2. Classification and diagnosis of diabetes: Standards of Care in Diabetes—2022. Diabetes Care 2022;45(Suppl. 1):S17–S38 [DOI] [PubMed] [Google Scholar]
- 18. World Health Organization . International Guide for Monitoring Alcohol Consumption and Related Harm. Geneva, Switzerland, World Health Organization, 2000 [Google Scholar]
- 19. Wells GA, Wells G, Shea B, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottowa, Canada, The Ottowa Hospital, 2021. Accessed 25 October 2022. Available from https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [Google Scholar]
- 20. Shaper AG, Wannamethee G, Walker M. Alcohol and mortality in British men: explaining the U-shaped curve. Lancet 1988;2:1267–1273 [DOI] [PubMed] [Google Scholar]
- 21. Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw 2010;36:1–48 [Google Scholar]
- 22. Harrell FE. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. New York, Springer-Verlag, 2010 [Google Scholar]
- 23. Kanazawa M, Yoshiike N, Osaka T, Numba Y, Zimmet P, Inoue S. Criteria and classification of obesity in Japan and Asia-Oceania. World Rev Nutr Diet 2005;94:1–12 [DOI] [PubMed] [Google Scholar]
- 24. The Expert Committee on the Diagnosis Classification of Diabetes Mellitus . Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1997;20:1183–1197 [DOI] [PubMed] [Google Scholar]
- 25. Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 1998;15:539–553 [DOI] [PubMed] [Google Scholar]
- 26. Crippa A, Orsini N. Multivariate dose-response meta-analysis: the dosresmeta R package. Journal of Statistical Software 2016;72:1–15 [Google Scholar]
- 27. Harrer M, Cuijpers P, Furukawa TA, Ebert DD. Doing Meta-analysis With R: A Hands-On Guide. New York, Chapman and Hall/CRC, 2021 [Google Scholar]
- 28. Agardh EE, Lundin A, Lager A, et al. Alcohol and type 2 diabetes: the role of socioeconomic, lifestyle and psychosocial factors. Scand J Public Health 2019;47:408–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Facchini F, Chen YD, Reaven GM. Light-to-moderate alcohol intake is associated with enhanced insulin sensitivity. Diabetes Care 1994;17:115–119 [DOI] [PubMed] [Google Scholar]
- 30. Mayer EJ, Newman B, Quesenberry CP Jr, Friedman GD, Selby JV. Alcohol consumption and insulin concentrations. Role of insulin in associations of alcohol intake with high-density lipoprotein cholesterol and triglycerides. Circulation 1993;88:2190–2197 [DOI] [PubMed] [Google Scholar]
- 31. Schrieks IC, Heil AL, Hendriks HF, Mukamal KJ, Beulens JW. The effect of alcohol consumption on insulin sensitivity and glycemic status: a systematic review and meta-analysis of intervention studies. Diabetes Care 2015;38:723–732 [DOI] [PubMed] [Google Scholar]
- 32. Joosten MM, Beulens JWJ, Kersten S, Hendriks HFJ. Moderate alcohol consumption increases insulin sensitivity and ADIPOQ expression in postmenopausal women: a randomised, crossover trial. Diabetologia 2008;51:1375–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Paulson QX, Hong J, Holcomb VB, Nunez NP. Effects of body weight and alcohol consumption on insulin sensitivity. Nutr J 2010;9:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Frezza M, di Padova C, Pozzato G, Terpin M, Baraona E, Lieber CS. High blood alcohol levels in women. The role of decreased gastric alcohol dehydrogenase activity and first-pass metabolism. N Engl J Med 1990;322:95–99 [DOI] [PubMed] [Google Scholar]
- 35. Suter PM, Schutz Y, Jequier E. The effect of ethanol on fat storage in healthy subjects. N Engl J Med 1992;326:983–987 [DOI] [PubMed] [Google Scholar]
- 36. Stockwell T, Zhao J, Sherk A, Rehm J, Shield K, Naimi T. Underestimation of alcohol consumption in cohort studies and implications for alcohol’s contribution to the global burden of disease. Addiction 2018;113:2245–2249 [DOI] [PubMed] [Google Scholar]
- 37. Midanik LT. Validity of self-reported alcohol use: a literature review and assessment. Br J Addict 1988;83:1019–1030 [DOI] [PubMed] [Google Scholar]
- 38. Burton R, Sheron N. No level of alcohol consumption improves health. Lancet 2018;392:987–988 [DOI] [PubMed] [Google Scholar]
- 39. Rehm J, Gmel GE Sr, Gmel G, et al. The relationship between different dimensions of alcohol use and the burden of disease-an update. Addiction 2017;112:968–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Global Burden of Disease Collaborative Network . Global Burden of Disease Study 2019 (GBD 2019) Relative Risks, 2020. Accessed 24 April 2023. Available from https://ghdx.healthdata.org/record/ihme-data/gbd-2019-relative-risks
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contribution presented in the study are included in the article and supplementary material. Further inquiries can be directed to the corresponding author.