Abstract
Abnormal retention of mitochondria in mature red blood cells (RBC) has been recently reported in sickle cell anemia (SCA) but their functionality and their role in the pathophysiology of SCA remain unknown. The presence of mitochondria within RBC was determined by flow cytometry in 61 SCA patients and ten healthy donors. Patients were classified according to the percentage of mature RBC with mitochondria contained in the whole RBC population: low (0-4%), moderate (>4% and <8%), or high level (>8%). RBC rheological, hematological, senescence and oxidative stress markers were compared between the three groups. RBC senescence and oxidative stress markers were also compared between mature RBC containing mitochondria and those without. The functionality of residual mitochondria in sickle RBC was measured by high-resolution respirometry assay and showed detectable mitochondrial oxygen consumption in sickle mature RBC but not in healthy RBC. Increased levels of mitochondrial reactive oxygen species were observed in mature sickle RBC when incubated with Antimycin A versus without. In addition, mature RBC retaining mitochondria exhibited greater levels of reactive oxygen species compared to RBC without mitochondria, as well as greater Ca2+, lower CD47 and greater phosphatidylserine exposure. Hematocrit and RBC deformability were lower, and the propensity of RBC to sickle under deoxygenation was higher, in the SCA group with a high percentage of mitochondria retention in mature RBC. This study showed the presence of functional mitochondria in mature sickle RBC, which could favor RBC sickling and accelerate RBC senescence, leading to increased cellular fragility and hemolysis.
Introduction
Sickle cell anemia (SCA) is an inherited hemoglobinopathy caused by a single point mutation in the β-globin gene which leads to the synthesis of an abnormal hemoglobin, called hemoglobin S (HbS). When deoxygenated, HbS polymerizes causing a mechanical distortion (i.e., sickling) of red blood cells (RBC).1 Sickle RBC are less deformable and more fragile than normal RBC, resulting in chronic anemia2 and frequent vaso-occlusive crises.3 Moreover, the accumulation of free hemoglobin and heme in the plasma promotes oxidative stress, inflammation and endothelial dysfunction, which contribute to the pathophysiology of SCA and the development of chronic complications.4
Recent studies reported the presence of mitochondria in mature RBC in patients with SCA.5-7 Mitophagy is an important step for the survival of RBC during erythroid maturation.8 It has been demonstrated that the abnormal mitochondria retention in sickle RBC would be the result of a deficient mitophagy pathway throughout erythropoiesis.5 Jagadeeswaran et al.9 demonstrated that the use of the lysine-specific demethylase 1A (LSD1) inhibitor (RN-1), and the use of a mitophagy-inducing agent mammalian target of rapamycin (mTOR) inhibitor (sirolimus) increased RBC lifespan in a sickle cell mouse model. Of note, LSD1 inhibitor RN-1 could also improve RBC lifespan through its effects on HbF synthesis, as reported in non-human primates.10 In another study, it has been reported that SCA patients with a high percentage of mature RBC containing mitochondria exhibited higher levels of reticulocytes and total bilirubin, suggesting increased hemolysis in these patients compared to those with a low percentage of mature RBC with mitochondria.5 Indeed, although not formally proved, it has been hypothesized that the retention of mitochondria in mature RBC would favor hemolysis.9 It has been proposed that mitochondria retention in mature RBC could cause a shortening of their lifespan because of the accumulation of reactive oxygen species (ROS) generated by metabolically active mitochondria11 that would damage the cell.12 Promoting mitophagy in sickle cell mice was accompanied by a decrease in RBC ROS content.9 However, Martino et al.5 did not show any difference in the mitochondrial membrane potential using Mitrotracker orange between patients with mature RBC containing mitochondria compared to those without. Moreover, transmission electron microscopy experiments revealed that mitochondria in mature RBC were swollen, small and disorganized.5 It was concluded that mitochondria in mature RBC in SCA patients are not functional. However, another recent study supports the opposite.6 Proteomic, metabolomic and lipidomic analyses of mature RBC containing mitochondria and of mature RBC without mitochondria were performed, and higher levels of mitochondria metabolites were observed in the first population.6 Subsequent analyses in one patient with SCA showed detectable levels of oxidative phosphorylation activity and the presence of mitochondrial electron chain components in mature RBC containing mitochondria, supporting the idea that mitochondria would be functional. Indeed, the question of the functionality of mitochondria in mature RBC of SCA patients is still debated, and how mitochondria retention in mature RBC could be responsible for a rise in hemolysis in SCA remains unclear. The aim of this study was to test the associations between sickling tendency, RBC senescence, oxidative stress, hematological markers and the levels of mature RBC containing mitochondria in SCA patients and to investigate the functionality of these mitochondria.
