Abstract
Angelman syndrome (AS) is an imprinted neurodevelopmental disorder that lacks a cure, characterized by developmental delay, intellectual impairment, seizures, ataxia, and paroxysmal laughter. The condition arises due to the loss of the maternally inherited copy of the UBE3A gene in neurons. The paternally inherited UBE3A allele is unable to compensate because it is silenced by the expression of an antisense transcript (UBE3A-ATS) on the paternal chromosome. UBE3A, encoding enigmatic E3 ubiquitin ligase variants, regulates target proteins by either modifying their properties/functions or leading them to degradation through the proteasome. Over time, animal models, particularly the Ube3a mat−/pat+ Knock-Out (KO) mice, have significantly contributed to our understanding of the molecular mechanisms underlying AS. However, a shift toward human pluripotent stem cell models (PSCs), such as human embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs), has gained momentum. These stem cell models accurately capture human genetic and cellular characteristics, offering an alternative or a complement to animal experimentation. Human stem cells possess the remarkable ability to recapitulate neurogenesis and generate “brain-in-a-dish” models, making them valuable tools for studying neurodevelopmental disorders like AS. In this review, we provide an overview of the current state-of-the-art human stem cell models of AS and explore their potential to become the preclinical models of choice for drug screening and development, thus propelling AS therapeutic advancements and improving the lives of affected individuals.
Keywords: Angelman syndrome (AS), genomic imprinting, UBE3A, pluripotent stem cells (PSCs), disease modeling, brain organoids, antisense oligonucleotides (ASOs)
Introduction
Angelman Syndrome (AS) (OMIM#105830) is a rare neurodevelopmental disorder estimated to affect between 1 in 12,000 and 1 in 20,000 live births (Buiting et al., 2016). It presents a diverse symptomatology, including severe developmental delay, speech impairment, movement disorders ranging from tremors to ataxia, epilepsy, and atypical episodes of laughter and smiling. Typically, these symptoms begin to emerge between 6–9 months of age. However, it is important to note that a definitive diagnosis of AS may take some time, usually within the first 3 years of a child’s life. Several characteristic features commonly linked to AS may intersect with symptoms seen in other neurodevelopmental disorders. Therefore, a precise AS diagnosis requires validation through molecular testing (Margolis et al., 2015; Maranga et al., 2021).
AS results from the absence or deficiency of Ubiquitin Protein Ligase E3A (UBE3A) protein function in neurons. By integrating the ubiquitin-proteasome protein degradation pathway and possibly other regulatory processes, UBE3A regulates protein function and/or degradation of several specific targets through its ubiquitination activity. As a result, disruption of normal UBE3A expression is thought to affect several key neuronal processes necessary for normal synaptic function and plasticity. While UBE3A is biallelically expressed in most human tissues, only the maternal copy of this gene is expressed in neurons, constituting an example of a gene that is regulated by a cell type-specific form of genomic imprinting (Williams et al., 2010; Margolis et al., 2015; MedlinePlus, 2022). The lack of function of the maternal UBE3A copy in neurons is sufficient for the manifestation of AS.
Our current understanding of AS has been built upon studies using different models. Postmortem analysis of human AS tissues, animal models such as mouse, rat, or Drosophila, and in vitro cellular studies, have all furthered the knowledge of this disease and its mechanisms (Jay et al., 1991; Jiang et al., 1998; Miura et al., 2002; Wu et al., 2008; Chamberlain et al., 2010; Jiang et al., 2010; Berg et al., 2020; Dodge et al., 2020). The most useful of all has been the Ube3a mat−/pat+ Knock-Out (KO) mouse (Jiang et al., 1998) which advanced our knowledge of the pathophysiological mechanisms of the disease. Despite overall milder symptomatology when compared to human AS individuals, this KO mouse has also been an important preclinical model for drug development. More recently, the advent of human embryonic stem cells (ESCs) and patient-derived induced Pluripotent stem cells (iPSCs) have provided the possibility of new avenues of research for AS, which overcomes some of the limitations regarding phenotype recapitulation and ethical concerns presented by animal models (reviewed in Maranga et al., 2021). Here, we aim to provide a comprehensive overview of stem cell-focused research on AS. We will delve into the advantages offered by these cellular models in terms of disease phenotyping, identification of druggable targets, and their exceptional utility as a preclinical model for drug screening.
Research milestones in Angelman syndrome
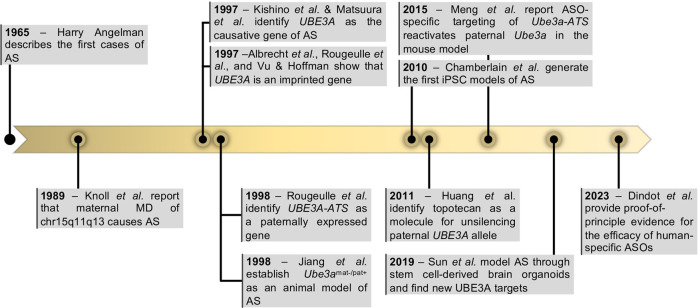
AS was first described in 1965 by an English pediatrician, Harry Angelman, in ‘Puppet’ children. A report on three cases (Figure 1). In this report, Angelman described the symptoms of three patients, which he considered similar enough to justify combining them in a “specific group, as yet of unknown cause”. They shared common symptoms such as depression in the occipital region of the skull, brachycephaly associated with microcephaly, severe intellectual disability, easily provoked and prolonged paroxysms of laughter, ataxia like the one observed in cerebellar deficiency and unusually protruding tongues, among other features (Angelman, 1965). Not long after, more cases had been reported and the “Happy Puppet syndrome” was renamed Angelman syndrome, honoring Harry Angelman as the discoverer of this new human condition (Berg and Pakula, 1972).
FIGURE 1.
Milestones in Angelman Syndrome research. Highlighted years represent the year of the relevant publication. Abbreviations: UBE3A—Ubiquitin Protein Ligase E3A; UBE3A-ATS—ubiquitin-protein ligase E3A anti-sense; ASOs—antisense oligonucleotides.
It was not until the late 1980s that the first genetic aberration was linked to AS. Two studies reported megabase deletions (herein named megadeletions or MDs) within the chr15q11-q13 region present in individuals with AS (Kaplan et al., 1987; Magenis et al., 1987). At the time, this was an intriguing discovery since MDs of the same region were already associated with Prader-Willi syndrome (PWS), a very different condition characterized by mild-to-moderate intellectual impairment, constant feeling of hunger, and obesity (OMIM#176270). The mystery was later solved by the discovery that the parental origin of the chr15q11-q13 MD dictated the disease presentation: inheritance of the paternal deletion results in PWS, while maternal deletion results in AS (Knoll et al., 1989) (Figure 1). These findings suggested that chr15q11-q13 was regulated by genomic imprinting, an epigenetic phenomenon that regulates monoallelic expression of genes according to their parental origin. Therefore, PWS and AS were proposed to be the first examples of imprinting disorders (Hall, 1990; Williams et al., 1990). This was further supported by the discovery that inheritance of two paternal chromosomes 15 (patUPD15) also causes AS (Malcolm et al., 1991), while maternal uniparental disomy was a frequent cause of PWS (Butler, 1983). Once the importance of DNA methylation in regulating genomic imprinting was established (Li et al., 1993), several reports soon found differential methylated regions (DMRs) between the two parental alleles at the chr15q11-q13 region (Dittrich et al., 1992; Driscoll et al., 1992; Clayton-Smith et al., 1993). Unusual and contrasting DNA methylation patterns were then reported in both PWS and AS patients with no obvious genetic abnormality (Reis et al., 1994). This revealed that the abnormal establishment of DNA methylation at DMRs was sufficient to cause these imprinting diseases. The important genetic elements, also known as imprinting centers (IC), were later mapped to a region including “D15S63 (PW71) and SNRPN” thanks to microdeletions found in AS and PWS individuals and further fine-tuned to two regions now known as AS and PWS ICs (AS-IC and PWS-IC). PWS-IC, also known as SNURF TSS-DMR, was then confirmed to hold a DMR inheriting the methylation mark only from the maternal germline (Buiting et al., 1995; Shemer et al., 2000).
While it was becoming evident that AS was an imprinting disorder affecting the chr15q11-13 region, the causing gene(s) were yet to be identified. In 1997, two back-to-back publications undoubtedly pinpointed UBE3A as the gene implicated in AS (Kishino et al., 1997; Matsuura et al., 1997) (Figure 1). Both studies report distinct mutations in the UBE3A gene as the cause of AS in non-MD/non-UPD/non-imprinting defect AS individuals. Shortly after, UBE3A was confirmed to be an imprinted gene, expressed only from the maternal allele (Figure 1). However, in contrast to most genes known at the time, imprinting of UBE3A was restricted to the brain (Albrecht et al., 1997; Rougeulle et al., 1997; Vu and Hoffman, 1997). In 1998, Rougeulle et al. identified the antisense non-coding transcript of human UBE3A, commonly referred today as UBE3A-ATS, which was reciprocally imprinted, being expressed only from the paternal allele in the brain (Rougeulle et al., 1998) (Figure 1). This antisense RNA was later shown to belong to a large polycistronic transcript originated from the unmethylated paternally inherited PWS-IC region and encoding several distinct transcripts including SNRPN/SNURF, IPW, PWAR1 and tandemly repeated C/D snoRNA genes, besides the antisense RNA to UBE3A (Runte et al., 2001) (Figure 2A). Since its discovery, UBE3-ATS has been anticipated to be a putative regulator of paternal UBE3A silencing (Chamberlain and Brannan, 2001). Formal proof of that was first shown thanks to the addition of a transcription termination cassette that halted UBE3A-ATS expression and resulted in the unsilencing of UBE3A from the paternal allele (Meng et al., 2012).
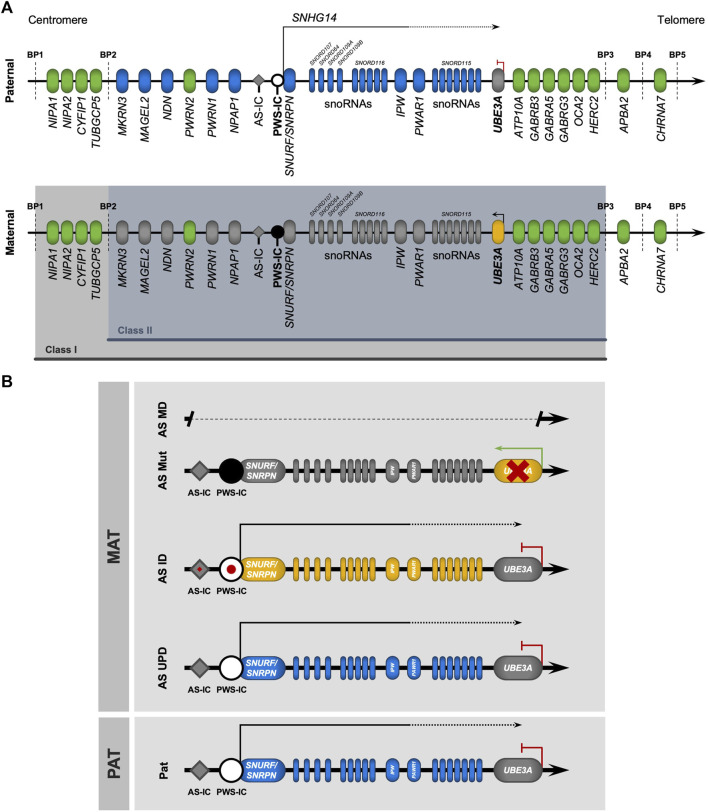
FIGURE 2.
The (epi)genetics of Angelman syndrome. (A) Genomic map of the chr15q11-q13 region. Parental-of-origin specific DNA methylation (black circle) occurs on the CpG-rich locus known as the Prader-Willi imprinting center (PWS-IC), only on the maternal allele. From the unmethylated paternal allele, PWS-IC serves as a promotor for a large transcription unit (small nucleolar host gene 14, SNHG14) containing the transcripts of some genes (SNURF/SNURPN, IPW) and a group of C/D small nucleolar RNAs (snoRNAs) with the SNORD116 host transcript, expressed only from the paternal allele (in blue). In neurons, the loss of an insulator element at the IPW and PWAR1 locus results in the extension of SNHG14, which now contains SNORD115 (also containing C/D snoRNAs) and UBE3A-ATS, an antisense transcript that silences the paternal UBE3A allele by transcriptional interference. Thus, UBE3A is only expressed from the maternal allele (in yellow) in neurons. Biallelically expressed genes are represented in green. The two most common types of large deletions that lead to Angelman syndrome (AS) are highlighted between breakpoints (BP) BP1-BP3 (class I) and BP2-BP3 (class II). (B) Genomic map of the (epi)genetic causes of Angelman syndrome. AS is caused by four main causes relating to the maternal chr15, all leading to a loss-of-function of UBE3A: megadeletions in the chr15q11-q13 region (AS MD); deleterious mutations in the UBE3A allele (AS Mut); imprinting defects (red spot) at the PWS-IC locus causing an absence of DNA methylation (AS ID); and paternal uniparental disomy of chr15 (AS UPD).
