Venetoclax (Ven) in combination with hypomethylating agents (HMA) is Food and Drug Administration-approved for elderly/unfit acute myeloid leukemia (AML) patients. In the phase III VIALE-A study, complete remission (CR) with or without count recovery (CRi) was achieved in 66.4% of previously untreated patients with AML receiving Ven and azacitidine. CR/CRi was superior in the presence of IDH1/2, NPM1, FLT3, and TP53 mutations in the Ven and azacitidine arm compared to treatment with azacitidine alone.1 However, disease progression or relapse was documented in 42% of patients on Ven and azacitidine therapy.1 Similarly, in a separate study of 95 newly diagnosed AML patients treated on frontline Ven+HMA clinical trials, 41 (43%) of patients experienced relapsed or refractory disease; median overall survival after Ven+HMA failure was considerably inferior at 2.4 months, particularly in patients that did not receive salvage therapy (1.3 months).2 On the other hand, outcomes of AML patients following failure of upfront Ven+HMA therapy outside the context of clinical trials have not been well-studied. Accordingly, in the current study, our primary objective was to describe the clinical outcomes of patients with AML following failure of frontline Ven+HMA therapy in routine clinical practice and identify clinical and molecular predictors of survival after Ven+HMA failure.
Patients with newly diagnosed AML treated with frontline Ven+HMA outside clinical trials at the Mayo Clinic between 2018 and 2020 were retrospectively recruited after Institutional Review Board approval. Follow-up was updated in November 2022. All patients received at least one cycle of either azacitidine 75 mg/m2 days 1-7 or decitabine 20 mg/m2 days 1-5 with Ven dose-adjusted based on azole antifungal prophylaxis.3 Bone marrow biopsy was obtained after either cycle 1 or 2 based on treating physician discretion with response assessed according to the 2017 European Leukemia Net (ELN) criteria.4 Treatment failure was defined as inability to achieve CR/CRi (refractory) or loss of CR/CRi (relapsed). Cytogenetic and molecular studies were performed at the time of AML diagnosis by conventional karyotype, and next-generation sequencing (42-gene panel), respectively in all patients, while a subset of patients underwent molecular testing at the time of relapsed/refractory disease. Survival was calculated from the time of treatment initiation and from onset of relapsed or refractory disease to last follow-up or death and survival curves prepared by the Kaplan-Meier method and compared by the log-rank test. Cox proportional hazard model was used for multivariable analysis. JMP Pro 16.0.0 software package, SAS Institute, Cary, NC was used for statistical analysis.
Outcomes from initial treatment with venetoclax in combination with hypomethylating agents
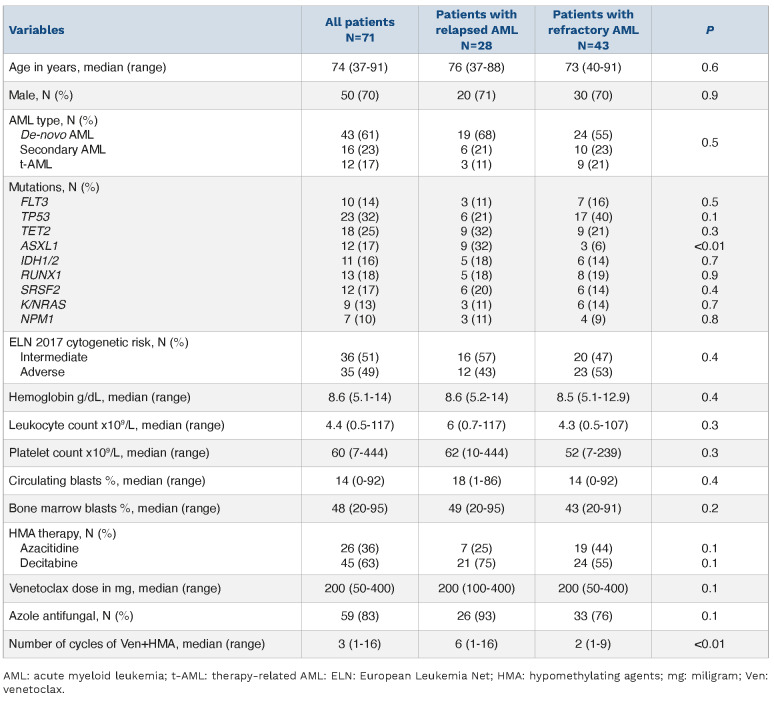