Methods
More details on the methods are given in the Online Supplementary Appendix. Blood samples from 61 SCA patients (HbSS genotype, 29 men and 32 women, at steady state, 23.2±14.2 years, non-transfused) and ten healthy race-matched donors (AA) were collected in EDTA tubes. The study was conducted in accordance with the guidelines set by the Declaration of Helsinki, and all subjects gave informed written consent. The study was approved by the Regional Ethics Committees (L16-47, CPP Sud-Est IV, Hospices Civils de Lyon).
Image Stream (Amnis, MK II) and Mitotracker Red CMXRos Dye (Invitrogen) were used to label active mitochondria. Flow cytometry (MACSQuant 16, Miltenyi), MitoTracker(R) Deep Red probe (MTKdr, Sigma-aldrich) and anti-CD71 antibody were used to determine the percentages of reticulocytes and mature RBC containing mitochondria. Since no criteria or threshold exist in the literature to decide which patient has high or low mitochondria retention in mature RBC, patients were divided into three groups using terciles according to the percentages of mature RBC containing mitochondria in the whole RBC population: “high”, “moderate” and “low” percentage of RBC containing mitochondria. Triple staining on blood samples from ten SCA patients was performed with anti-CD235a (Miltenyi) and anti-CD41 (Miltenyi) antibodies and Mitotracker Deep Red probe, to gate on the RBC+/platelet- population.
Blood samples from eight patients and five AA were used for percoll gradient separation. The second layer containing mature RBC was collected and the presence of reticulocytes was assessed by flow cytometry by using an anti-CD71 antibody (Miltenyi). Then, we investigated the functionality of mitochondria in mature RBC from SCA patients using a high-resolution respirometry protocol for intact blood cells.13,14 Endogenous O2 consumption was recorded before inhibiting ATP-dependent O2 consumption with Oligomycin (2.5 μM), an inhibitor of ATP synthase. The mitochondrial uncoupler FCCP (carbonyl cyanide-p-trifluoro-methoxyphenyl-hydrazone) was then titrated in 0.05 μM steps until the maximal uncoupled O2 consumption was reached. Finally, mitochondrial O2 consumption was fully inhibited by adding Antimycin A (2.5 μM). Flow cytometry was also used to determine the percentage of RBC exposing phosphatidylserine (PS) (Annexin-V-PE, Miltenyi 130-118-363), the anti-phagocytic CD47 antigen (anti-CD47-PE antibody, Miltenyi) and intracellular ROS levels (2’,7’–dichlorofluorescin diacetate [DCFDA], Sigma-Aldrich) of the three groups categorized according to the percentages of mature sickle RBC containing mitochondria. Double staining for mitochondria retention (Mitotracker Deep Red) and i) intracellular Ca2+ (Fluo3 AM, ThermoFicher), ii) CD47, iii) PS or iv) intracellular ROS, was performed to compare these parameters between mature RBC containing mitochondria and those without. Mitochondria superoxide production in sickle RBC was assessed using the MitoSOX Red mitochondrial superoxide indicator (Invitrogen, M36008) and flow cytometry, with and without Antimycin A, an inhibitor of the Respiratory Complex III.
Reduced (GSH) and total (GSSG+GSH) intracellular glutathione were measured using the luminescence-based assay GSH-Glo glutathione Assay (Promega). Oxygen gradient ektacytometry was performed using the Oxygenscan protocol of the LORRCA Maxsis (Mechatronics) to measure RBC deformability in an oxygen gradient.15,16 The maximum RBC deformability (EImax) reached in normoxia and the oxygen pressure at which RBC start to sickle (point of sickling [PoS]) were determined.
Results
Presence of functional mitochondria in mature sickle red blood cells
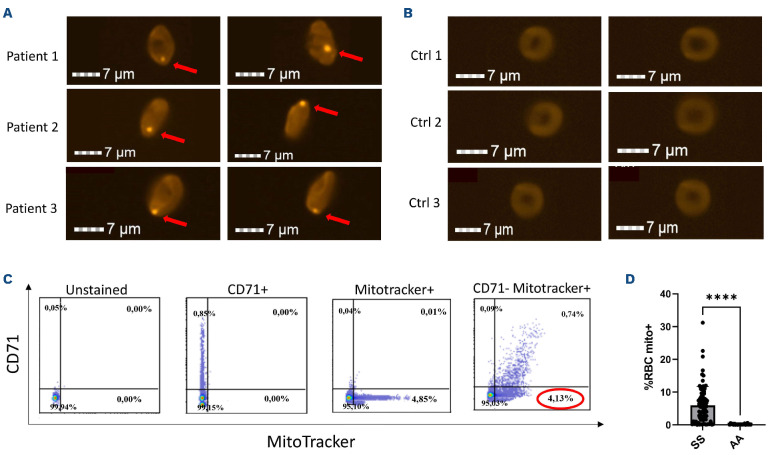
Image Stream analysis showed the presence of mitochondria in mature RBC isolated from SCA patients, but not in RBC from healthy donors. Representative images of RBC containing mitochondria from three SCA patients are shown in Figure 1A. Figure 1B shows the lack of mitochondria in RBC from three healthy donors. Flow cytometry analyses (gating strategy in Figure 1C) demonstrated no mitochondria in mature RBC from healthy individuals and highly variable percentages of mature RBC containing mitochondria in patients with SCA (Figure 1D). The mean percentages of mature RBC containing mitochondria in the three SCA groups categorized according to the percentages of mature RBC containing mitochondria in the whole RBC population were: low group 1.40±1.31%, moderate group 6.2±1.02% and high group 13.02±5.84%.