Advances in AS research would have only been possible with the use of a variety of research models. Through the years, researchers have privileged the use of mouse models with AS-like phenotypes. The first AS mouse model was described by Cattanach et al. in 1997 and consisted of a paternal duplication of the murine homologous region of the human chr15q11-q13 (Cattanach et al., 1997). Although these mice exhibited AS traits, they needed complex breeding schemes and were never extensively used for dissecting pathophysiological mechanisms of disease. With the discovery that loss of function of the maternal copy of UBE3A gene causes AS, Jiang et al. created the Ube3a mat−/pat+ KO mice, with a deletion on the maternally inherited Ube3a allele, which presents ataxia, inducible seizures, and sleep alterations, all features shown by AS individuals (Jiang et al., 1998; Colas et al., 2005; Dindot et al., 2007). Other AS mouse models have been generated with similar phenotypes (Miura et al., 2002; Jiang et al., 2010), but the original Ube3a mat−/pat+ mice from Jiang et al. (1998) has remained the preferential AS mouse model used by researchers. Other important mouse models comprise the Ube3a Stop/p+; Cre ERT+ mouse allowing for temporal control of Ube3a reinstatement to discern the critical developmental time windows for therapeutic intervention (Silva-Santos et al., 2015; Gu et al., 2018; Rotaru et al., 2023), the conditional Ube3a floxed allele, Ube3a fl/+ (Bruinsma et al., 2015; Gu et al., 2018) used to investigate the cell/region-specific contribution to the disease phenotype or the Ube3a m+/pYFP knock-in mice (Dindot et al., 2007) which provide a useful read-out for drug screening strategies aiming at unsilencing paternal Ube3a allele as a therapeutic option to treat AS (Huang et al., 2012; Meng et al., 2015). Recently, AS rat models with a complete KO of the maternal Ube3a copy have been developed (Berg et al., 2020; Dodge et al., 2020; Born et al., 2021). These larger rodents, while sharing phenotypic similarities with Ube3a mat−/pat+ KO mice, displayed distinctive behaviors and previously unseen changes in neuroanatomy (Berg et al., 2020; Dodge et al., 2020; Born et al., 2021), bringing an added value to AS research.
After the seminal studies from Thomson et al. (1998) on the derivation of human embryonic stem cells (ESCs) and from Yamanaka and others in the mid-2000s (Takahashi and Yamanaka, 2006; Takahashi et al., 2007) on the generation of induced pluripotent stem cells (iPSCs), PSC models emerged as alternative humanized and personalized cellular systems for disease modeling. The first AS iPSC models were soon generated (Chamberlain et al., 2010), initiating a new era of research on AS based on the use of stem cell-derived neurons and, later on, brain organoid models to reveal new pathophysiologic mechanisms of the disease, find new ubiquitination targets of UBE3A and validate potential therapeutic approaches (Fink et al., 2017; Sun et al., 2019; Pandya et al., 2021; Dindot et al., 2023).
Disease management for AS relies on approaches that ameliorate the most detrimental symptoms such as seizures or sleep abnormalities. None of these therapeutic interventions targets the cause of the disease, which is the loss of function of the UBE3A gene. In recent years, many hopes have been put on emerging strategies to reinstate UBE3A expression as a therapeutic option. The favored strategy has not been the ectopic expression of UBE3A (Daily et al., 2011), amid fears of elevating the dosage of UBE3A, a known cause of the autism and epilepsy-related Dup15q syndrome (Lusk et al., 2021), but rather the reactivation of the intact, albeit silenced paternal copy of the UBE3A gene. Using primary cortical neurons from Ube3a m+/pYFP mice, a study identified inhibitors of topoisomerase I and II as molecules that reactivate the paternal Ube3a-YFP allele (Huang et al., 2012). The most promising compound was topotecan, a clinical-grade topoisomerase I inhibitor, that was shown to reactivate paternal Ube3a through the reduction of transcription of the polycistronic transcription unit containing Ube3a-ATS. Although topotecan has a generalized effect on long genes associated with autism (King et al., 2013) that may halt its widespread use as a therapeutic agent to treat AS, this seminal study showed that pharmacological perturbation of UBE3A-ATS transcription is a feasible approach to reinstate UBE3A expression in AS individuals. This idea was further explored by Meng et al. (2015) who screened for antisense oligonucleotides (ASOs) capable of specifically downregulating Ube3a-ATS. This downregulation was able to activate the paternal Ube3a allele (Meng et al., 2015) resulting in phenotypic rescue of cognitive and behavior deficits in the Ube3a mat−/pat+ KO mouse (Meng et al., 2015; Milazzo et al., 2021). This study was later translated to human cells with the identification of primate-specific ASOs able to reactive paternal UBE3A not only in iPSC-derived neurons in vitro but also in vivo by lumbar puncture in cynomolgus macaques (Dindot et al., 2023). These findings support the molecular basis for an ongoing clinical trial (ClinicalTrials.gov, NCT04259281), soon followed by another one also using oligonucleotide-based therapeutics (NCT04428281). Other attempts to perturb UBE3A-ATS transcript, by taking advantage of the CRISPR/Cas9 genetic editing technology, are under development with promising results achieved in the mouse model (Wolter et al., 2020; Schmid et al., 2021; Li et al., 2023).
More than 50 years after the initial description of the ‘Puppet’ children by Dr. H. Angelman, a promising therapy targeting the molecular cause of the disease has reached the clinical stage (Dindot et al., 2023). However, many challenges in AS research and treatment remain, justifying continuous efforts in investigating further the multiple aspects of this disease for which stem cell models are becoming increasingly important as research tools.
Clinical hallmarks of Angelman syndrome
From the 1960s through the 1990s several studies further characterized the symptoms of the disorder first reported by Dr. Harry Angelman (Bower and Jeavons, 1967; Berg and Pakula, 1972; Williams et al., 1982; Robb et al., 1989; Dickinson et al., 1990). This ultimately led to a clinical consensus concerning the symptomatology of AS individuals. The diverse symptoms observed in AS individuals have been divided into three categories: consistent, frequent, and associated features. Consistent features are those present in all AS patients and include functionally severe developmental delay, a movement or balance disorder (usually ataxia), a combination of frequent laughter and smiling and hypermotoric behavior, and absent or impaired speech. Frequent characteristics are present in ≥80% of AS patients, and include microcephaly, early onset seizures, and a specific and abnormal electroencephalogram pattern. The remaining shared traits, affecting from 20% to 80% of patients, include the occipital groove and protruding tongue observed by Harry Angelman, as well as a wide variety of symptoms, among them feeding problems, prognathia, an uplifted and flexed arm position during ambulation, wide-based gait, abnormal sleep cycles and food-related behaviors, and attraction/fascination with water (Williams et al., 2006). Some of these symptoms become apparent as early as 6–9 months old, with most AS diagnoses happening between 9 months and 6 years of age (Mayo Clinic, 2022; NHS, 2023). AS patients have a reasonably long lifespan, with some patients living past 70 years of age. Reduced lifespan of some patients is mostly associated with epilepsy (severe convulsions) and lack of balance/coordination (ambulatory accidents), combined with a hyperactive and exploratory personality often seen in children with AS (Buiting et al., 2016).
AS shares similarities with other neurodevelopmental disorders with mutations in other genes, which could cause difficulty in early diagnosis. These include Rett syndrome (MECP2, OMIM#312750), early infantile epileptic encephalopathy (CDKL5, OMIM#300672), FOXG1 syndrome (OMIM#613454), Christianson syndrome (SLC9A6, OMIM#300243), or Pitt-Hopkins syndrome (TCF4, OMIM#610954) (McKnight et al., 2022). Given the overlapping manifestations, a definitive diagnosis relies on molecular testing, which in the case of AS may need several independent tests depending on the molecular cause of the disease.
Molecular causes of Angelman syndrome
The chr15q11-q13 region, where the UBE3A gene is located, is regulated by genomic imprinting (Figure 2A). This epigenetic mechanism of gene regulation selectively silences one of the two parental alleles, resulting in a parental-of-origin monoallelic expression of the imprinted genes (reviewed in da Rocha and Gendrel, 2019). Imprinting regulation in the chr15q11-q13 region is ensured by the PWS-IC, which is characterized by a dense CpG sequence with maternal allele-specific DNA methylation, established in the germline (da Rocha and Gendrel, 2019). Methylation of the PWS-IC in the maternal germline is established by the transcription of upstream exons of the SNURF/SNRPN bicistronic gene, driven by a promoter element known as AS-IC, which induces transcription-associated CpG methylation at the maternal PWS-IC (Horsthemke and Wagstaff, 2008). The unmethylated paternal PWS-IC serves as a promoter of a large polycistronic transcription unit, also known as SNHG14 (small nucleolar RNA host gene 14), exclusively expressed from the paternal allele. SNHG14 encodes the bicistronic SNURF/SNRPN gene pair and several long and small RNAs. These include IPW, PWAR1 long noncoding RNAs (lncRNAs), and tandem-repeated C/D snoRNA genes clustered in two domains, known as SNORD116, which is ubiquitously expressed, and SNORD115. At the 3’ end tip of the SNHG14 transcript unit sits the UBE3A-ATS lncRNA which overlaps with the UBE3A gene (reviewed in Maranga et al., 2020) (Figure 2A). In non-neuronal cells, UBE3A is biallelically expressed, as UBE3A-ATS is absent. However, in neurons, loss of an insulator element at the IPW and PWAR1 genes, composed of poly(A), conserved sites, and CTCF (CCCTC-binding factor) binding motifs, results in the extension of the SNHG14 transcript (Vu and Hoffman, 1997; Hsiao et al., 2019), that, in its full form, includes SNORD115 and UBE3A-ATS, which silences the paternal allele of UBE3A by transcription interference (Meng et al., 2012; Meng et al., 2013). As such, UBE3A expression in neurons is exclusively ensured by the maternal allele, which leads to AS when absent, not expressed, or mutated.
Loss-of-function of maternal UBE3A may result from four main (epi)genetic defects (Figure 2B), with varying degrees of disease manifestation and severity (reviewed in Maranga et al., 2020; Yang et al., 2021): megadeletions in the maternal chr15q11-q13 region—AS MD (60%–70%); deleterious mutations in the maternal UBE3A gene—AS Mut (10%); paternal uniparental disomy of chromosome 15—AS UPD (10%); imprinting defects on the maternal PWS-IC—AS ID (3%–5%). Some cases (<10%), despite having AS-like clinical diagnosis, are not attributed to any of the four known (epi)genetic causes and may arise from genetic abnormalities in other genes (Aguilera et al., 2021) or are misdiagnosed by another neurodevelopmental disorder with similar disease presentation (McKnight et al., 2022).
Significant heterogeneity in disease severity is observed across the different (epi)genetic origins of AS (Keute et al., 2021). AS MD presents the most severe manifestations, with stark development delays, as well as more frequent and grave seizures, when compared to the other causes (Gentile et al., 2010). Hypopigmentation is also a characteristic of individuals carrying MDs (Luk and Lo, 2016), likely associated with the haploinsufficiency of the OCA2 and GABRB3 genes (Delahanty et al., 2016) (Figure 2A). The vast majority of AS MD cases (95%) (Yang et al., 2021) falls under either class I (BP1-BP3, ∼6 Mb/16 genes) or class II (BP2-BP3, ∼5 Mb/12 genes) MDs (Figures 2A, B). In these cases, the absent chromosomal region includes UBE3A and several other imprinted and non-imprinted genes. While paternally imprinted genes remain unaffected, non-imprinted genes have half of their normal expression levels. Besides UBE3A, class I and II megadeletions cause the absence of the maternally inherited copies of three GABAA receptor subunit genes (GABRB3, GABRA5, and GABRG3), which are genes implicated in neuronal development, synaptic function, and epilepsy (Tang et al., 2021). Another gene absent in both megadeletions is HERC2. This gene encodes for a protein that interacts with UBE3A and is also involved in ubiquitination (Galligan et al., 2015). Defects on this gene cause an autosomal recessive AS-like syndrome named intellectual developmental disorder, autosomal recessive 38 (OMIM#615516) (Kühnle et al., 2011; Puffenberger et al., 2012; Cubillos-Rojas et al., 2016). Class I deletions have an additional deleted region (BP1-BP2) that encompasses four evolutionarily conserved genes (NIPA1, NIPA2, CYF1P1, and TUBGCP5) involved in brain development and function (Burnside et al., 2011; Vanlerberghe et al., 2015). The difference in deletion size suggests class I MD should result in the most severe phenotypes, with some evidence in favor (Valente et al., 2013). However, analyses show disagreement, with recent data suggesting only minor phenotypic differences between individuals carrying class I or II MDs, as measured by scales of development, in terms of cognitive ability, motor, social, and communication skills (Keute et al., 2021). Further comparative studies may be needed for a definitive answer on the potential differences between class I and II MDs.
Up to ten percent of AS cases are AS UPD that lack the maternal copy of chr15, having instead two paternal copies of this chromosome. This results in the complete silencing of UBE3A, and results in overexpression of paternally expressed imprinted genes, such as SNURF/SNRPN, IPW, SNORD115/116, and UBE3A-ATS (Figure 2B). These patients have milder disease manifestations compared to AS MD cases, with less prevalence and severity of seizures, but still have severe development delay and more pronounced sleep problems (Gentile et al., 2010; Yang et al., 2021). Interestingly, AS UPD individuals tend to present hyperphagia and a higher risk of obesity (Brennan et al., 2015).
