Abstract
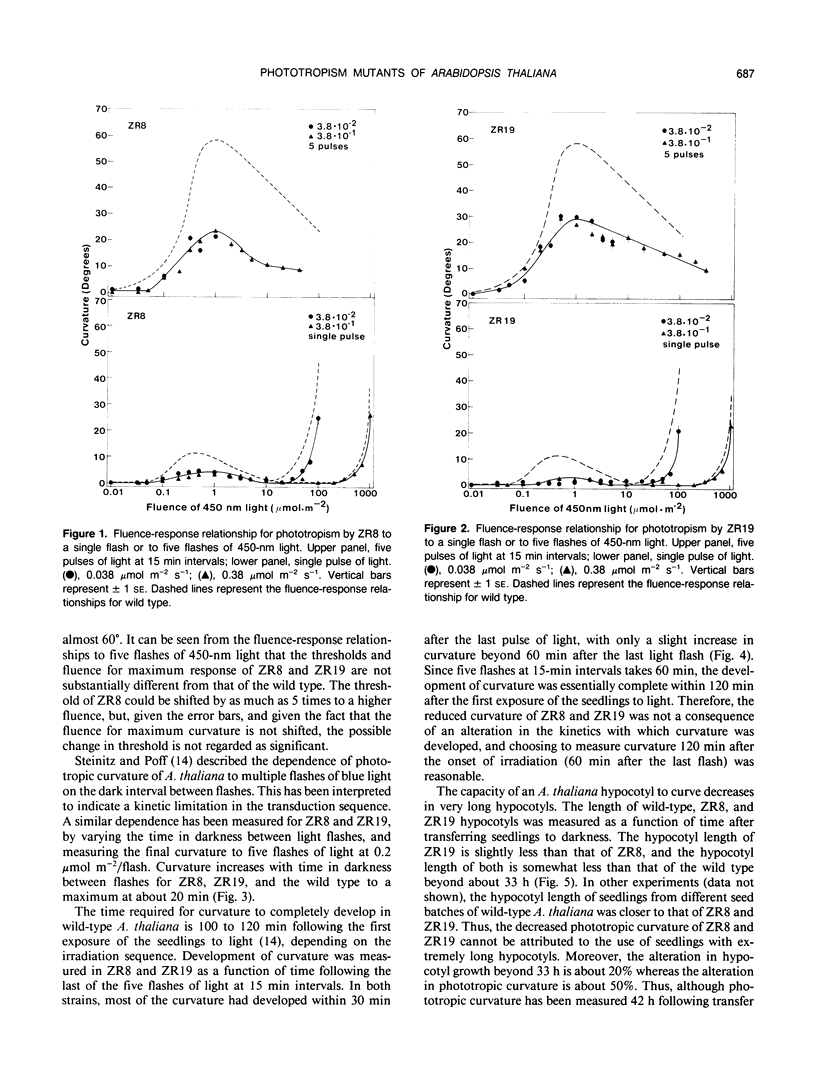
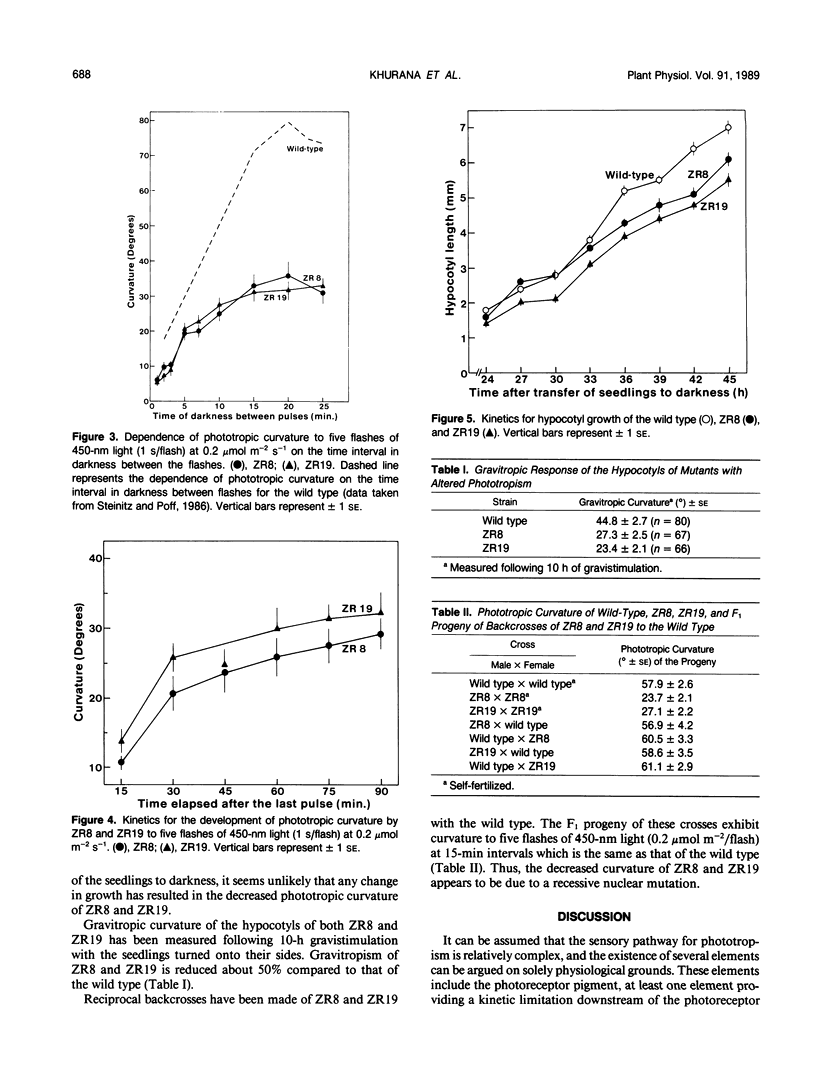
Two mutants of Arabidopsis thaliana have been identified with decreased phototropism to 450-nanometer light. Fluence-response relationships for these strains (ZR8 and ZR19) to single and multiple flashes of light show thresholds, curve shapes, and fluence for maximum curvature in `first positive' phototropism which are the same as those of the wild type. Similarly, there is no alteration from the wild type in the kinetics of curvature or in the optimum dark period separating sequential flashes in a multiple flash regimen. In addition, in both strains, gravitropism is decreased compared to the wild type by an amount which is comparable to the decrease in phototropism. Based on reciprocal backcrosses, it appears that the alteration is due to a recessive nuclear mutation. It is suggested that ZR8 and ZR19 represent alterations in some step analogous to an amplifier, downstream of the photoreceptor pigment, and common to both phototropism and gravitropism.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brain R. D., Freeberg J. A., Weiss C. V., Briggs W. R. Blue light-induced Absorbance Changes in Membrane Fractions from Corn and Neurospora. Plant Physiol. 1977 May;59(5):948–952. doi: 10.1104/pp.59.5.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke P. V., Bullen B. L., Poff K. L. Frequency distribution histograms for the rapid analysis of data. Plant Physiol. 1988;87:797–798. doi: 10.1104/pp.87.4.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galland P., Lipson E. D. Action spectra for phototropic balance in Phycomyces blakesleeanus: dependence on reference wavelength and intensity range. Photochem Photobiol. 1985 Mar;41(3):323–329. doi: 10.1111/j.1751-1097.1985.tb03492.x. [DOI] [PubMed] [Google Scholar]