Figure 1.
Presence of mitochondria in mature sickle red blood cells. (A) ImageStream and staining with Mitotracker Red CMRXRos dye of mitochondria in mature sickle red blood cells (RBC) of three sickle cell (SCA) patients. (B) ImageStream and staining with Mitotracker Red CMRXRos dye show no mitochondria in RBC from healthy donors. (C) Gating strategy for flow cytometry analysis to discriminate mature sickle RBC retaining mitochondria (CD71- Mitotracker+). (D) Mean percentages of mature RBC with mitochondria (mito) in 61 SCA (SS) patients and 10 healthy (AA) donors. Significant difference: ****P<0.0001.
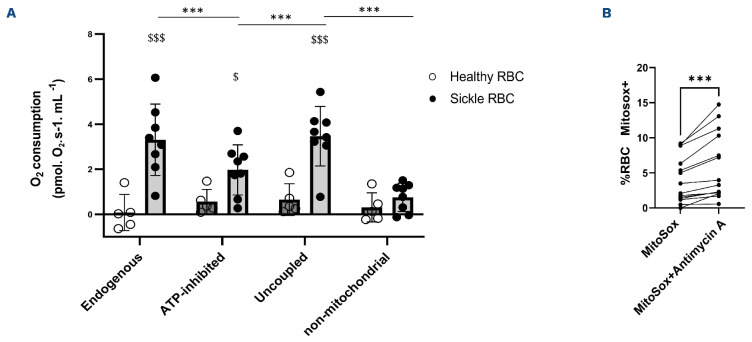
The high-resolution respirometry assay showed active oxygen consumption being responsive to specific mitochondrial inhibitors (Figure 2A). Flow cytometry analysis of the isolated RBC fraction obtained after the Percoll separation gradient showed a very low percentage of residual reticulocytes (<0.6%) and platelets (<0.5%), indicating that the respirometry results were mainly attributable to sickle RBC. Specifically, oxygen consumption was observed in mature sickle RBC but not in healthy RBC (Figure 2A, interaction patient status *respiratory rate; P<0.001). As expected, respiration was undetectable and unaffected by the mitochondrial inhibitors/uncoupler (Oligomycin, FCCP, and Antimycin A; all P>0.24) in healthy controls. In SCA patients, endogenous respiration was detectable and decreased after adding Oligomycin (Figure 2A; P<0.001), an inhibitor of ATP synthase (ATP-inhibited), showing that approximately half of the endogenous oxygen consumption was coupled to ATP synthesis in mature sickle RBC. Following the addition of the uncoupler FCCP thereby reducing the proton force in the inner mitochondrial membrane (i.e., uncoupled O2 consumption), the cellular respiration of mature sickle RBC increased back to the endogenous level (P<0.001). Finally, the use of Antimycin A, an inhibitor of Mitchondrial Complex III, caused a marked reduction of O2 consumption in mature sickle RBC (i.e., non-mitochondrial O2 consumption; P<0.001). Superoxide anion accumulation from mitochondria in mature RBC from 14 SCA patients was detected by the MitoSox Red probe with and without the use of Antimycin A. The percentages of mature sickle RBC accumulating mitochondrial superoxide anion increased after the incubation with Antimycin A (Figure 2B).
Figure 2.
Mitochondrial respiration in mature red blood cells from sickle cell disease patients. (A) Comparison of mitochondrial respiration between 5 healthy and 8 mature sickle red blood cells (RBC). (B) Effect of Antimycin A on superoxide anion generated by mitochondria in mature sickle RBC (N=13). Endogenous: basal mitochondrial O2 consumption; ATP-inhibited: mitochondrial O2 consumption after the use of ATP-synthase inhibitor (Oligomycin); uncoupled: maximal uncoupled O2 consumption obtained with the mitochondrial uncoupler (carbonyl cyanide-p-trifluoro-methoxyphenyl-hydrazone [FCCP]); non-mitochondrial: O2 consumption after adding the Antimycin A. Difference between healthy and mature sickle RBC: $P>0.05; $$$P<0.001. The addition of mitochondrial inhibitors or uncoupler had no effects on healthy RBC while changes were observed on mature sickle RBC: ***P<0.001. Difference between Mitosox and Mitosox+Antimycin A conditions: ***P<0.001. %RBC Mitosox+: percentages of RBC accumulating superoxide anion.