In individuals with AS ID, the maternal copy of the chr15q11-q13 region suffers an epigenotype switch to become indistinguishable from the paternal copy (Figure 2B). In other words, the PWS-IC of both chromosomes lacks DNA methylation, thus resulting in expression from the PWS-IC region and silencing of not only the paternal but also the maternal copy of UBE3A. The ID arises from a failure to establish the maternal imprint at PWS-IC during female germline development, but the reasons for this failure are not always clear. In certain instances, imprinting defects on the PWS-IC arise from mutations and microdeletions at the AS-IC or PWS-IC affecting the ability to create the imprint in the maternal germline (Horsthemke and Buiting, 2006; Beygo et al., 2020). At the molecular level, AS ID and AS UPD share the same transcriptional profile at chr15q11-q13 (Figure 2B), with the most relevant difference being the fact that AS UPD are homozygous for all loci on chr15 when this is not the case for AS ID. At the clinical level, AS ID individuals share similar phenotypes and disease progression with individuals with AS UPD, often showing milder characteristic impairments (Gentile et al., 2010; Yang et al., 2021). Additionally, similarly to AS UPD individuals, AS ID individuals also tend to have hyperphagia and an increased risk of obesity (Brennan et al., 2015).
There is a wide range of reported adverse mutations in the maternal copy of the UBE3A gene that cause AS (AS Mut), most of which are nonsense mutations that lead to frameshifts and premature stop codons (Sadikovic et al., 2014). Besides mutations that result in the truncation of the maternal UBE3A transcript, loss of function of UBE3A may also result from missense mutations affecting active domains and protein stability (Beasley et al., 2020), intracellular localization (Bossuyt et al., 2021), and even gain of function (Weston et al., 2021), although the latter is often associated with Dup15q syndrome and not AS (Copping et al., 2017; Xing et al., 2023). AS individuals carrying UBE3A mutations often present the mildest phenotypes out of the four (epi)genetic causes, with less pronounced development delay (Gentile et al., 2010; Yang et al., 2021), as seen by clinical scales of development in infancy (Keute et al., 2021). Epileptic episodes are an exception, as these tend to be more severe for AS Mut than AS UPD or AS ID individuals (Luk and Lo, 2016; Yang et al., 2021).
Molecular diagnosis of AS is important not only to rule out other clinically similar diseases but also to understand the (epi)genetic cause underlying the clinical AS diagnosis, which could impact disease management. Given the distinct (epi)genetic causes of AS, more than one molecular diagnostic test is needed (Bird, 2014; Maranga et al., 2020; Yang et al., 2021). If an individual has clinical symptoms of AS, the first step is to evaluate the DNA methylation status at the PWS-IC locus, usually by methylation-specific PCR or methylation-specific multiplex ligation-dependent probe (MS-MPLA), the latter of which also detects large deletions in genomic DNA. If normal DNA methylation is detected, the UBE3A gene is sequenced to screen for potential pathogenic mutations (AS Mut), and if negative, AS-like syndromes should be investigated. Instead, if DNA methylation is anomalous, the second step is to determine whether or not the patient has a large deletion of the chr15q11-13 region, by MPLA, fluorescence in situ hybridization (FISH) for chr15q11-q13, or a comparative genomic hybridization array (array CGH). If a maternal MD is detected, the patient has AS MD. If instead, no megadeletion is present, microsatellites or nucleotide polymorphism markers for chr15 are used to assess if the patient has AS UPD (only paternal markers) or AS ID (maternal and paternal markers).
Pathophysiological mechanisms of Angelman syndrome
UBE3A, also known as E6-associated protein (E6AP), is a 100 kDa protein that tags proteins for proteasomal degradation or for acquiring novel properties through target-specific ubiquitination (Huibregtse et al., 1993; Avagliano Trezza et al., 2021) (Figure 3A). Ubiquitination is a type of post-translational modification mediated by a three-step enzyme cascade, E1-E2-E3 [reviewed in Damgaard (2021)], that adds the small peptide ubiquitin (Ub) to a target protein. The first enzyme (E1) is responsible for the activation of free ubiquitin, in an ATP-dependent manner, and then transfer it to the E2 enzyme. Finally, the E2-Ub complex mediates the transfer of Ub to the E3 enzymes, which then covalently links it to specific target proteins, mediated by the HECT (Homologous to the E6-AP Carboxyl Terminus) domain of E3. As the final mediators of the cascade, E3 ligases are the most specific of the three types of enzymes, having individualized targets, and are abundant in the human genome (Medvar et al., 2016), with UBE3A being one example. Although first identified as a marker for proteasomal degradation, ubiquitination is a cellular tool for the regulation of protein activity and is involved in many cellular processes including cell cycle control, apoptosis, signal transduction, intracellular traffic, DNA repair, and more (Damgaard, 2021; Mathieu et al., 2021).
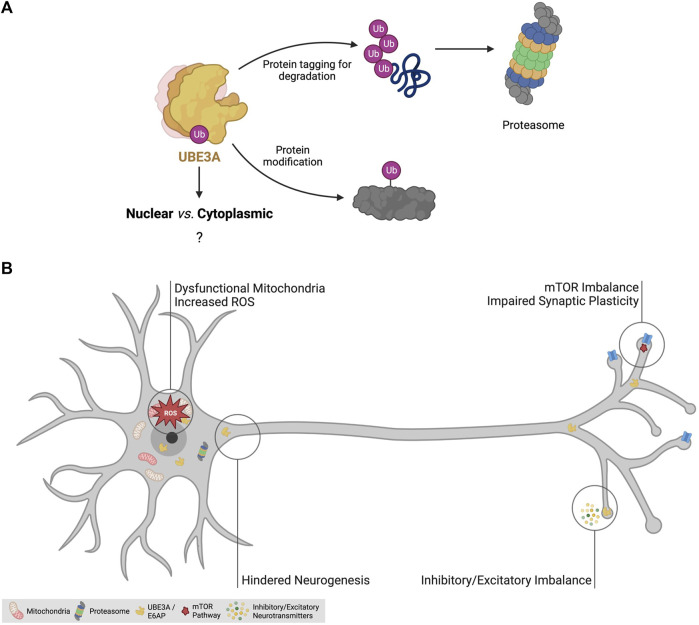
FIGURE 3.
The disease mechanisms of Angelman syndrome. (A) Roles of ubiquitin-protein ligase E3A (UBE3A). UBE3A is an E3 ligase responsible for the addition of ubiquitin to specific targets. Polyubiquitination is known to be a mechanism to tag proteins for proteasomal degradation. UBE3A is also known to mediate monoubiquitination to modulate the protein activity of certain targets. The function of its three isoforms and the role of nuclear and cytoplasmic UBE3A remain elusive. (B) Identified dysregulated cellular processes in AS models. AS mouse models have been crucial to unravel pathophysiological mechanisms of the disease and have allowed the identification of signaling pathway imbalance, dysfunctional mitochondria, increased oxidative stress, impairments in neurogenesis and synaptic plasticity, and inhibitory/excitatory imbalance as features of the AS brain, likely contributing to the clinical manifestations of the disease. Abbreviations: Ub - ubiquitination; ROS—reactive oxygen species; mTOR—mechanistic target of rapamycin pathway. Created with Biorender.com.
Given the pivotal role of UBE3A loss-of-function in AS, its downstream targets may be relevant to uncover pathophysiological mechanisms and potential therapeutic targets for the disease. Examples of these ubiquitination targets include tumor suppressor p53 (Huibregtse et al., 1993), synaptic regulator ARC (Greer et al., 2010), small-conductance potassium channel 2 (SK2) (Sun and Zhu, 2015), voltage-dependent big potassium (BK) (Sun et al., 2019), and RPH3A (Avagliano Trezza et al., 2021). However, some targets may not actually be direct targets of UBE3A. Such is the example of synaptic regulator ARC, which was initially reported as a ubiquitination target of UBE3A (Greer et al., 2010), but more recent data suggest it is not a UBE3A target (Pastuzyn and Shepherd, 2017). As such, rigorous and controlled ubiquitination experiments should be performed to confirm a potential candidate as a ubiquitination target of UBE3A.
UBE3A has three known isoforms, with isoform 1 being the most abundant, and all three can be localized in both the cytoplasm and the nucleus in human ESCs and ESC-derived neurons (Sirois et al., 2020). This contrasts with the situation in the mouse where isoforms with clear nuclear and cytoplasmic localization have been found (Avagliano Trezza et al., 2019). Interestingly, KO mice for the nuclear isoform of Ube3a showed severe behavior impairments and synaptic defects, while KO mice for the cytoplasmic isoform were asymptomatic (Avagliano Trezza et al., 2019). As such, these results suggest loss of nuclear activity of UBE3A may be critical in AS (Sirois et al., 2020; Bossuyt et al., 2021), but its function remains elusive.
Over the decades, many studies have identified disrupted molecular pathways and mechanisms in the hippocampus, cortex, striatum (Rotaru et al., 2023), and cerebellum of AS models [reviewed in Maranga et al. (2020)] (Figure 3B). The dysregulation of the mechanistic target of rapamycin (mTOR) pathway in AS mice has been widely reported (Sun and Liu, 2015; Sun et al., 2016; Pastuzyn and Shepherd, 2017). The mTOR pathway, comprising the two complex families mTORC1 and mTORC2, is implicated in a plethora of cellular processes such as cell growth, lipid synthesis, mitochondria biogenesis, and apoptosis. This pathway is important for neuronal activity as it regulates autophagy, lysosome biogenesis, and actin dynamics (Costa-Mattioli and Monteggia, 2013; Liu and Sabatini, 2020). In the brain of AS mice, an imbalance of the mTOR pathway, expressed in the form of increased mTORC1 activity and decreased mTORC2 activity, leads to increased levels of the ARC protein and impaired actin remodeling (Sun and Liu, 2015; Sun et al., 2016; Pastuzyn and Shepherd, 2017), potentially contributing to the cognitive and behavioral impairments observed in AS.
Mitochondrial dysfunction and increased oxidative stress have been described as hallmarks of AS animal models (Su et al., 2011; Llewellyn et al., 2015; Santini et al., 2015; Berkowitz et al., 2017). In fact, impairments in oxidative phosphorylation and increased levels of reactive oxygen species (ROS) are associated with several neurodevelopmental and neurodegenerative disorders, appearing as a common theme in brain diseases (Keating, 2008; Norat et al., 2020; Anitha et al., 2023). In AS models, increased levels of ROS have been linked to compromised hippocampal synaptic function (Su et al., 2011; Santini et al., 2015). They affect neurodevelopment by causing mitochondrial malfunctioning in neural precursor cells leading to excessive ROS and increased apoptosis (Simchi et al., 2023). However, how UBE3A is influencing mitochondrial redox homeostasis is still unclear and might prove insightful to better understand the pathophysiology of AS and other neurodevelopmental diseases.
Epilepsy is another consistent feature of AS and it is hypothesized to result from dysfunctional GABAergic circuitry (Gu et al., 2018), likely suggesting a distinctive imbalance between inhibitory and excitatory signals (Yashiro et al., 2009; Wallace et al., 2012; Fink et al., 2017; Rotaru et al., 2018). Deficits in inhibitory and excitatory neuronal circuits have been reported in the AS mouse brain (Egawa et al., 2012; Wallace et al., 2012; Rotaru et al., 2018), suggested to be a consequence of impaired synaptic plasticity and dendritic spine formation. Coupled with this, long-term potentiation (LTP), an example of synaptic plasticity associated with learning and memory, has been shown to be impaired in AS mice (Kaphzan et al., 2011; Sun and Liu, 2015). In short, over the years, many studies have pointed out several impaired cellular mechanisms and neuronal functions, including synaptic plasticity, mitochondrial dysfunction, increased oxidative stress, and excitatory/inhibitory imbalance, that overall contribute to the pathophysiology of AS (Maranga et al., 2020) (Figure 3B).
Pluripotent stem cell models of Angelman syndrome
Animal models of AS have been pivotal for the understanding of disease mechanisms and as preclinical models to advance new therapeutics [reviewed in Maranga et al. (2020)]. However, they have several limitations when used to model human diseases (Kelley and Pașca, 2022). First, they exhibit significant biological differences with humans and may not reflect accurately the disease phenotypes and/or differ in their responses to therapeutic agents. Second, mouse models do not capture the genetic and phenotypic heterogeneity seen in the human population. This is well illustrated in the case of AS where different molecular causes give rise to symptoms of diverse severity. The most commonly used AS animal models represent the loss of function of Ube3a alone (Jiang et al., 1998; Miura et al., 2002; Berg et al., 2020; Dodge et al., 2020). However, the majority of AS individuals have MDs associated with the loss of UBE3A plus haploinsufficiency of dozens of genes. This, in part, could also explain the milder phenotypes observed in these animal models when compared to AS individuals. Third, the use of animals for experimentation raises ethical concerns due to the potential harm inflicted on them. For these reasons, alternative research models that can complement or replace the use of animals are very welcomed. Human ESCs and iPSCs represent potent tools for bridging gaps in existing animal-based disease models, yielding supplementary insights into human biology by exploring human neurodevelopment in vitro. Nevertheless, stem cell models also raise ethical considerations, notably stemming from the embryonic provenance of hESCs and the potential for misuse of human PSCs, and they do not supplant the indispensable role of animal models in behavior assessment.