Seventy-one of 103 (69%) patients with treatment-naïve AML (median age 74 years; range, 37-91; 70% males; 61% de novo, 23% secondary, 17% therapy-related) were either refractory (n=43, 61%) or relapsed (n=28, 39%) following Ven+HMA therapy. Initial treatment consisted of decitabine in 45 of 71 (63%) patients and the remainder received azacitidine with median Ven dose of 200 mg (range, 50-400 mg) for a median of three cycles (range, 1-16 cycles). In addition, 59 (83%) of patients received concomitant azole antifungal prophylaxis. Relapsed and refractory patients received a median of six cycles (range, 1-16 cycles) and two cycles (range, 1-19 cycles) of Ven+HMA, respectively. Median duration of CR (n=15) or CRi (n=13) was 5 months (range, 1-22 months); moreover, three of eight evaluable patients were measurable residual disease (MRD)-positive by multi-parametric flow cytometry testing. Treatment-related toxicities were noted in 51 (72%) of patients which included prolonged cytopenias (n=26), infections (n=20), major hemorrhage (n=3), and tumor lysis syndrome (n=1). Table 1 provides detailed clinical characteristics at time of initiation of Ven+HMA for AML patients that were relapsed or refractory to frontline Ven+HMA. ELN 2017 cytogenetic risk included intermediate (51%, n=36) or adverse (49%, n=35). Mutations involved TP53 in 23 patients (32%), ASXL1 in 12 (17%), IDH1/2 in 11 (16%), FLT3 in ten (14%), N/KRAS in nine (13%) (NRAS, n=5) and NPM1 in seven (10%). All TP53 mutations except one were classified as multi-hit based on the 2022 International Consensus classification5, with median variant allele frequency of 44.5% (range, 6-96%). A comparison of clinical characteristics of refractory versus relapsed patients revealed similarities in age (median age 73 vs. 76 years; P=0.6), secondary/therapy-related AML (44% vs. 32%; P=0.5), ELN adverse cytogenetics (53% vs. 43%; P=0.4), FLT3 (16% vs. 11%; P=0.5), NPM1 (9% vs. 11%; P=0.8), IDH1/2 (14% vs. 18%; P=0.7), and N/KRAS mutations (14% vs. 11%; P=0.7). On the other hand, patients that were refractory to Ven+HMA were more likely to harbor TP53 mutations (40% vs. 21%; P=0.1) while ASXL1 mutations were predominant in relapsed patients (32% vs. 6%; P=0.01). At a median follow-up of 6 months (range, 1-40 months) from initial treatment with Ven+HMA, 67 (94%) deaths were recorded. Median overall survival was 5.9 months (95% confidence interval [CI]: 2.7-14.1), and was superior in patients that were relapsed versus those refractory to Ven+HMA (median overall survival 11.2 vs. 3.1 months; P<0.01).
Table 1.
Clinical characteristics at time of initiation of venetoclax plus hypomethylating agents including treatment details for 71 patients with acute myeloid leukemia relapsed or refractory to frontline therapy with venetoclax plus hypomethylating agents.
Online Supplementary Table S1 provides a description of 103 AML patients treated with frontline Ven+HMA including a comparison of clinical and laboratory characteristics of patients with or without relapsed/refractory disease following Ven+HMA. As expected, patients relapsed/refractory to Ven+HMA compared to responders were more likely to harbor adverse cytogenetics (49% vs. 34%; P=0.15), TP53 (32% vs. 6%; P=0.002) and FLT3-internal tandem duplications (ITD) (14% vs. 0%; P=0.005) mutations.