Mitochondria retention in mature sickle red blood cells (RBC) is associated with increased RBC senescence markers and increased propensity of RBC to sickle under deoxygenation
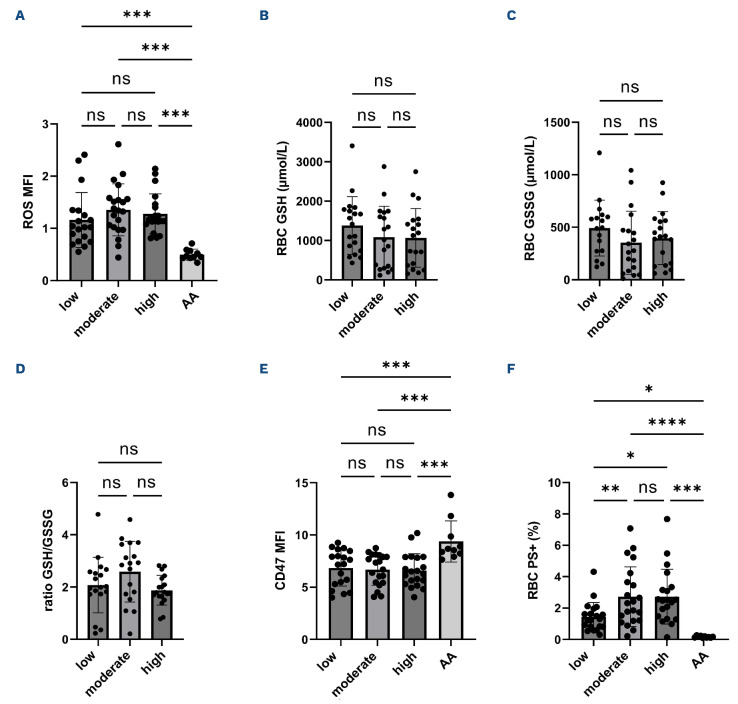
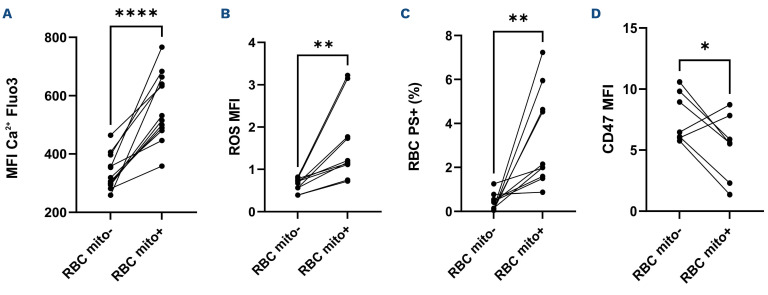
The comparisons of the three groups categorized according to the percentages of mature sickle RBC retaining mitochondria contained in the whole RBC population showed no difference in intracellular ROS (Figure 3A), reduced (GSH, Figure 3B), oxidized (GSSG, Figure 3C) glutathione levels and the ratio between the two forms (Figure 3D). No difference in anti-phagocytic CD47 expression was observed between the three groups (Figure 3E). The percentages of RBC with externalized PS were greater in the groups with moderate and high percentages of mature RBC containing mitochondria (Figure 3F). The comparisons between mature sickle RBC with or without mitochondria showed higher Ca2+ levels, intracellular ROS, and lower CD47 expression in RBC retaining mitochondria (Figure 4A-C). Figure 4D shows greater percentages of RBC with externalized PS in mature sickle RBC with mitochondria compared to those without. No platelets contamination (CD41+) within the RBC population was observed.
Figure 3.
Comparisons of red blood cells oxidative stress and senescence markers between the three groups of sickle cell patients categorized according to the percentage of mature sickle red blood cell containing mitochondria (low group N=20, moderate group N=21, high group N=21) and healthy individuals (AA group N=10). (A-D) Red blood cell (RBC) oxidative stress markers, (E) CD47 expression and (F) percentages of RBC exposing phosphatidylserine (PS). ROS: reactive oxygen species; GSH: reduced glutathione; GSSG: oxidized glutathione; MFI: mean fluorescence intensity; ns: no significant difference. Significant difference: *P<0.05; **P<0.01; ***P<0.001; ****P< 0.0001.
Figure 4.
Comparisons of oxidative stress and senescence markers between mature sickle red blood cells containing mitochondria and mature sickle red blood cells without. (A) Ca2+ levels, (B) intracellular reactive oxygen species (ROS), (C) red blood cells (RBC) exposing phosphatidylserine (PS) and (D) CD47 expression. MFI: mean fluorescence intensity. mito: mitochondria. Significant difference: *P<0.05; **P<0.01; ****P<0.0001.