In the last decade, a great effort has been made to generate PSC models of AS. Apart from the derivation of iPSCs from AS patients, advances are being made in the genetic editing of UBE3A in iPSCs or ESCs (Tables 1, 2). Stem cell models have also been engineered to study the molecular mechanisms regulating imprinting at the PWS/AS cluster (Hsiao et al., 2019). The first stem models of AS were iPSCs derived from skin fibroblasts of male and female individuals with AS MD (Chamberlain et al., 2010). In the past decade, many other AS iPSC lines were derived from different somatic origins (fibroblasts, peripheral blood mononuclear cells, or B lymphocytes) and encompassing the four main molecular causes of the disease (Table 1). Initially, these iPSCs were generated using retroviral/lentiviral vectors that integrated the Yamanaka factors or analogs into the genome (Chamberlain et al., 2010; Stanurova et al., 2016; Fink et al., 2017; Pólvora-Brandão et al., 2018). More recently, iPSC models have been generated using non-integrative methods such as Sendai viruses or episomal vectors (Takahashi et al., 2017; Neureiter et al., 2018; Niki et al., 2019; Li et al., 2022; Maranga et al., 2022).
TABLE 1.
Angelman syndrome individual-derived induced pluripotent stem cell lines (iPSCs). The table features the reference article where each iPSC line was generated, the biological sex of the individual with Angelman syndrome (AS) from which the line was derived, cell donor source used for reprogramming, details on the molecular causes of AS and the method used for reprogramming. n.s. Means not specified; * information of the exact mutation could not be retrieved; ** information on whether the megadeletion (MD) was class I or II or other could not be found; Abbreviations: MD - megadeletion; OSKM - short for the Yamanaka cocktail composed of OCT4, SOX2, KLF4, and c-MYC); Mut - mutation; UPD - uniparental disomy; ID - imprinting defect.
| References | Biological sex | Cell donor source | The molecular cause of AS | Method of reprogramming |
|---|---|---|---|---|
| Chamberlain et al. (2010) | Male and Female | Fibroblasts | Class II MD of the maternal chr15q11-q13 | Retroviral vectors for OSKM and LIN28 |
| Stanurova et al. (2016) | Female | Fibroblasts | UBE3A Mut: in-frame 3bp deletion (p.G538del, c.1613-1615delGAG) | Lentiviral excisable vector for OSKM |
| Fink et al. (2017) | Male | n.s. | UBE3A frameshift mutation (2 bp deletion)* | Retroviral and lentiviral vectors for OSKM and LIN28 |
| Takahashi et al. (2017) | Male | B lymphocytes | Paternal UPD for chr15 | Episomal vectors for OSKM |
| Pólvora-Brandão et al. (2018) | Female | Fibroblasts | Class II MD of the maternal chr15q11-q13 | Lentiviral vector for OSKM |
| Neureiter et al. (2018) | Female | Fibroblasts | ID at the PWS-IC | Episomal vectors for OSKM, LIN28, and a shRNA against p53 |
| Niki et al. (2019) | Female | PBMCs | MD of chr15q11.2–q13** | Episomal vectors for OSKM, LIN28, EBNA1 and p53 carboxy-terminal dominant-negative fragment |
| Maranga et al. (2022) | Female | Fibroblasts | Class II MD of the maternal chr15q11-q13 | Sendai virus for OSKM |
| Li et al. (2022) | Female | PBMCs | UBE3A Mut: missense mutation (p.Asp563GLy, c.1688 A > G) | Episomal vectors for pCE-hOCT3/4, pCE-hSK, pCE-hUL, pCE-mP53DD and pCXB-EBNA1 |
TABLE 2.
UBE3A Knock-Out (KO) stem cell models. The table displays the information on the reference article, cell line, gene editing approach, and genetic modification for each UBE3A Knock-Out (KO) stem cell model. Abbreviations: iPSC - induced pluripotent stem cell; CRISPR/Cas9 - clustered regularly interspaced short palindromic repeats/CRISPR-associated protein 9; KO - Knock-Out; TSS - transcription start site; ESC - embryonic stem cell; ssODN - single-stranded oligodeoxynucleotides; Iso1 - isoform 1; Iso2 - isoform 2; Iso3 -isoform 3. * The reference of the original non-AS iPSC could not be retrieved.
| References | Cell line | Gene editing approach | Genetic modification |
|---|---|---|---|
| Fink et al. (2017) | iPSC* | CRISPR/Cas9 | UBE3A KO (1 bp G) insertion at the TSS) |
| Sun et al. (2019) | H1 and H9 ESCs | CRISPR/Cas9 | UBE3A KO (5bp deletion on exon 6 causing a frameshift and early translational termination) |
| Sirois et al. (2020) | H9 ESC | CRISPR/Cas9 in the presence of ssODN | UBE3A Iso1 KO, Iso2 KO, and Iso3 KO (by disruption of each TSS) |
Besides iPSC models, genetically edited stem cell lines targeting the UBE3A gene have also been engineered (Table 2). A UBE3A KO cell line was generated from a male iPSC line through the insertion of 1 bp at the translational start site of the UBE3A protein isoform 1 in both maternal and paternal alleles (Fink et al., 2017). More recently, two ESC lines, the male H1, and the female H9, were edited at the UBE3A locus using a single guide RNA targeting exon 6 that led to a 5 base pair deletion in exon 6 of this gene, causing a frameshift and early translational termination in the best characterized H9 clone (Sun et al., 2019). Furthermore, isogenic H9 ESCs that specifically lack one of the three individual UBE3A protein isoforms were generated through mutation of their independent translational start sites using CRISPR/Cas9 and single-stranded oligodeoxynucleotides (ssODN) templates to discern the relative contribution of each isoform for the building up of the AS phenotype (Sirois et al., 2020). These CRISPR/Cas9-engineered ESC models targeting UBE3A as a whole or its individual isoforms are powerful models for studying the localization and function of UBE3A (Sirois et al., 2020). However, they lack UBE3A expression already in the stem cell state and do not recapitulate the developmental path leading to neuronal-specific loss of UBE3A expression due to imprinting, reproduced in iPSC models derived from AS individuals (Chamberlain et al., 2010; Stanurova et al., 2016). Therefore, this should be considered when comparing the results acquired using non-edited iPSCs versus UBE3A-edited iPSC/ESC models.
With the increased use of stem cell models, researchers have noticed that reprogramming and long-term in vitro culture lead to the accumulation of genetic and epigenetic defects (Bar et al., 2017; Halliwell et al., 2020; Bansal et al., 2021). This includes imprinting defects which occur mainly during the process of iPSC reprogramming (Nazor et al., 2012; Ma et al., 2014; Bar et al., 2017; Arez et al., 2022). This is particularly concerning when using stem cell models, especially iPSCs, to model imprinting disorders such as AS. Fortunately, methylation profiles at PWS-IC or SNURF TSS-DMR, are not prone to reprogramming-induced errors in contrast to other imprinted loci such as PEG3, IGF2-H19, and DLK1-DIO3 regions (Nazor et al., 2012; Ma et al., 2014; Bar et al., 2017; Klobučar et al., 2020). Indeed, all published AS iPSC lines have been confirmed to preserve the original methylation pattern at PWS-IC following iPSC reprogramming (Table 1) and recapitulate neuron-specific imprinting of UBE3A upon neuronal differentiation (Chamberlain et al., 2010; Stanurova et al., 2016). Nonetheless, Pólvora-Brandão et al. (2018) have reported a loss of maternal methylation at the PWS-IC in one out of five non-AS iPSC clones, originating a cell line mimicking the imprinting defect typical of AS ID iPSCs (Pólvora-Brandão et al., 2018). Validation of the correct methylation pattern at the PWS-IC is therefore mandatory to ensure that imprinting has not been lost in PSC models of AS and their controls.
Although a reasonable number of stem cell lines covering the major causes of the disease have been created to model AS, these cellular models have their own limitations. First, their number can be considered low, especially for AS UPD and ID cell lines, with only one of each having been generated (Takahashi et al., 2017; Neureiter et al., 2018). Second, by coincidence, there is a sex bias trend with fewer male than female AS iPSC lines (Table 1). Third, most of these lack appropriate controls such as CRISPR/Cas9 gene-corrected clones or familiar controls. Gene-corrected isogenic controls are ideal but in the context of AS are almost only applicable for UBE3A Mut cases. AS MD and AS IC could, in theory, also be recreated using non-AS iPSCs/ESCs through genetic or epigenetic editing (Qian et al., 2023; Zhou et al., 2023), respectively. This is not an option for AS UPD cases, where the genetic error (inheritance of two paternal chr15s) cannot be rescued with current editing techniques. In this case, the best available option to act as a control iPSC would be from non-affected parents or siblings. All in all, these points highlight the importance of enlarging the current portfolio of stem cell lines available for AS research, with an unbiased representation of both biological sexes and with appropriate genetically matched controls. These will guarantee the accuracy and reproducibility of the results gathered using AS stem cell models.
Advances in Angelman syndrome research using human pluripotent stem cells
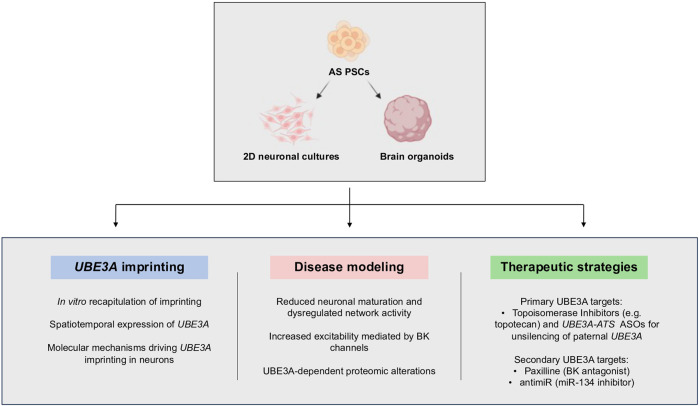
Stem-cell-based research on AS is still in its infancy but has already led to innovative studies that increased our knowledge about this syndrome. To model AS, iPSCs/ESCs are usually submitted to neuronal differentiation either as a simple monolayer culture or more complex self-organized organ-like structures known as brain organoids (Pașca et al., 2022). Organoids have been defined as “in vitro-generated cellular systems that emerge by self-organization, include multiple cell types, and exhibit some cytoarchitectural and functional features reminiscent of an organ or organ region” (Pașca et al., 2022). Several protocols, relying mostly on intrinsic factors and spontaneous differentiation (unguided) or with controlled addition of external factors to direct the differentiation process (guided) can be followed to generate whole or region-specific brain organoids. Both 2D and 3D differentiation protocols resort to specific media and supplements/specific pathways inhibitors, to promote the development of neural progenitors and at later stages of differentiation, mature neurons and astrocytes aiming to recreate in vitro the developmental path of brain regions [reviewed in Paşca (2019)]. In particular, brain organoids have helped to overcome one of the biggest challenges associated with investigating neurological disorders, the difficulty of studying the human brain. While post-mortem samples and medical imaging techniques were and still are a source of information, they can only monitor disease progression and cannot track pathological processes at the cellular level (Eichmüller and Knoblich, 2022). A great part of AS research has also used mouse models to study the disease, although there are limitations here as well. The mouse brain differs from the human in key aspects such as size, architecture, and gyrification of the cortex. Furthermore, the regulation of some conserved pathways is different, leading to morphological, architectural, and connectivity differences between species (Eichmüller and Knoblich, 2022). Brain organoids fill the gap between these different models, allowing the study of neurodevelopmental disorders from early stages, at cellular and molecular levels, using human cells. In the case of AS, stem cell models have been used in three main contexts: 1) to understand the process of imprinting regulation at the chr15q11-q13; 2) as a disease model to find molecular/functional signatures of the disease; 3) for preclinical development of existing and novel therapeutic agents for future treatment for AS (Figure 4).
FIGURE 4.
Applications of human pluripotent stem cells (PSCs) in Angelman syndrome research. Neuronal differentiation of iPSCs in 2D neuronal cultures and brain organoids has allowed the study of UBE3A imprinting in vitro, to discover neuronal phenotypes of the disease, and evaluate the potential of several therapeutic strategies. Abbreviations: BK—big potassium channels; ASOs—antisense oligonucleotides. PSCs—pluripotent stem cells. Created with Biorender.com.