Outcomes from the time of relapsed or refractory disease
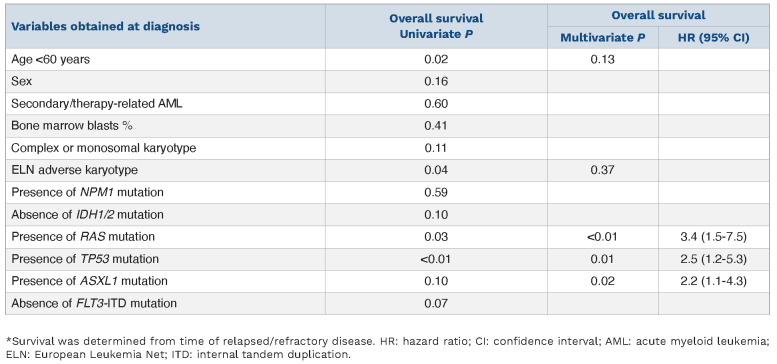
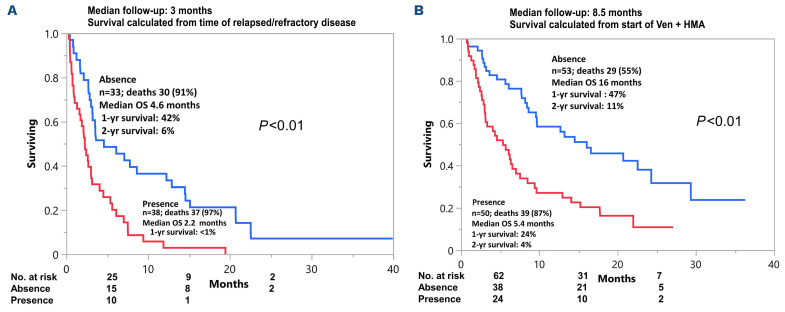
Molecular testing at the time of relapsed/refractory disease was obtained in a subset of patients (n=18) which revealed persistence of TP53 in all six (100%) TP53-mutated patients and one of two (50%) NRAS-mutated patients that were tested. New emergent clones in the remainder of ten patients tested included mutations in NRAS, CEPBA and BCOR in one patient each. Salvage therapy was pursued in 11 of 71 (15%) patients with gilteritinib (n=6), ivosidenib (n=2), Ven+gilteritinib (n=1), CPX-351 (n=1), and clinical trial (n=1) of which three patients (27%) achieved CR with one patient proceeding to allogeneic stem cell transplant. Notably, none of the patients received standard induction salvage chemotherapy which was not surprising given the median age of study patients was 74 years. On the other hand, in a recent single institution series of younger patients (median age 62 years) and predominantly ELN adverse risk disease (79%), 19 of 208 (9%) went on to receive intensive chemotherapy following failure of Ven+HMA.6 A total of 21 patients had FLT3-ITD (n=10) and IDH1/2 mutations (n=11), of which two patients were co-mutated for FLT3-ITD and IDH. Seven of ten FLT3-ITD-mutated patients received FLT3 inhibitors, three patients did not receive salvage therapy because of mortality from sepsis (n=2) and fungal pneumonia (n=1). On the other hand, two of 11 IDH1/2 mutated patients received IDH inhibitors, the remainder of patients passed away from sepsis (n=3), fungal pneumonia (n=1), intracranial hemorrhage (n=1) or transitioned to hospice due to poor performance status/medical comorbidities (n=4). However, it is to be noted that although survival was not significantly different in patients that received or did not receive targeted therapy (8.6 vs. 3.0 months; P=0.71), the observed difference was likely a reflection of the superior performance status/medical condition of patients that went on to receive salvage therapy. At a median follow up duration of 3 months from the time of relapsed or refractory disease, median overall survival was similar for relapsed versus refractory patients (median survival 3.1 vs. 2.8 months; P=0.82). In univariate survival analysis, age <60 years (2.5 vs. 3.2 months; P=0.02), presence of TP53 mutation (2.2 vs. 3.6 months; P=0.01), presence of K/NRAS mutation (0.7 vs. 3.2 months; P<0.01), presence of