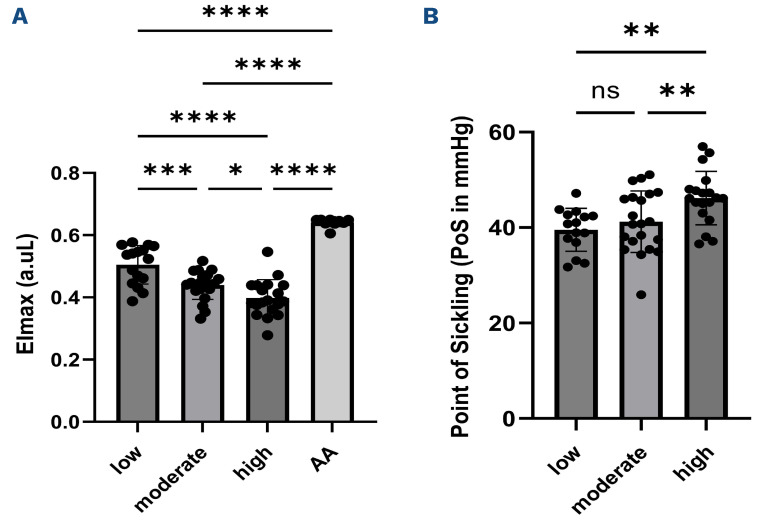
Rheological parameters of the total RBC population showed different tendencies according to the presence of mitochondria. EImax was lower in the two groups with the highest percentages of mature RBC containing mitochondria, with a further reduction in the third (high) group (Figure 5A). The PoS was greater in patients with a high percentage of mature RBC with mitochondria compared to the two other groups (Figure 5B).
Figure 5.
Comparisons of red blood cell rheological parameters between the three groups of sickle cell patients categorized according to the percentage of mature sickle red blood cells containing mitochondria (low group N=20, moderate group N=21, high group N=21) and healthy individuals (AA group N=10). (A) EImax and (B) point of sickling (PoS). Elmax: red blood cell (RBC) deformability in normoxia; ns: no significant difference. Significant difference: *P<0.05; **P<0.01; ***P<0.001; ****<0.0001.
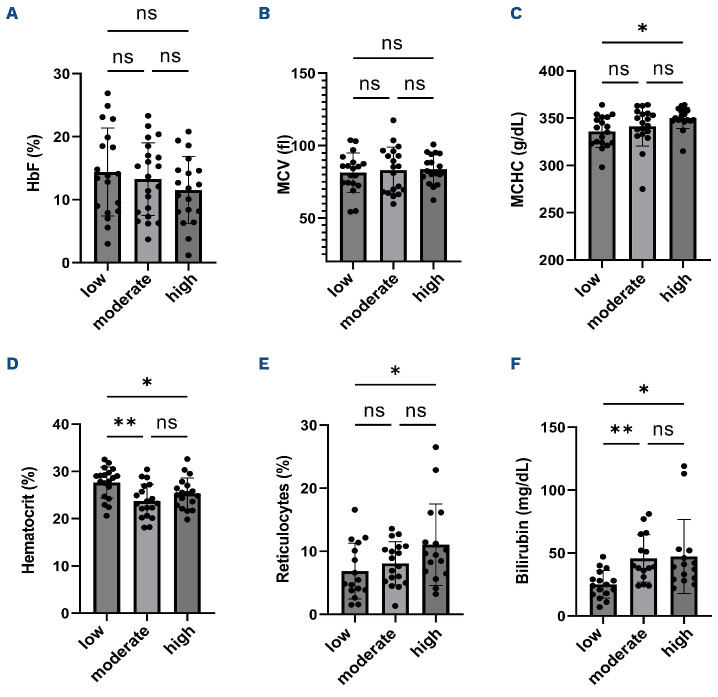
Hematocrit was lower and hemolytic markers were greater in patients with high percentages of mature red blood cells containing mitochondria
No difference in fetal hemoglobin (HbF) and mean corpuscular volume was observed between the three groups (Figures 6A, B). The mean corpuscular hemoglobin concentration was slightly but significantly increased in the group showing a higher percentage of mitochondrial retention compared to the group with a low percentage (Figure 6C). Percentages of reticulocytes and bilirubin levels were greater, and hematocrit was lower, in the group of patients with a high percentage of mature RBC with mitochondria (Figure 6D-F).
Figure 6.
Influence of the presence of mitochondria in mature sickle red blood cells on hematological parameters. (A-D) Comparison of hematological parameters and (E-F) hemolytic markers between the 3 groups (low group N=20, moderate N=21, high N=21). HbF: percentage of fetal hemoglobin; MCV: mean corpuscular volume; MCHC: mean corpuscular hemoglobin concentration; ns: no significant difference. Significant difference: *P<0.05; **P<0.01.