Several studies have been conducted in stem cells to unveil when and how UBE3A imprinting arises during in vitro differentiation. On one hand, these cell models provide an accessible system to study imprinting establishment. On the other hand, UBE3A imprinting is a prerequisite to validate their applicability for studying AS. UBE3A imprinting has been confirmed to occur during in vitro differentiation of AS iPSCs in both 2D cultures and organoids (Chamberlain et al., 2010; Stanurova et al., 2016; Sen et al., 2020). This has been perceived by the downregulation of UBE3A RNA and protein levels with a concomitant upregulation of UBE3A-ATS when the first neurons emerge in the culture. Formal proof of loss of the paternal UBE3A allele was obtained by Stanurova et al. (2016), who determined allelic ratios between the maternal and paternal alleles thanks to genetic variation caused by a missense 3 bp deletion in UBE3A gene in an AS Mut iPSC line. These studies pointed to a correlation between UBE3A-ATS expression and paternal UBE3A silencing and proved that iPSC-derived neuronal differentiation recapitulates UBE3A imprinting and can be an adequate model to study AS.
The group of S. Chamberlain has pioneered the use of engineered stem cell models to study the molecular mechanisms regulating imprinting at the chr15q11-q13 region (Chamberlain et al., 2010; Martins-Taylor et al., 2014; Hsiao et al., 2019). In one of their studies, they investigated the reasons explaining why UBE3A imprinting is restricted to neurons. Through a series of CRISPR/Cas9-induced deletions and inversions in iPSCs, they identified a boundary element around IPW and PWAR1 genes that is responsible for terminating SNHG14 transcription in non-neuronal cells. Ablation of such a genomic element enables transcription to extend beyond this boundary and leads to an earlier onset of paternal UBE3A silencing during neuronal differentiation (Hsiao et al., 2019). This study illustrates the capacity of genetic manipulation of stem cells to gain mechanistic insights into the regulation of neuronal-specific imprinting expression of UBE3A.
Neuronal differentiation of both iPSC and ESC has provided important insights into the spatiotemporal expression pattern of UBE3A (Sen et al., 2020), as well as on differences in location and neuronal function of several human UBE3A isoforms (Sirois et al., 2020). In H9 ESC-derived whole-brain organoids, nuclear UBE3A increases with the progress of differentiation, with a decreasing ratio of cytoplasmic to nuclear UBE3A. The protein appeared to be predominantly nuclear in neurons after only 3 weeks in culture, with this location increasing over time (Sen et al., 2020), matching previous results in mice (Judson et al., 2014). In another study, monitoring isogenic mutated ESC lines throughout neuronal differentiation further revealed that UBE3A isoform 1 predominates in both undifferentiated cells and neurons (Sirois et al., 2020). Loss of isoform 1 led to a significant reduction in total and cytoplasmic UBE3A levels (but not nuclear), while loss of isoforms 2 and 3 did not induce significant alterations in UBE3A levels. By Western blot, UBE3A was found to localize predominantly in the cytoplasm, in both ESC and neurons in this study. However, by immunofluorescence, the strongest UBE3A signal in neurons appears to be in the nucleus, although there was also a signal detected in neurites and the soma, outside the nucleus (Sirois et al., 2020). Future experiments are needed to gain insights into these apparently contradictory findings.
Elegant experiments using stem cell models of AS uncovered novel molecular/functional signatures of the disease. In contrast to the absence of significant morphological and functional changes at early differentiation stages, both in 2D (Fink et al., 2017) and 3D models (Sun et al., 2019), important alterations were observed at later time points, coinciding with the appearance of functionally mature neurons in culture (Fink et al., 2017; Sun et al., 2019). The first study that extensively characterized AS phenotype in long-term neuronal cultures (over 20 weeks) was developed by Fink et al. (2017), using AS iPSC-derived forebrain neurons. The authors used three AS-iPSC lines (two AS MD and one AS Mut), and an engineered UBE3A KO iPSC line. AS and control cultures presented similar cell composition throughout differentiation, with no significant differences between the proportion of glutamatergic and GABAergic neurons, astrocytes, or between upper and deep cortical layer markers. Nonetheless, alterations in neuronal excitability, functionality, and synaptic plasticity were detected, in line with previous research in mice models (Mardirossian et al., 2009; Kaphzan et al., 2011; Campbell et al., 2022; O’Geen et al., 2023). These effects included more depolarized resting membrane potentials (RMPs), a lower proportion of cells firing action potential (AP) spike trains, more immature APs, and fewer calcium transients. These effects were also proven to be UBE3A-dependent, as they were observed in UBE3A KO lines or when control iPSCs were treated with UBE3A-targeting ASOs to knockdown UBE3A expression (Fink et al., 2017). More precisely, isoform 1 is thought to be the most related to the observed neuronal phenotypes, as a KO line showed similar RMP depolarization (Sirois et al., 2020). Overall, these results point to a reduced developmental maturation, as well as dysregulated network activity and excitability features in AS, and prove the usefulness of AS stem cell models to find functional phenotypes and readouts of the disease.
Sun et al. (2019) developed the first study where two pairs of UBE3A KO hESCs and an AS MD iPSC line were differentiated not only in 2D but also in 3D, into cortical organoids. They uncovered a channelopathy mediated by an increase in BK channel density leading to increased neuronal excitability. This increase was due to the lack of UBE3A-mediated ubiquitination and proteasomal degradation of BK channels, as confirmed by in vitro and in vivo ubiquitination assays. Therefore, BK channels are a substrate of UBE3A and a putative therapeutic target of AS. All in all, this study has contributed to a better understanding of the mechanisms underlying network hyperactivity and epilepsy susceptibility in AS patients (Sun et al., 2019).
In another study, Pandya et al. (2021) took advantage of AS iPSC-derived neurons to perform a proteomic study and identify proteins whose abundance was responsive to changes in UBE3A levels. After an initial comparison between AS and control iPSC-derived neurons, the authors modulated the expression of UBE3A using ASOs against UBE3A (to reduce expression) or against UBE3-ATS (to increase expression) and repeated their proteomic workflow. A range from 70 to 225 proteins were identified with the main outcome being the discovery of the secreted retrovirus-like GAG-domain-containing protein PEG10 and associated proteins as being upregulated in AS neurons (Pandya et al., 2021). This was also confirmed in post-mortem brain samples of AS individuals. Curiously, this was not observed in mice, which could suggest a relevant mechanistic difference between AS humans and AS mouse models. Although PEG10 was found not to be a ubiquitin target of UBE3A, it was still targeted to the proteasome in a UBE3A-dependent manner. Interestingly, PEG10 downregulation in AS iPSC-derived neurons results in a transcriptomic response similar to what happens upon UBE3A reinstatement, suggesting that PEG10 could contribute to the pathophysiology of AS (Pandya et al., 2021). Interestingly, PEG10, also encoded by a paternally imprinted gene, is recruited to stress granules where it interacts with several RNAs and is secreted in extracellular vesicles. How this could be related to its putative role in AS pathogenesis still needs to be further explored. As a secreted protein, PEG10 is also a promising biomarker to consider for AS therapeutics currently advancing in clinical trials.
Stem cell models of AS are also becoming key for the testing and development of potential new therapeutic strategies. They have been used for either 1) testing inhibitors of UBE3A targets or 2) using molecules targeting the molecular cause of the disease. To exemplify the first case, Sun et al. (2019) tested paxilline, a BK blocker, after they discovered that these channels were increased in AS iPSC-derived neurons. They showed that paxilline was able to revert altered excitability and abnormal AP firing in both AS 2D neuronal cultures and cortical organoids. This finding was further supported by the amelioration of seizure threshold and susceptibility when administered in a mouse model of AS (Sun et al., 2019). These results position BK channels as promising therapeutic targets for the treatment of seizures in AS individuals (Sun et al., 2019). Another interesting study explored the idea that UBE3A mRNA might function as a sponge for the microRNA miR-134 (Campbell et al., 2022). An antimiR oligonucleotide inhibitor of miR-134 was shown to upregulate its targets in neurons differentiated from AS MD iPSCs and ameliorate AS phenotypes in UBE3A mat-/pat+ mice upon intracerebroventricular injection. These findings give the prospect that microRNA modulation could be beneficial in treating some clinically relevant symptoms affecting AS individuals.
The most widely investigated therapeutic option is the reinstatement of UBE3A through unsilencing of the paternal copy of the gene. This is achieved by disrupting the UBE3A-ATS RNA and can be achieved in multiple ways (Huang et al., 2012; Meng et al., 2015; Wolter et al., 2020; Schmid et al., 2021; Dindot et al., 2023; Li et al., 2023; O’Geen et al., 2023). One such way uses topoisomerase inhibitors, such as topotecan or indotecan (Huang et al., 2012; Lee et al., 2018). Administration of 1 µM topotecan to in vitro iPSC-derived neuronal cultures led to a ∼50% increase in UBE3A mRNA expression and rescued abnormal AP firing, RMP depolarization, and synaptic frequency (Fink et al., 2017). Administration of 1 µM topotecan or indotecan to AS cerebral organoids had similar effects, resulting in a knockdown of UBE3A-ATS and increased UBE3A in neurons, with a single dose of indotecan being able to persistently rescue UBE3A for 10-to-17 days after exposure. As a result, calcium transient phenotypes in AS organoids were also reverted (Sen et al., 2020). The problem with topoisomerase inhibitors is that they disrupt the full transcriptional unit that contains SNURF/SNRPN and snoRNA genes which are involved in PWS, besides causing the downregulation of several long RNA transcripts (Huang et al., 2012; King et al., 2013; Tan and Bird, 2016; Lee et al., 2018). A preferential strategy will hit specifically the UBE3A-ATS gene only.
Targeting the UBE3A-ATS specifically using modified ASOs was found to successfully unsilence paternal UBE3A without disrupting the expression of the other transcripts implicated in PWS and rescue several disease phenotypes in the AS mouse model (Meng et al., 2015). This proof-of-principle experiment showed the unprecedented ability of this approach to target the molecular cause of AS. However, the efficient ASOs found in mice hold no homology in the human genome, therefore, human sequence-specific ASOs needed to be tested in human cells. The optimal cellular system for this purpose would be human ESC/iPSC-derived neurons. An additional advantage is that iPSCs can be derived from individuals with different molecular causes of the disease, enabling the monitoring of ASO efficacy in distinct (epi)genetic backgrounds of the disease. An earlier publication showed that human-specific ASOs were able to downregulate UBE3A-ATS and activate UBE3A in iPSC-derived neurons (Pandya et al., 2021). More recently, Dindot et al. (2023) have perfected ASO chemistry to target an evolutionary conserved region at the start of UBE3A-ATS to efficiently repress UBE3-ATS transcription and reactivate paternal UBE3A. After this screening study in iPSC-derived neurons, the best ASO candidates were administered by lumbar intrathecal injections in cynomolgus monkeys, and promising results were obtained with minor adverse effects (Dindot et al., 2023). These findings drove the first molecular therapy for AS to go into clinical development in phase I/II (ClinicalTrials.gov, NCT04259281). A similar trial from Roche using the same technology also reached phase I clinical trials (ClinicalTrials.gov, NCT04428281). This is illustrative of the power of stem cell-based research to study diseases such as AS.
Conclusion remarks and future perspectives
In this article, we provide a comprehensive overview of stem cell-based research in AS and contextualize it within the historical advancements made over the past ∼50/60 years in understanding this disease. Although the use of human stem cell models of AS is relatively recent, it already provided significant scientific progress in uncovering novel pathophysiological aspects of the disorder, as well as in identifying new proteins affected by UBE3A that could be targets for future therapeutic interventions. Additionally, stem cell models are serving as valuable preclinical tools for evaluating human sequence-specific genetic therapies such as ASOs or CRISPR-based gene editing. In the forthcoming years, the AS stem cell model portfolio will likely expand, hopefully encompassing the full spectrum of (epi)genetic variability observed in this condition. Current concerns in the epigenetic and genetic fidelity of human stem cells are being tackled (Pham et al., 2022) and hopefully will be solved in the near future. Also, there are grand expectations for further development of stem cell-based disease modeling with the potential to revolutionize biomedical research, drug development, and, ultimately, patient care. More complex multi-organ models such as assembloids or organ-on-chip systems will provide a more comprehensive understanding of disease pathogenesis, and also a better prediction of drug responses in the human body (Zhang et al., 2018; Miura et al., 2022; Tenreiro et al., 2023). Improvement of long-term organoid culture by optimizing dynamic culture methods and incorporating supportive cell types and biomaterials would also aid in the study of disease pathology providing a deeper insight into the mechanisms underlying disease development (Giandomenico et al., 2021). Advances are also being made in the automation and miniaturization of stem cell-based assays to improve high-throughput screening and accelerate drug discovery processes (Brandenberg et al., 2020). The constant refinement in gene editing technologies (Anzalone et al., 2020) as well as the production of single-cell and spatial transcriptomics data (Vandereyken et al., 2023) would also produce novel cellular models and datasets that would accelerate our collective understanding of human diseases, such as AS. In conclusion, stem cell-based models hold immense promise to revolutionize the lives of AS patients by paving the way toward transformative treatment addressing the root causes of the disease, and offering hope for improved cognitive, motor, and behavioral outcomes.
Funding Statement
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was funded by the Angelman Syndrome Alliance (Research Grant 2022). STdR is supported by an assistant research contract 2021.00660.CEECIND from Fundação para Ciência e Tecnologia/Ministério da Ciência, Tecnologia e Ensino Superior (FCT/MCTES). This work is also financed by national funds from FCT-Fundação para a Ciência e a Tecnologia, I.P., in the scope of the project UIDB/04565/2020 of the Research Unit Institute for Bioengineering and Biosciences-iBB and the project LA/P/0140/2020 of the Associate Laboratory Institute for Health and Bioeconomy-i4HB.
