Abstract
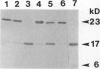
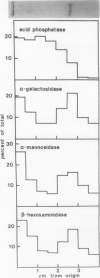
During seed development, various storage proteins and hydrolases accumulate in specialized storage vacuoles, the protein bodies, via an elaborate intracellular transport system involving the rough endoplasmic reticulum, the Golgi apparatus, and transit vesicles. Clathrin-coated vesicles, similar to those which transport lysosomal proteins to lysosomes, an organelle analogous to the vacuole, in animal cells, could be involved in this intracellular transport mechanism. Clathrin-coated vesicles have been isolated from cotyledons of developing pea (Pisum sativum L.) seeds at the time of rapid protein accumulation and analyzed for the presence of protein body constitutents. A 23,000 Mr polypeptide, corresponding to pea lectin precursor, was found associated with the vesicles, as determined by immunoblotting. The lectin precursor was apparently sequestered within the vesicles, as the polypeptide was only susceptible to proteolysis if detergents were included in the digestion buffer. A number of glycosidase activities, including α-mannosidase, α-galactosidase, and β-N-acetylhexosaminidase, were also associated with the vesicles. Thus, it appears that clathrin-coated vesicles are involved in the intracellular transport of storage proteins during seed development.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altstiel L., Branton D. Fusion of coated vesicles with lysosomes: measurement with a fluorescence assay. Cell. 1983 Mar;32(3):921–929. doi: 10.1016/0092-8674(83)90077-6. [DOI] [PubMed] [Google Scholar]
- Beevers L., Poulson R. Protein Synthesis in Cotyledons of Pisum sativum L: I. Changes in Cell-Free Amino Acid Incorporation Capacity during Seed Development and Maturation. Plant Physiol. 1972 Apr;49(4):476–481. doi: 10.1104/pp.49.4.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Campbell C. H., Fine R. E., Squicciarini J., Rome L. H. Coated vesicles from rat liver and calf brain contain cryptic mannose 6-phosphate receptors. J Biol Chem. 1983 Feb 25;258(4):2628–2633. [PubMed] [Google Scholar]
- Campbell C. H., Rome L. H. Coated vesicles from rat liver and calf brain contain lysosomal enzymes bound to mannose 6-phosphate receptors. J Biol Chem. 1983 Nov 10;258(21):13347–13352. [PubMed] [Google Scholar]
- Harley S. M., Beevers L. Isozymes of beta-N-Acetylhexosaminidase from Pea Seeds (Pisum sativum L.). Plant Physiol. 1987 Dec;85(4):1118–1122. doi: 10.1104/pp.85.4.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman E. M., Shannon L. M. Accumulation and Subcellular Localization of alpha-Galactosidase-Hemagglutinin in Developing Soybean Cotyledons. Plant Physiol. 1985 Apr;77(4):886–890. doi: 10.1104/pp.77.4.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins T. J., Chrispeels M. J., Chandler P. M., Spencer D. Intracellular sites of synthesis and processing of lectin in developing pea cotyledons. J Biol Chem. 1983 Aug 10;258(15):9550–9552. [PubMed] [Google Scholar]
- Hodges T. K., Leonard R. T. Purification of a plasma membrane-bound adenosine triphosphatase from plant roots. Methods Enzymol. 1974;32:392–406. doi: 10.1016/0076-6879(74)32039-3. [DOI] [PubMed] [Google Scholar]
- Keen J. H. Clathrin assembly proteins: affinity purification and a model for coat assembly. J Cell Biol. 1987 Nov;105(5):1989–1998. doi: 10.1083/jcb.105.5.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen J. H., Willingham M. C., Pastan I. H. Clathrin-coated vesicles: isolation, dissociation and factor-dependent reassociation of clathrin baskets. Cell. 1979 Feb;16(2):303–312. doi: 10.1016/0092-8674(79)90007-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Marquardt T., Braulke T., Hasilik A., von Figura K. Association of the precursor of cathepsin D with coated membranes. Kinetics and carbohydrate processing. Eur J Biochem. 1987 Oct 1;168(1):37–42. doi: 10.1111/j.1432-1033.1987.tb13383.x. [DOI] [PubMed] [Google Scholar]
- Nagahashi J., Beevers L. Subcellular Localization of Glycosyl Transferases Involved in Glycoprotein Biosynthesis in the Cotyledons of Pisum sativum L. Plant Physiol. 1978 Mar;61(3):451–459. doi: 10.1104/pp.61.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. A simplified method for analysis of inorganic phosphate in the presence of interfering substances. Anal Biochem. 1978 Jan;84(1):164–172. doi: 10.1016/0003-2697(78)90495-5. [DOI] [PubMed] [Google Scholar]
- Robinson M. S. Coated vesicles and protein sorting. J Cell Sci. 1987 Mar;87(Pt 2):203–204. doi: 10.1242/jcs.87.2.203. [DOI] [PubMed] [Google Scholar]
- Rubenstein J. L., Fine R. E., Luskey B. D., Rothman J. E. Purification of coated vesicles by agarose gel electrophoresis. J Cell Biol. 1981 May;89(2):357–361. doi: 10.1083/jcb.89.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]