No association between mature sickle RBC mitochondria retention and previous history of clinical manifestations (i.e., vaso-occlusive crises and acute chest syndrome rates in the 3 preceding years, glomerulopathy, priapism, leg ulcers, osteonecrosis, stroke or pulmonary hypertension) was observed. Glucose-6-phosphate dehydrogenase deficiency frequency did not differ between the three groups (15%, 14% and 10% in the low, moderate, and high group, respectively; χ=0.39; P= 0.82).
Discussion
The present study investigated the functionality of mitochondria in mature RBC from SCA patients and the associations between RBC mitochondria retention, the propensity of RBC to sickle under deoxygenation, hemolytic markers, RBC senescence and RBC oxidative stress. Our findings support the fact that mitochondria in mature RBC from SCA patients are still functional and able to produce ATP. In addition, we found that i) patients with a higher percentage of mitochondria retention in mature RBC were those exhibiting higher hemolytic rate, increased RBC senescence and greater propensity of RBC to sickle under deoxygenation, ii) oxidative stress and senescence markers were greater in mature sickle RBC containing mitochondria compared to those without.
Martino et al.5 reported that the abnormal retention of mitochondria in sickle RBC was the result of a lower expression of mitophagy inducers PINK1, NIX and of a higher expression of HSP90 chaperone. The interest in studying mitochondria retention in sickle RBC is based on the fact that these organelles may be a source of ROS that could damage RBC and accelerate their death/removal from blood circulation. Our results did not show any difference in RBC oxidative stress markers between patients with high mitochondria retention in mature RBC and those with low retention. The lack of association between RBC oxidative stress level and the degree of mitochondria retention in mature RBC is in agreement with the study of Martino et al.5 However, the intracellular environment of sickle RBC is already characterized by a high level of oxidative stress caused by a high HbS auto-oxidation rate, increased NADPH oxidase activity and low antioxidant defense.17 In addition, the probe used to assess intracellular ROS in our study (i.e., DCFDA), although having several advantages, also has some limitations. DCFDA reacts with both nitric oxide and ROS18 and DCF formation is increased in the presence of heme-containing molecules, like peroxidase, hematin or metal ions with redox action.19,20 Thus, even if the mitochondria in sickle RBC were functional, it is tempting to hypothesize that their contribution to the whole intracellular ROS production and RBC oxidative stress is too weak to be probed specifically by DCFDA. Nevertheless, the comparison of intracellular ROS levels between mature RBC containing mitochondria and mature RBC without, showed higher ROS content in the former RBC subpopulation. Indeed, the lack of intracellular ROS difference between the three groups could be due to the fact that the higher intracellular ROS content of the mature RBC containing mitochondria was diluted in the whole RBC population (i.e., mature RBC with and without mitochondria retention). The use of a more specific probe for mitochondrial superoxide anion detection (i.e., MitoSox Red) indicated the capability of these RBC mitochondria to produce superoxide anions, especially after the incubation with Antimycin A, a blocker of Mitochondrial Complex III, which resulted in increased mitochondrial ROS accumulation. Indeed, we suspect that mitochondria in mature sickle RBC could contribute to the production of ROS and thus explain why inducing mitophagy in a sickle mice model resulted in a decrease of RBC ROS levels and increased RBC survival.9
The functionality of mitochondria in mature RBC from sickle cell patients was confirmed by the respiration assay experiments. Indeed, we observed a mitochondria-dependent oxygen consumption in sickle RBC and the use of oligomycin demonstrated that about 48.7% of endogenous oxygen consumption was related to the production of ATP. Image Stream experiments also showed that active mitochondria are present in sickle RBC, further supporting mitochondrial functionality in mature sickle RBC. Although we were not able to measure individually the deformability of mature sickle RBC with mitochondria and of mature sickle RBC without mitochondria, we observed that patients with a high percentage of mitochondria retention in mature RBC had a lower mean RBC deformability and a greater propensity of RBC to sickle under deoxygenation compared to patients with a lower percentage of mature RBC containing mitochondria. Indeed, it is possible that a higher rate of oxygen consumption by mitochondria contained in mature RBC from SCA patients could facilitate sickling by decreasing the amount of oxygen available for HbS. The subsequent greater rheological alterations would then increase RBC fragility,2 which may explain the lower hematocrit and greater hemolytic rate found in the patients with high mitochondria retention in mature RBC, as previously reported.5 Moreover, the production of ROS by mitochondria could affect the RBC membrane and increase the risk of RBC membrane disruption. Alternatively, a high rate of hemolysis could induce abnormal erythropoiesis and altered RBC maturation process, which would lead in turn to a greater retention of mitochondria in mature RBC. Further studies are needed to investigate the mechanisms at the origin of the relationships between mitochondria retention in mature RBC and increased hemolysis in SCA patients.