Author contributions
JC: Conceptualization, Writing–original draft. CA: Conceptualization, Writing–original draft. FC: Conceptualization, Writing–original draft. RC: Conceptualization, Writing–original draft. EB: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing–review and editing. SR: Conceptualization, Funding acquisition, Resources, Supervision, Writing–review and editing, Project administration.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Aguilera C., Gabau E., Ramirez-Mallafré A., Brun-Gasca C., Dominguez-Carral J., Delgadillo V., et al. (2021). New genes involved in Angelman syndrome-like: expanding the genetic spectrum. PLOS ONE 16 (10), e0258766. 10.1371/journal.pone.0258766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht U., Sutcliffe J. S., Cattanach B. M., Beechey C. V., Armstrong D., Eichele G., et al. (1997). Imprinted expression of the murine Angelman syndrome gene, Ube3a, in hippocampal and Purkinje neurons. Nat. Genet. 17 (1), 75–78. 10.1038/ng0997-75 [DOI] [PubMed] [Google Scholar]
- Angelman H. (1965). Puppet children A report on three cases. Dev. Med. Child Neurology 7 (6), 681–688. 10.1111/j.1469-8749.1965.tb07844.x [DOI] [PubMed] [Google Scholar]
- Anitha A., Thanseem I., Iype M., Thomas S. V. (2023). Mitochondrial dysfunction in cognitive neurodevelopmental disorders: cause or effect? Mitochondrion 69, 18–32. 10.1016/j.mito.2023.01.002 [DOI] [PubMed] [Google Scholar]
- Anzalone A. V., Koblan L. W., Liu D. R. (2020). Genome editing with CRISPR–Cas nucleases, base editors, transposases and prime editors. Nat. Biotechnol. 38 (7), 824–844. 10.1038/s41587-020-0561-9 [DOI] [PubMed] [Google Scholar]
- Arez M., Eckersley-Maslin M., Klobučar T., von Gilsa Lopes J., Krueger F., Mupo A., et al. (2022). Imprinting fidelity in mouse iPSCs depends on sex of donor cell and medium formulation. Nat. Commun. 13 (1), 5432. 10.1038/s41467-022-33013-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avagliano Trezza R., Punt A. M., Mientjes E., van den Berg M., Zampeta F. I., de Graaf I. J., et al. (2021). Mono-ubiquitination of Rabphilin 3A by UBE3A serves a non-degradative function. Sci. Rep. 11 (1), 3007. 10.1038/s41598-021-82319-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avagliano Trezza R., Sonzogni M., Bossuyt S. N. V., Zampeta F. I., Punt A. M., van den Berg M., et al. (2019). Loss of nuclear UBE3A causes electrophysiological and behavioral deficits in mice and is associated with Angelman syndrome. Nat. Neurosci. 22 (8), 1235–1247. 10.1038/s41593-019-0425-0 [DOI] [PubMed] [Google Scholar]
- Bansal P., Ahern D. T., Kondaveeti Y., Qiu C. W., Pinter S. F. (2021). Contiguous erosion of the inactive X in human pluripotency concludes with global DNA hypomethylation. Cell. Rep. 35 (10), 109215. 10.1016/j.celrep.2021.109215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar S., Schachter M., Eldar-Geva T., Benvenisty N. (2017). Large-Scale analysis of loss of imprinting in human pluripotent stem cells. Cell. Rep. 19 (5), 957–968. 10.1016/j.celrep.2017.04.020 [DOI] [PubMed] [Google Scholar]
- Beasley S. A., Kellum C. E., Orlomoski R. J., Idrizi F., Spratt D. E. (2020). An Angelman syndrome substitution in the HECT E3 ubiquitin ligase C-terminal Lobe of E6AP affects protein stability and activity. PLOS ONE 15 (7), e0235925. 10.1371/journal.pone.0235925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg E. L., Pride M. C., Petkova S. P., Lee R. D., Copping N. A., Shen Y., et al. (2020). Translational outcomes in a full gene deletion of ubiquitin protein ligase E3A rat model of Angelman syndrome. Transl. Psychiatry 10 (1), 39. 10.1038/s41398-020-0720-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg J. M., Pakula Z. (1972). Angelman’s (“Happy puppet”) syndrome. Archives Pediatr. Adolesc. Med. 123 (1), 72–74. 10.1001/archpedi.1972.02110070122020 [DOI] [PubMed] [Google Scholar]
- Berkowitz B. A., Lenning J., Khetarpal N., Tran C., Wu J. Y., Berri A. M., et al. (2017). In vivo imaging of prodromal hippocampus CA1 subfield oxidative stress in models of Alzheimer disease and Angelman syndrome. FASEB J. 31 (9), 4179–4186. 10.1096/fj.201700229R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beygo J., Grosser C., Kaya S., Mertel C., Buiting K., Horsthemke B. (2020). Common genetic variation in the Angelman syndrome imprinting centre affects the imprinting of chromosome 15. Eur. J. Hum. Genet. 28 (6), 835–839. 10.1038/s41431-020-0595-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird L. (2014). Angelman syndrome: review of clinical and molecular aspects. Appl. Clin. Genet. 7, 93–104. 10.2147/TACG.S57386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Born H. A., Martinez L. A., Levine A. T., Harris S. E., Mehra S., Lee W. L., et al. (2021). Early developmental EEG and seizure phenotypes in a full gene deletion of ubiquitin protein ligase E3A rat model of angelman syndrome. eneuro 8 (2), ENEURO.0345–20.2020. 10.1523/ENEURO.0345-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossuyt S. N. V., Punt A. M., de Graaf I. J., van den Burg J., Williams M. G., Heussler H., et al. (2021). Loss of nuclear UBE3A activity is the predominant cause of Angelman syndrome in individuals carrying UBE3A missense mutations. Hum. Mol. Genet. 30 (6), 430–442. 10.1093/hmg/ddab050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower B. D., Jeavons P. M. (1967). The “happy puppet” syndrome. Archives Dis. Child. 42 (223), 298–302. 10.1136/adc.42.223.298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandenberg N., Hoehnel S., Kuttler F., Homicsko K., Ceroni C., Ringel T., et al. (2020). High-throughput automated organoid culture via stem-cell aggregation in microcavity arrays. Nat. Biomed. Eng. 4 (9), 863–874. 10.1038/s41551-020-0565-2 [DOI] [PubMed] [Google Scholar]
- Brennan M. L., Adam M. P., Seaver L. H., Myers A., Schelley S., Zadeh N., et al. (2015). Increased body mass in infancy and early toddlerhood in Angelman syndrome patients with uniparental disomy and imprinting center defects. Am. J. Med. Genet. Part A 167 (1), 142–146. 10.1002/ajmg.a.36831 [DOI] [PubMed] [Google Scholar]
- Bruinsma C. F., Schonewille M., Gao Z., Aronica E. M. A., Judson M. C., Philpot B. D., et al. (2015). Dissociation of locomotor and cerebellar deficits in a murine Angelman syndrome model. J. Clin. Investigation 125 (11), 4305–4315. 10.1172/JCI83541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buiting K., Saitoh S., Gross S., Dittrich B., Schwartz S., Nicholls R. D., et al. (1995). Inherited microdeletions in the Angelman and Prader–Willi syndromes define an imprinting centre on human chromosome 15. Nat. Genet. 9 (4), 395–400. 10.1038/ng0495-395 [DOI] [PubMed] [Google Scholar]
- Buiting K., Williams C., Horsthemke B. (2016). Angelman syndrome — insights into a rare neurogenetic disorder. Nat. Rev. Neurol. 12 (10), 584–593. 10.1038/nrneurol.2016.133 [DOI] [PubMed] [Google Scholar]
- Burnside R. D., Pasion R., Mikhail F. M., Carroll A. J., Robin N. H., Youngs E. L., et al. (2011). Microdeletion/microduplication of proximal 15q11.2 between BP1 and BP2: a susceptibility region for neurological dysfunction including developmental and language delay. Hum. Genet. 130 (4), 517–528. 10.1007/s00439-011-0970-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler M., Palmer C. G. (1983). Parental origin of chromosome 15 deletion in Prader-Willi Syndrome. Lancet 321 (8336), 1285–1286. 10.1016/S0140-6736(83)92745-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell A., Morris G., Sanfeliu A., Augusto J., Langa E., Kesavan J. C., et al. (2022). AntimiR targeting of microRNA-134 reduces seizures in a mouse model of Angelman syndrome. Mol. Ther. - Nucleic Acids 28, 514–529. 10.1016/j.omtn.2022.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattanach B. M., Barr J. A., Beechey C. V., Martin J., Noebels J., Jones J. (1997). A candidate model for angelman syndrome in the mouse. Mamm. Genome 8 (7), 472–478. 10.1007/s003359900479 [DOI] [PubMed] [Google Scholar]
- Chamberlain S. J., Brannan C. I. (2001). The prader–willi syndrome imprinting center activates the paternally expressed murine Ube3a antisense transcript but represses paternal Ube3a. Genomics 73 (3), 316–322. 10.1006/geno.2001.6543 [DOI] [PubMed] [Google Scholar]
- Chamberlain S. J., Chen P. F., Ng K. Y., Bourgois-Rocha F., Lemtiri-Chlieh F., Levine E. S., et al. (2010). Induced pluripotent stem cell models of the genomic imprinting disorders Angelman and Prader-Willi syndromes. Proc. Natl. Acad. Sci. U. S. A. 107 (41), 17668–17673. 10.1073/pnas.1004487107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton-Smith J., Driscoll D. J., Waters M. F., Webb T., Andrews T., Malcolm S., et al. (1993). Difference in methylation patterns within the D15S9 region of chromosome 15q11-13 in first cousins with Angelman syndrome and Prader-Willi syndrome. Am. J. Med. Genet. 47 (5), 683–686. 10.1002/ajmg.1320470519 [DOI] [PubMed] [Google Scholar]
- Colas D., Wagstaff J., Fort P., Salvert D., Sarda N. (2005). Sleep disturbances in Ube3a maternal-deficient mice modeling Angelman syndrome. Neurobiol. Dis. 20 (2), 471–478. 10.1016/j.nbd.2005.04.003 [DOI] [PubMed] [Google Scholar]
- Copping N. A., Christian S. G. B., Ritter D. J., Islam M. S., Buscher N., Zolkowska D., et al. (2017). Neuronal overexpression of Ube3a isoform 2 causes behavioral impairments and neuroanatomical pathology relevant to 15q11.2-q13.3 duplication syndrome. Hum. Mol. Genet. 26 (20), 3995–4010. 10.1093/hmg/ddx289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa-Mattioli M., Monteggia L. M. (2013). mTOR complexes in neurodevelopmental and neuropsychiatric disorders. Nat. Neurosci. 16 (11), 1537–1543. 10.1038/nn.3546 [DOI] [PubMed] [Google Scholar]
- Cubillos-Rojas M., Schneider T., Hadjebi O., Pedrazza L., de Oliveira J. R., Langa F., et al. (2016). The HERC2 ubiquitin ligase is essential for embryonic development and regulates motor coordination. Oncotarget 7 (35), 56083–56106. 10.18632/oncotarget.11270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Rocha S. T., Gendrel A. V. (2019). The influence of DNA methylation on monoallelic expression. Essays Biochem. 63 (6), 663–676. 10.1042/EBC20190034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daily J. L., Nash K., Jinwal U., Golde T., Rogers J., Peters M. M., et al. (2011). Adeno-associated virus-mediated rescue of the cognitive defects in a mouse model for angelman syndrome. PLoS ONE 6 (12), e27221. 10.1371/journal.pone.0027221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damgaard R. B. (2021). The ubiquitin system: from cell signalling to disease biology and new therapeutic opportunities. Cell. Death Differ. 28 (2), 423–426. 10.1038/s41418-020-00703-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delahanty R. J., Zhang Y., Bichell T. J., Shen W., Verdier K., Macdonald R. L., et al. (2016). Beyond epilepsy and autism: disruption of GABRB3 causes ocular hypopigmentation. Cell. Rep. 17 (12), 3115–3124. 10.1016/j.celrep.2016.11.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson A. J., Fielder A. R., Young I. D., Duckett D. P. (1990). Ocular findings in angelman’s (happy puppet) syndrome. Ophthalmic Paediatr. Genet. 11 (1), 1–6. 10.3109/13816819009012942 [DOI] [PubMed] [Google Scholar]
- Dindot S. V., Antalffy B. A., Bhattacharjee M. B., Beaudet A. L. (2007). The Angelman syndrome ubiquitin ligase localizes to the synapse and nucleus, and maternal deficiency results in abnormal dendritic spine morphology. Hum. Mol. Genet. 17 (1), 111–118. 10.1093/hmg/ddm288 [DOI] [PubMed] [Google Scholar]
- Dindot S. V., Christian S., Murphy W. J., Berent A., Panagoulias J., Schlafer A., et al. (2023). An ASO therapy for Angelman syndrome that targets an evolutionarily conserved region at the start of the UBE3A-AS transcript. Sci. Transl. Med. 15 (688), eabf4077. 10.1126/scitranslmed.abf4077 [DOI] [PubMed] [Google Scholar]
- Dittrich B., Robinson W. P., Knoblauch H., Buiting K., Schmidt K., Gillessen-Kaesbach G., et al. (1992). Molecular diagnosis of the Prader-Willi and Angelman syndromes by detection of parent-of-origin specific DNA methylation in 15q11-13. Hum. Genet. 90 (3), 313–315. 10.1007/BF00220089 [DOI] [PubMed] [Google Scholar]
- Dodge A., Peters M. M., Greene H. E., Dietrick C., Botelho R., Chung D., et al. (2020). Generation of a novel rat model of angelman syndrome with a complete Ube3a gene deletion. Autism Res. 13 (3), 397–409. 10.1002/aur.2267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll D. J., Waters M. F., Williams C. A., Zori R. T., Glenn C. C., Avidano K. M., et al. (1992). A DNA methylation imprint, determined by the sex of the parent, distinguishes the angelman and Prader-Willi syndromes. Genomics 13 (4), 917–924. 10.1016/0888-7543(92)90001-9 [DOI] [PubMed] [Google Scholar]