ASXL1 mutation (2.2 vs. 3.2 months; P=0.1), and ELN adverse cytogenetics (2.7 vs. 3.4 months; P=0.04) predicted inferior survival following Ven+HMA failure (Table 2). In subsequent multivariable analysis, presence of TP53 mutation (hazard ratio [HR]=3.1; 95% CI: 1.7-5.7; P<0.01), K/NRAS mutation (HR=3.2; 95% CI: 1.5-6.8; P<0.01) and ASXL1 mutation (HR=2.2; 95% CI: 1.1-4.3; P=0.03) retained significance. Accordingly, TP53/RAS/ASXL1 mutational status predicted survival with median survival of 4.6 months (1-year survival 42%) in the absence of all three mutations (n=33), versus 2.2 months (1-year survival <1%) in the presence of one or more mutations (n=38) (Figure 1A). Similar results were obtained when survival was assessed in 103 AML patients from time of initiation of frontline Ven+HMA with median survival of 16 months versus 5.4 months in the absence versus in the presence of TP53/RAS/ASXL1 mutations (P<0.01) (Figure 1B). The current study confirms the grim prognosis of AML patients that are relapsed/refractory to upfront Ven+HMA therapy. Moreover, salvage therapy was pursued in a minority (15%) of patients and demonstrated limited ability to induce CR with one patient proceeding to allogeneic stem cell transplant. The Mayo clinic cohort comprises AML patients treated outside the context of clinical trials and was enriched with secondary/therapy-related AML (40%) and adverse cytogenetics (49%). The corresponding figures for secondary AML and adverse cytogenetics in the VIALE-A study were lower at 25% and 36%, respectively.1 These differences explain the shorter CR duration and inferior median overall survival observed in our study in comparison to VIALE-A study.1,7
Table 2.
Predictors of inferior survival* following failure of venetoclax plus hypomethylating agents in 71 patients with acute myeloid leukemia relapsed or refractory to frontline therapy with venetoclax plus hypomethylating agents.
Figure 1.
Overall survival of patients with acute myleoid leukemia (AML) following frontline venetoclax plus hypomethylating agent stratified by presence/absence of TP53, K/NRAS, ASXL1 mutations. (A) Overall survival of 71 patients with acute myleoid leukemia (AML), relapsed/refractory following frontline venetoclax (Ven) plus hypomethylating agents (HMA) stratified by presence/absence of TP53, K/NRAS, ASXL1 mutations. (B) Overall survival of 103 patients with AML treated with frontline Ven plus HMA stratified by presence/absence of TP53, K/NRAS, ASXL1 mutations.