Patients with a high percentage of mature RBC containing mitochondria exhibited higher percentages of RBC with externalized PS, which could indicate an alteration of membrane asymmetry following scramblase stimulation and accelerated RBC senescence. The comparisons of mature RBC subpopulations according to the presence or not of mitochondria showed a higher percentage of RBC with externalized PS in mature RBC with mitochondria retention and that mature RBC with mitochondria had greater level of intracellular Ca2+ and lower membrane CD47 compared to mature RBC with no mitochondria. Of note, the evaluation of the percentages of RBC exposing PS was assessed using Annexin V, a protein with a high affinity for PS but that could also enter cells with membrane alterations and bind PS on the inner leaflet. This could have led to an overestimation of external PS and the use of lactadherin should be considered in future studies.
Increased cation permeability, and more particularly of Ca2+, has been reported in sickle RBC compared to normal RBC,21 notably in deoxygenated condition.22 Hanggi et al.23 reported increased Ca2+ conductance through NMDA receptors in sickle RBC and Wang et al.24 demonstrated increased Ca2+ accumulation in response to lysophosphatidic acid in sickle RBC compared to control RBC. Very recently, an important role of Piezo1 has been reported in SCA that may increase intracellular Ca2+ levels in sickle RBC.21 Intracellular accumulation of Ca2+ is known to stimulate scramblase, leading to a disruption of the membrane phospholipid asymmetry and PS externalization.25 The presence of mitochondria could also participate in the elevation of intracellular Ca2+ levels, as mitochondria have a remarkable ability to take up and store massive amounts of Ca2+,26 as well as in the release of it into the cytosol.27 Although mitochondria would provide RBC with ATP, the low residual mitochondria in sickle RBC are unable to maintain ATP levels high enough to ensure Ca2+ ATP-dependent extrusion.
It is well known that reticulocytes still have the endoplasmic reticulum, which is a major source of Ca2+.28 Reticulocytes normally lose all their remaining organelles during maturation. The abnormal retention of mitochondria and the increased Ca2+ and PS exposure in mature RBC could point to a defect in the final maturation of reticulocytes.29 Further studies are needed to analyze the content in endoplasmic reticulum proteins29 in mature RBC in SCA. Furthermore, Ca2+ may cause the activation of the Gardos channel pathway, which mediates rapid K+ and Cl- efflux and water loss.30 The resulting cellular dehydration facilitates HbS polymerization and RBC sickling, which could explain why SCA patients with the greatest percentage of mature RBC containing mitochondria were also those with a lower deformability, a greater MCHC and a higher point of sickling, i.e., with a greater propensity of RBC to sickle under deoxygenation. In conclusion, this study showed the presence of functional mitochondria in mature sickle RBC, which could favor RBC sickling and accelerate RBC senescence, leading to increased cellular fragility and hemolysis.
Supplementary Material
Funding Statement
Funding: This study was supported by the European Framework Horizon 2020 under grant agreement number 860436 (EVIDENCE).
References
- 1.Kato GJ, Gladwin MT, Steinberg MH. Deconstructing sickle cell disease: reappraisal of the role of hemolysis in the development of clinical subphenotypes. Blood Rev. 2007;21(1):37-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Connes P, Lamarre Y, Waltz X, et al. Haemolysis and abnormal haemorheology in sickle cell anaemia. Br J Haematol. 2014;165(4):564-572. [DOI] [PubMed] [Google Scholar]
- 3.Jang T, Poplawska M, Cimpeanu E, et al. Vaso-occlusive crisis in sickle cell disease: a vicious cycle of secondary events. J Transl Med. 2021;19(1):397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nader E, Romana M, Connes P. The red blood cell-inflammation vicious circle in sickle cell disease. Front Immunol. 2020;11:454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martino S, Arlet JB, Odievre MH, et al. Deficient mitophagy pathways in sickle cell disease. Br J Haematol. 2021;193(5):988-993. [DOI] [PubMed] [Google Scholar]