- Egawa K., Kitagawa K., Inoue K., Takayama M., Takayama C., Saitoh S., et al. (2012). Decreased tonic inhibition in cerebellar granule cells causes motor dysfunction in a mouse model of angelman syndrome. Sci. Transl. Med. 4 (163), 163ra157. 10.1126/scitranslmed.3004655 [DOI] [PubMed] [Google Scholar]
- Eichmüller O. L., Knoblich J. A. (2022). Human cerebral organoids — a new tool for clinical neurology research. Nat. Rev. Neurol. 18 (11), 661–680. 10.1038/s41582-022-00723-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink J. J., Robinson T. M., Germain N. D., Sirois C. L., Bolduc K. A., Ward A. J., et al. (2017). Disrupted neuronal maturation in Angelman syndrome-derived induced pluripotent stem cells. Nat. Commun. 8, 15038. 10.1038/ncomms15038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galligan J. T., Martinez-Noël G., Arndt V., Hayes S., Chittenden T. W., Harper J. W., et al. (2015). Proteomic analysis and identification of cellular interactors of the giant ubiquitin ligase HERC2. J. Proteome Res. 14 (2), 953–966. 10.1021/pr501005v [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentile J. K., Tan W. H., Horowitz L. T., Bacino C. A., Skinner S. A., Barbieri-Welge R., et al. (2010). A neurodevelopmental survey of angelman syndrome with genotype-phenotype correlations. J. Dev. Behav. Pediatr. 31 (7), 592–601. 10.1097/DBP.0b013e3181ee408e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giandomenico S. L., Sutcliffe M., Lancaster M. A. (2021). Generation and long-term culture of advanced cerebral organoids for studying later stages of neural development. Nat. Protoc. 16 (2), 579–602. 10.1038/s41596-020-00433-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer P. L., Hanayama R., Bloodgood B. L., Mardinly A. R., Lipton D. M., Flavell S. W., et al. (2010). The angelman syndrome protein Ube3A regulates synapse development by ubiquitinating arc. Cell. 140 (5), 704–716. 10.1016/j.cell.2010.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu B., Carstens K. E., Judson M. C., Dalton K. A., Rougié M., Clark E. P., et al. (2018). Ube3a reinstatement mitigates epileptogenesis in Angelman syndrome model mice. J. Clin. Investigation 129 (1), 163–168. 10.1172/JCI120816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J. G. (1990). Angelman’s syndrome, abnormality of 15q11-13, and imprinting. J. Med. Genet. 27 (2), 141. 10.1136/jmg.27.2.141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell J., Barbaric I., Andrews P. W. (2020). Acquired genetic changes in human pluripotent stem cells: origins and consequences. Nat. Rev. Mol. Cell. Biol. 21 (12), 715–728. 10.1038/s41580-020-00292-z [DOI] [PubMed] [Google Scholar]
- Horsthemke B., Buiting K. (2006). Imprinting defects on human chromosome 15. Cytogenet. Genome Res. 113 (1–4), 292–299. 10.1159/000090844 [DOI] [PubMed] [Google Scholar]
- Horsthemke B., Wagstaff J. (2008). Mechanisms of imprinting of the Prader-Willi/Angelman region. Am. J. Med. Genet. Part A 146A (16), 2041–2052. 10.1002/ajmg.a.32364 [DOI] [PubMed] [Google Scholar]
- Hsiao J. S., Germain N. D., Wilderman A., Stoddard C., Wojenski L. A., Villafano G. J., et al. (2019). A bipartite boundary element restricts UBE3A imprinting to mature neurons. Proc. Natl. Acad. Sci. 116 (6), 2181–2186. 10.1073/pnas.1815279116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H. S., Allen J. A., Mabb A. M., King I. F., Miriyala J., Taylor-Blake B., et al. (2012). Topoisomerase inhibitors unsilence the dormant allele of Ube3a in neurons. Nature 481 (7380), 185–189. 10.1038/nature10726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huibregtse J. M., Scheffner M., Howley P. M. (1993). Cloning and expression of the cDNA for E6-AP, a protein that mediates the interaction of the human papillomavirus E6 oncoprotein with p53. Mol. Cell. Biol. 13 (2), 775–784. 10.1128/mcb.13.2.775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay V., Becker L. E., Chan F. W., Perry T. L. (1991). Puppet-like syndrome of Angelman: a pathologic and neurochemical study. Neurology 41 (3), 416–422. 10.1212/WNL.41.3.416 [DOI] [PubMed] [Google Scholar]
- Jiang Y., Armstrong D., Albrecht U., Atkins C. M., Noebels J. L., Eichele G., et al. (1998). Mutation of the angelman ubiquitin ligase in mice causes increased cytoplasmic p53 and deficits of contextual learning and long-term potentiation. Neuron 21 (4), 799–811. 10.1016/S0896-6273(00)80596-6 [DOI] [PubMed] [Google Scholar]
- Jiang Y., Pan Y., Zhu L., Landa L., Yoo J., Spencer C., et al. (2010). Altered ultrasonic vocalization and impaired learning and memory in angelman syndrome mouse model with a large maternal deletion from Ube3a to Gabrb3. PLoS ONE 5 (8), e12278. 10.1371/journal.pone.0012278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judson M. C., Sosa-Pagan J. O., Del Cid W. A., Han J. E., Philpot B. D. (2014). Allelic specificity of Ube3a expression in the mouse brain during postnatal development. J. Comp. Neurology 522 (8), 1874–1896. 10.1002/cne.23507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaphzan H., Buffington S. A., Jung J. I., Rasband M. N., Klann E. (2011). Alterations in intrinsic membrane properties and the axon initial segment in a mouse model of angelman syndrome. J. Neurosci. 31 (48), 17637–17648. 10.1523/JNEUROSCI.4162-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan L. C., Wharton R., Elias E., Mandell F., Donlon T., Latt S. A. (1987). Clinical heterogeneity associated with deletions in the long arm of chromosome 15: report of 3 new cases and their possible genetic significance. Am. J. Med. Genet. 28 (1), 45–53. 10.1002/ajmg.1320280107 [DOI] [PubMed] [Google Scholar]
- Keating D. J. (2008). Mitochondrial dysfunction, oxidative stress, regulation of exocytosis and their relevance to neurodegenerative diseases. J. Neurochem. 104 (3), 298–305. 10.1111/j.1471-4159.2007.04997.x [DOI] [PubMed] [Google Scholar]
- Kelley K. W., Pasca S. P. (2022). Human brain organogenesis: toward a cellular understanding of development and disease. Cell. 185 (1), 42–61. 10.1016/j.cell.2021.10.003 [DOI] [PubMed] [Google Scholar]
- Keute M., Miller M. T., Krishnan M. L., Sadhwani A., Chamberlain S., Thibert R. L., et al. (2021). Angelman syndrome genotypes manifest varying degrees of clinical severity and developmental impairment. Mol. Psychiatry 26 (7), 3625–3633. 10.1038/s41380-020-0858-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King I. F., Yandava C. N., Mabb A. M., Hsiao J. S., Huang H. S., Pearson B. L., et al. (2013). Topoisomerases facilitate transcription of long genes linked to autism. Nature 501 (7465), 58–62. 10.1038/nature12504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishino T., Lalande M., Wagstaff J. (1997). UBE3A/E6-AP mutations cause Angelman syndrome. Nat. Genet. 15 (1), 70–73. 10.1038/ng0197-70 [DOI] [PubMed] [Google Scholar]
- Klobučar T., Kreibich E., Krueger F., Arez M., Pólvora-Brandão D., von Meyenn F., et al. (2020). IMPLICON: an ultra-deep sequencing method to uncover DNA methylation at imprinted regions. Nucleic Acids Res. 48 (16), E92. 10.1093/nar/gkaa567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll J. H. M., Nicholls R. D., Magenis R. E., Graham J. M., Lalande M., Latt S. A. (1989). Angelman and Prader-Willi syndromes share a common chromosome 15 deletion but differ in parental origin of the deletion. Am. J. Med. Genet. 32 (2), 285–290. 10.1002/ajmg.1320320235 [DOI] [PubMed] [Google Scholar]
- Kühnle S., Kogel U., Glockzin S., Marquardt A., Ciechanover A., Matentzoglu K., et al. (2011). Physical and functional interaction of the HECT ubiquitin-protein ligases E6AP and HERC2. J. Biol. Chem. 286 (22), 19410–19416. 10.1074/jbc.M110.205211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H. M., Clark E. P., Kuijer M. B., Cushman M., Pommier Y., Philpot B. D. (2018). Characterization and structure-activity relationships of indenoisoquinoline-derived topoisomerase I inhibitors in unsilencing the dormant Ube3a gene associated with Angelman syndrome. Mol. Autism 9 (1), 45. 10.1186/s13229-018-0228-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li E., Beard C., Jaenisch R. (1993). Role for DNA methylation in genomic imprinting. Nature 366 (6453), 362–365. 10.1038/366362a0 [DOI] [PubMed] [Google Scholar]
- Li J., Shen Z., Liu Y., Yan Z., Liu Y., Lin X., et al. (2023). A high-fidelity RNA-targeting Cas13 restores paternal Ube3a expression and improves motor functions in Angelman syndrome mice. Mol. Ther. 31 (7), 2286–2295. 10.1016/j.ymthe.2023.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Zhu Q., Cai Y., Yang Q. (2022). Generation of an induced pluripotent stem cell line from a patient with Angelman syndrome carrying UBE3A mutation. Stem Cell. Res. 62, 102791. 10.1016/j.scr.2022.102791 [DOI] [PubMed] [Google Scholar]
- Liu G. Y., Sabatini D. M. (2020). mTOR at the nexus of nutrition, growth, ageing and disease. Nat. Rev. Mol. Cell. Biol. 21 (4), 183–203. 10.1038/s41580-019-0199-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llewellyn K. J., Nalbandian A., Gomez A., Wei D., Walker N., Kimonis V. E. (2015). Administration of CoQ10 analogue ameliorates dysfunction of the mitochondrial respiratory chain in a mouse model of Angelman syndrome. Neurobiol. Dis. 76, 77–86. 10.1016/j.nbd.2015.01.005 [DOI] [PubMed] [Google Scholar]
- Luk H. M., Lo I. F. M. (2016). Angelman syndrome in Hong Kong Chinese: a 20 years’ experience. Eur. J. Med. Genet. 59 (6–7), 315–319. 10.1016/j.ejmg.2016.05.003 [DOI] [PubMed] [Google Scholar]
- Lusk L., Vogel-Farley V., DiStefano C. (2021). Maternal 15q duplication syndrome, GeneReviews®. Available at: https://www.ncbi.nlm.nih.gov/books/NBK367946/ (Accessed: August 1, 2023). [PubMed]
- Ma H., Morey R., O'Neil R. C., He Y., Daughtry B., Schultz M. D., et al. (2014). Abnormalities in human pluripotent cells due to reprogramming mechanisms. Nature 511 (7508), 177–183. 10.1038/nature13551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magenis R. E., Brown M. G., Lacy D. A., Budden S., LaFranchi S. (1987). Is angelman syndrome an alternate result of del(15)(qllql3)? Am. J. Med. Genet. 28 (4), 829–838. 10.1002/ajmg.1320280407 [DOI] [PubMed] [Google Scholar]
- Malcolm S., Clayton-Smith J., Nichols M., Robb S., Webb T., Armour J. A., et al. (1991). Uniparental paternal disomy in Angelman’s syndrome. Lancet 337 (8743), 694–697. 10.1016/0140-6736(91)90278-W [DOI] [PubMed] [Google Scholar]
- Maranga C., Fernandes T. G., Bekman E., da Rocha S. T. (2020). Angelman syndrome: a journey through the brain. FEBS J. 287 (11), 2154–2175. 10.1111/febs.15258 [DOI] [PubMed] [Google Scholar]
- Maranga C., Pereira C., Raposo A. C., Vieira A., Duarte S., Bekman E. P., et al. (2022). Generation and characterization of induced pluripotent stem cell line (IBBISTi004-A) from an Angelman syndrome patient carrying a class II deletion of the maternal chromosome 15q11.2-q13. Stem Cell. Res. 61, 102757. 10.1016/j.scr.2022.102757 [DOI] [PubMed] [Google Scholar]
- Maranga C., Vieira A. A., Bekman E. P., da Rocha S. T. (2021). Induced pluripotent stem cells for modeling Angelman syndrome. iPSCs Model. Central Nerv. Syst. Disord. 6, 217–238. 10.1016/B978-0-323-85764-2.00015-6 [DOI] [Google Scholar]
- Mardirossian S., Rampon C., Salvert D., Fort P., Sarda N. (2009). Impaired hippocampal plasticity and altered neurogenesis in adult Ube3a maternal deficient mouse model for Angelman syndrome. Exp. Neurol. 220 (2), 341–348. 10.1016/j.expneurol.2009.08.035 [DOI] [PubMed] [Google Scholar]
- Margolis S. S., Sell G. L., Zbinden M. A., Bird L. M. (2015). Angelman syndrome. Neurotherapeutics 12 (3), 641–650. 10.1007/s13311-015-0361-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins-Taylor K., Hsiao J. S., Chen P. F., Glatt-Deeley H., De Smith A. J., Blakemore A. I. F., et al. (2014). Imprinted expression of UBE3A in non-neuronal cells from a Prader–Willi syndrome patient with an atypical deletion. Hum. Mol. Genet. 23 (9), 2364–2373. 10.1093/hmg/ddt628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu N. A., Levin R. H., Spratt D. E. (2021). Exploring the roles of HERC2 and the NEDD4L HECT E3 ubiquitin ligase subfamily in p53 signaling and the DNA damage response. Front. Oncol. 11, 659049. 10.3389/fonc.2021.659049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura T., Sutcliffe J. S., Fang P., Galjaard R. J., Jiang Y. H., Benton C. S., et al. (1997). De novo truncating mutations in E6-AP ubiquitin-protein ligase gene (UBE3A) in Angelman syndrome. Nat. Genet. 15 (1), 74–77. 10.1038/ng0197-74 [DOI] [PubMed] [Google Scholar]
- Mayo Clinic (2022). Angelman syndrome. Available at: https://www.mayoclinic.org/diseases-conditions/angelman-syndrome/symptoms-causes/syc-20355621 (Accessed: July 7, 2023).