Our findings differ from an MD Anderson study which included 41 clinical trial patients, in which 24 of 41 (59%) of patients with AML that were relapsed/refractory disease following upfront Ven+HMA therapy received salvage therapy.2 However, akin to our study, only 21% of patients responded to salvage therapy and one patient underwent allogeneic stem cell transplant.2 Previous studies on the impact of mutations on response and survival in treatment-naïve and relapsed/refractory AML patients treated with Ven+HMA did not provide detailed information on survival following treatment failure. In our prior report on Ven+HMA-treated newly diagnosed AML patients, the presence of ASXL1 mutations and absence of FLT-ITD and TP53 mutations were associated with superior response; on the other hand, the presence of ASXL1 mutations, adverse karyotype and absence of CR/CRi predicted inferior survival.7 In contrast, in clinical trial patients with AML receiving frontline Ven+HMA or low-dose cytarabine, durable remissions and prolonged survival were reported with NPM1 and IDH2 mutations while TP53 and FLT3-ITD mutations were associated with adaptive resistance.8 Similarly, in relapsed/refractory AML patients treated with Ven combination therapy, responses were superior with NPM1 mutation while survival was shortened with TP53, K/NRAS and SF3B1 mutations.9 In addition, in a Mayo Clinic study of relapsed/refractory AML patients treated with Ven+HMA, the presence of ASXL1 mutation and absence of adverse karyotype predicted superior response, while survival was negatively impacted by the presence of TP53 mutations and absence of IDH1/2 mutations.10 In an exploratory analysis of AML patients treated on Ven+azacitidine clinical trials, stratified according to ELN 2017 risk, adverse risk patients with TP53 mutation had distinctly poor outcome with median overall survival of 5.42 months.11 The current study unveils the prognostic impact of TP53, RAS and ASXL1 mutations on survival in the setting of Ven+HMA failure in treatment-naïve patients with AML. Our observations provide a practically relevant survival prediction model based on the presence versus absence of TP53, RAS and ASXL1 mutations (1-year survival <1% vs. 42% in their presence vs. absence) which should be incorporated in patient counseling. However, whether these findings are specific to Ven+HMA therapy remains to be determined. Taken together, the current study underscores the prognostic relevance of TP53, RAS and ASXL1 mutations in treatment-naïve AML patients following failure of frontline Ven+HMA therapy.
Supplementary Material
References
- 1.DiNardo CD, Jonas BA, Pullarkat V, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020;383(7):617-629. [DOI] [PubMed] [Google Scholar]
- 2.Maiti A, Rausch CR, Cortes JE, et al. Outcomes of relapsed or refractory acute myeloid leukemia after frontline hypomethylating agent and venetoclax regimens. Haematologica. 2021;106(3):894-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agarwal SK, DiNardo CD, Potluri J, et al. Management of venetoclax-posaconazole interaction in acute myeloid leukemia patients: evaluation of dose adjustments. Clin Ther. 2017;39(2):359-367. [DOI] [PubMed] [Google Scholar]
- 4.Dohner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arber DA, Hasserjian RP, Orazi A, et al. Classification of myeloid neoplasms/acute leukemia: Global perspectives and the international consensus classification approach. Am J Hematol. 2022;97(5):514-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McMahon CM, Gil K, Amaya ML, et al. Response to intensive induction chemotherapy after failure of frontline azacitidine+venetoclax in acute myeloid leukemia. Blood. 2022;140(Suppl 1):S6185-6186. [DOI] [PubMed] [Google Scholar]
- 7.Gangat N, Johnson I, McCullough K, et al. Molecular predictors of response to venetoclax plus hypomethylating agent in treatment-naïve acute myeloid leukemia. Haematologica. 2022;107(10):2501-2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DiNardo CD, Tiong IS, Quaglieri A, et al. Molecular patterns of response and treatment failure after frontline venetoclax combinations in older patients with AML. Blood. 2020;135(11):791-803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stahl M, Menghrajani K, Derkach A, et al. Clinical and molecular predictors of response and survival following venetoclax therapy in relapsed/refractory AML. Blood Adv. 2021;5(5):1552-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McKerrow Johnson I, Ilyas R, McCullough K, et al. Molecular predictors of response and survival in patients with relapsed/refractory acute myeloid leukemia following venetoclax plus hypomethylating agent therapy. Blood. 2022;140(Suppl 1):S3233-3234. [Google Scholar]
- 11.Döhner H, Pratz KW, DiNardo CD, et al. ELN risk stratification is not predictive of outcomes for treatment-naïve patients with acute myeloid leukemia treated with venetoclax and azacitidine. Blood. 2022;140(Suppl 1):S1441-1444. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.