- 6.Moriconi C, Dzieciatkowska M, Roy M, et al. Retention of functional mitochondria in mature red blood cells from patients with sickle cell disease. Br J Haematol. 2022;198(3):574-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tumburu L, Ghosh-Choudhary S, Seifuddin FT, et al. Circulating mitochondrial DNA is a proinflammatory DAMP in sickle cell disease. Blood. 2021;137(22):3116-3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mortensen M, Ferguson DJ, Edelmann M, et al. Loss of autophagy in erythroid cells leads to defective removal of mitochondria and severe anemia in vivo. Proc Natl Acad Sci U S A. 2010;107(2):832-837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jagadeeswaran R, Vazquez BA, Thiruppathi M, et al. Pharmacological inhibition of LSD1 and mTOR reduces mitochondrial retention and associated ROS levels in the red blood cells of sickle cell disease. Exp Hematol. 2017;50:46-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rivers A, Jagadeeswaran R, Lavelle D. Potential role of LSD1 inhibitors in the treatment of sickle cell disease: a review of preclinical animal model data. Am J Physiol Regul Integr Comp Physiol. 2018;315(4):r840-r847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nohl H, Gille L, Staniek K. Intracellular generation of reactive oxygen species by mitochondria. Biochem Pharmacol. 2005;69(5):719-723. [DOI] [PubMed] [Google Scholar]
- 12.Barodka VM, Nagababu E, Mohanty JG, et al. New insights provided by a comparison of impaired deformability with erythrocyte oxidative stress for sickle cell disease. Blood Cells Mol Dis. 2014;52(4):230-235. [DOI] [PubMed] [Google Scholar]
- 13.Sjovall F, Ehinger JK, Marelsson SE, et al. Mitochondrial respiration in human viable platelets - methodology and influence of gender, age and storage. Mitochondrion. 2013;13(1):7-14. [DOI] [PubMed] [Google Scholar]
- 14.Stier A, Romestaing C, Schull Q, et al. How to measure mitochondrial function in birds using red blood cells: a case study in the king penguin and perspectives in ecology and evolution. Meth Ecol Evol. 2017;8(10):1172-1182. [Google Scholar]
- 15.Boisson C, Rab MAE, Nader E, et al. Effects of genotypes and treatment on oxygenscan parameters in sickle cell disease. Cells. 2021;10(4):811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rab MAE, Kanne CK, Bos J, et al. Oxygen gradient ektacytometry-derived biomarkers are associated with vasoocclusive crises and correlate with treatment response in sickle cell disease. Am J Hematol. 2021;96(1):e29-e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hebbel RP. Reconstructing sickle cell disease: a data-based analysis of the "hyperhemolysis paradigm" for pulmonary hypertension from the perspective of evidence-based medicine. Am J Hematol. 2011;86(2):123-154. [DOI] [PubMed] [Google Scholar]
- 18.Aslan M, Thornley-Brown D, Freeman BA. Reactive species in sickle cell disease. Ann N Y Acad Sci. 2000;899:375-391. [DOI] [PubMed] [Google Scholar]
- 19.Fuloria S, Subramaniyan V, Karupiah S, et al. Comprehensive review of methodology to detect reactive oxygen species (ROS) in mammalian species and establish its relationship with antioxidants and cancer. Antioxidants (Basel). 2021;10(1):128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tarpey MM, Wink DA, Grisham MB. Methods for detection of reactive metabolites of oxygen and nitrogen: in vitro and in vivo considerations. Am J Physiol Regul Integr Comp Physiol. 2004;286(3):R431-444. [DOI] [PubMed] [Google Scholar]
- 21.Nader E, Conran N, Leonardo FC, et al. Piezo1 activation augments sickling propensity and the adhesive properties of sickle red blood cells in a calcium-dependent manner Br J Haematol. 2023 Apr 3. 2023;202(3):657-668. [DOI] [PubMed] [Google Scholar]
- 22.Brugnara C. Sickle cell dehydration: pathophysiology and therapeutic applications. Clin Hemorheol Microcirc. 2018;68(2-3):187-204. [DOI] [PubMed] [Google Scholar]
- 23.Hanggi P, Makhro A, Gassmann M, et al. Red blood cells of sickle cell disease patients exhibit abnormally high abundance of N-methyl D-aspartate receptors mediating excessive calcium uptake. Br J Haematol. 2014;167(2):252-264. [DOI] [PubMed] [Google Scholar]
- 24.Wang J, Hertz L, Ruppenthal S, et al. Lysophosphatidic acid-activated calcium signaling is elevated in red cells from sickle cell disease patients. Cells. 2021;10(2):456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bevers EM, Williamson PL. Phospholipid scramblase: an update. FEBS Lett. 2010;584(13):2724-2730. [DOI] [PubMed] [Google Scholar]
- 26.Strubbe-Rivera JO, Schrad JR, Pavlov EV, et al. The mitochondrial permeability transition phenomenon elucidated by cryo-EM reveals the genuine impact of calcium overload on mitochondrial structure and function. Sci Rep. 2021;11(1):1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takeuchi A, Kim B, Matsuoka S. The destiny of Ca(2+) released by mitochondria. J Physiol Sci. 2015;65(1):11-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moras M, Lefevre SD, Ostuni MA. From erythroblasts to mature red blood cells: organelle clearance in mammals. Front Physiol. 2017;8:1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brusson M, Cochet S, Leduc M, et al. Enhanced calreticulin expression in red cells of polycythemia vera patients harboring the JAK2(V617F) mutation. Haematologica. 2017;102(7):e241-e244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maher AD, Kuchel PW. The Gardos channel: a review of the Ca2+-activated K+ channel in human erythrocytes. Int J Biochem Cell Biol. 2003;35(8):1182-1197. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.