- Galland P., Lipson E. D. Blue-light reception in Phycomyces phototropism: evidence for two photosystems operating in low- and high-intensity ranges. Proc Natl Acad Sci U S A. 1987 Jan;84(1):104–108. doi: 10.1073/pnas.84.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galland P., Lipson E. D. Modified action spectra of photogeotropic equilibrium in Phycomyces blakesleeanus mutants with defects in genes madA, madB, madC, and madH. Photochem Photobiol. 1985 Mar;41(3):331–335. doi: 10.1111/j.1751-1097.1985.tb03493.x. [DOI] [PubMed] [Google Scholar]
- Muñoz V., Butler W. L. Photoreceptor Pigment for Blue Light in Neurospora crassa. Plant Physiol. 1975 Feb;55(2):421–426. doi: 10.1104/pp.55.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poff K. L., Butler W. L. Absorbance changes induced by blue light in Phycomyces blakesleeanus and Dictyostelium discoideum. Nature. 1974 Apr 26;248(5451):799–801. doi: 10.1038/248799a0. [DOI] [PubMed] [Google Scholar]
- Springer M. S., Goy M. F., Adler J. Sensory transduction in Escherichia coli: two complementary pathways of information processing that involve methylated proteins. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3312–3316. doi: 10.1073/pnas.74.8.3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinitz B., Ren Z., Poff K. L. Blue and Green Light-Induced Phototropism in Arabidopsis thaliana and Lactuca sativa L. Seedlings. Plant Physiol. 1985 Jan;77(1):248–251. doi: 10.1104/pp.77.1.248. [DOI] [PMC free article] [PubMed] [Google Scholar]