- McKnight D., Bean L., Karbassi I., Beattie K., Bienvenu T., Bonin H., et al. (2022). Recommendations by the ClinGen Rett/Angelman‐like expert panel for gene‐specific variant interpretation methods. Hum. Mutat. 43 (8), 1097–1113. 10.1002/humu.24302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MedlinePlus (2022). UBE3A gene: medlinePlus genetics. Available at: https://medlineplus.gov/genetics/gene/ube3a/ (Accessed: July 6, 2023).
- Medvar B., Raghuram V., Pisitkun T., Sarkar A., Knepper M. A. (2016). Comprehensive database of human E3 ubiquitin ligases: application to aquaporin-2 regulation. Physiol. Genomics 48 (7), 502–512. 10.1152/physiolgenomics.00031.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng L., Person R. E., Beaudet A. L. (2012). Ube3a-ATS is an atypical RNA polymerase II transcript that represses the paternal expression of Ube3a. Hum. Mol. Genet. 21 (13), 3001–3012. 10.1093/hmg/dds130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng L., Person R. E., Huang W., Zhu P. J., Costa-Mattioli M., Beaudet A. L. (2013). Truncation of ube3a-ATS unsilences paternal Ube3a and ameliorates behavioral defects in the angelman syndrome mouse model. PLoS Genet. 9 (12), e1004039. 10.1371/journal.pgen.1004039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng L., Ward A. J., Chun S., Bennett C. F., Beaudet A. L., Rigo F. (2015). Towards a therapy for Angelman syndrome by targeting a long non-coding RNA. Nature 518 (7539), 409–412. 10.1038/nature13975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milazzo C., Mientjes E. J., Wallaard I., Rasmussen S. V., Erichsen K. D., Kakunuri T., et al. (2021). Antisense oligonucleotide treatment rescues UBE3A expression and multiple phenotypes of an Angelman syndrome mouse model. JCI Insight 6 (15), e145991. 10.1172/jci.insight.145991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura K., Kishino T., Li E., Webber H., Dikkes P., Holmes G. L., et al. (2002). Neurobehavioral and electroencephalographic abnormalities in Ube3aMaternal-deficient mice. Neurobiol. Dis. 9 (2), 149–159. 10.1006/nbdi.2001.0463 [DOI] [PubMed] [Google Scholar]
- Miura Y., Li M. Y., Revah O., Yoon S. J., Narazaki G., Pașca S. P. (2022). Engineering brain assembloids to interrogate human neural circuits. Nat. Protoc. 17 (1), 15–35. 10.1038/s41596-021-00632-z [DOI] [PubMed] [Google Scholar]
- Nazor K. L., Altun G., Lynch C., Tran H., Harness J. V., Slavin I., et al. (2012). Recurrent variations in DNA methylation in human pluripotent stem cells and their differentiated derivatives. Cell. Stem Cell. 10 (5), 620–634. 10.1016/j.stem.2012.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neureiter A., Brändl B., Hiber M., Tandon R., Müller F. J., Steenpass L. (2018). Generation of an iPSC line of a patient with Angelman syndrome due to an imprinting defect. Stem Cell. Res. 33, 20–24. 10.1016/j.scr.2018.09.015 [DOI] [PubMed] [Google Scholar]
- NHS (2023). Angelman syndrome. Available at: https://www.nhs.uk/conditions/angelman-syndrome/ (Accessed: July 7, 2023).
- Niki T., Imamura K., Enami T., Kinoshita M., Inoue H. (2019). Establishment of human induced pluripotent stem cell line from a patient with Angelman syndrome carrying the deletion of maternal chromosome 15q11.2-q13. Stem Cell. Res. 34, 101363. 10.1016/j.scr.2018.101363 [DOI] [PubMed] [Google Scholar]
- Norat P., Soldozy S., Sokolowski J. D., Gorick C. M., Kumar J. S., Chae Y., et al. (2020). Mitochondrial dysfunction in neurological disorders: exploring mitochondrial transplantation. npj Regen. Med. 5 (1), 22. 10.1038/s41536-020-00107-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Geen H., Beitnere U., Garcia M. S., Adhikari A., Cameron D. L., Fenton T. A., et al. (2023). Transcriptional reprogramming restores UBE3A brain-wide and rescues behavioral phenotypes in an Angelman syndrome mouse model. Mol. Ther. 31 (4), 1088–1105. 10.1016/j.ymthe.2023.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya N. J., Wang C., Costa V., Lopatta P., Meier S., Zampeta F. I., et al. (2021). Secreted retrovirus-like GAG-domain-containing protein PEG10 is regulated by UBE3A and is involved in Angelman syndrome pathophysiology. Cell. Rep. Med. 2 (8), 100360. 10.1016/j.xcrm.2021.100360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasca S. P., Arlotta P., Bateup H. S., Camp J. G., Cappello S., Gage F. H., et al. (2022). A nomenclature consensus for nervous system organoids and assembloids. Nature 609 (7929), 907–910. 10.1038/s41586-022-05219-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paşca S. P. (2019). Assembling human brain organoids. Science 363 (6423), 126–127. 10.1126/science.aau5729 [DOI] [PubMed] [Google Scholar]
- Pastuzyn E. D., Shepherd J. D. (2017). Activity-dependent arc expression and homeostatic synaptic plasticity are altered in neurons from a mouse model of angelman syndrome. Front. Mol. Neurosci. 10, 234. 10.3389/fnmol.2017.00234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham A., Selenou C., Giabicani E., Fontaine V., Marteau S., Brioude F., et al. (2022). Maintenance of methylation profile in imprinting control regions in human induced pluripotent stem cells. Clin. Epigenetics 14 (1), 190. 10.1186/s13148-022-01410-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pólvora-Brandão D., Joaquim M., Godinho I., Aprile D., Álvaro A. R., Onofre I., et al. (2018). Loss of hierarchical imprinting regulation at the Prader-Willi/Angelman syndrome locus in human iPSCs. Hum. Mol. Genet. 27 (23), 3999–4011. 10.1093/hmg/ddy274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puffenberger E. G., Jinks R. N., Wang H., Xin B., Fiorentini C., Sherman E. A., et al. (2012). A homozygous missense mutation in HERC2 associated with global developmental delay and autism spectrum disorder. Hum. Mutat. 33 (12), 1639–1646. 10.1002/humu.22237 [DOI] [PubMed] [Google Scholar]
- Qian J., Guan X., Xie B., Xu C., Niu J., Tang X., et al. (2023). Multiplex epigenome editing of MECP2 to rescue Rett syndrome neurons. Sci. Transl. Med. 15 (679), eadd4666. 10.1126/scitranslmed.add4666 [DOI] [PubMed] [Google Scholar]
- Reis A., Dittrich B., Greger V., Buiting K., Lalande M., Gillessen-Kaesbach G., et al. (1994). Imprinting mutations suggested by abnormal DNA methylation patterns in familial Angelman and Prader-Willi syndromes. Am. J. Hum. Genet. 54 (5), 741–747. [PMC free article] [PubMed] [Google Scholar]
- Robb S. A., Pohl K. R., Baraitser M., Wilson J., Brett E. M. (1989). The “happy puppet” syndrome of Angelman: review of the clinical features. Archives Dis. Child. 64 (1), 83–86. 10.1136/adc.64.1.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotaru D. C., van Woerden G. M., Wallaard I., Elgersma Y. (2018). Adult Ube3a gene reinstatement restores the electrophysiological deficits of prefrontal cortex layer 5 neurons in a mouse model of angelman syndrome. J. Neurosci. 38 (37), 8011–8030. 10.1523/JNEUROSCI.0083-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotaru D. C., Wallaard I., de Vries M., van der Bie J., Elgersma Y. (2023). UBE3A expression during early postnatal brain development is required for proper dorsomedial striatal maturation. JCI Insight 8 (4), e166073. 10.1172/jci.insight.166073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rougeulle C., Cardoso C., Fontés M., Colleaux L., Lalande M. (1998). An imprinted antisense RNA overlaps UBE3A and a second maternally expressed transcript. Nat. Genet. 19 (1), 15–16. 10.1038/ng0598-15 [DOI] [PubMed] [Google Scholar]
- Rougeulle C., Glatt H., Lalande M. (1997). The Angelman syndrome candidate gene, UBE3A/E6-AP, is imprinted in brain. Nat. Genet. 17 (1), 14–15. 10.1038/ng0997-14 [DOI] [PubMed] [Google Scholar]
- Runte M., Hüttenhofer A., Gross S., Kiefmann M., Horsthemke B., Buiting K. (2001). The IC-SNURF-SNRPN transcript serves as a host for multiple small nucleolar RNA species and as an antisense RNA for UBE3A. Hum. Mol. Genet. 10 (23), 2687–2700. 10.1093/hmg/10.23.2687 [DOI] [PubMed] [Google Scholar]
- Sadikovic B., Fernandes P., Zhang V. W., Ward P. A., Miloslavskaya I., Rhead W., et al. (2014). Mutation update for UBE3A variants in angelman syndrome. Hum. Mutat. 35 (12), 1407–1417. 10.1002/humu.22687 [DOI] [PubMed] [Google Scholar]
- Santini E., Turner K. L., Ramaraj A. B., Murphy M. P., Klann E., Kaphzan H. (2015). Mitochondrial superoxide contributes to hippocampal synaptic dysfunction and memory deficits in angelman syndrome model mice. J. Neurosci. 35 (49), 16213–16220. 10.1523/JNEUROSCI.2246-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid R. S., Deng X., Panikker P., Msackyi M., Breton C., Wilson J. M. (2021). CRISPR/Cas9 directed to the Ube3a antisense transcript improves Angelman syndrome phenotype in mice. J. Clin. Investigation 131 (5), e142574. 10.1172/JCI142574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen D., Voulgaropoulos A., Drobna Z., Keung A. J. (2020). Human cerebral organoids reveal early spatiotemporal dynamics and pharmacological responses of UBE3A. Stem Cell. Rep. 15 (4), 845–854. 10.1016/j.stemcr.2020.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shemer R., Hershko A. Y., Perk J., Mostoslavsky R., Tsuberi B., Cedar H., et al. (2000). The imprinting box of the Prader-Willi/Angelman syndrome domain. Nat. Genet. 26 (4), 440–443. 10.1038/82571 [DOI] [PubMed] [Google Scholar]
- Silva-Santos S., van Woerden G. M., Bruinsma C. F., Mientjes E., Jolfaei M. A., Distel B., et al. (2015). Ube3a reinstatement identifies distinct developmental windows in a murine Angelman syndrome model. J. Clin. Investigation 125 (5), 2069–2076. 10.1172/JCI80554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simchi L., Gupta P. K., Feuermann Y., Kaphzan H. (2023). Elevated ROS levels during the early development of Angelman syndrome alter the apoptotic capacity of the developing neural precursor cells. Mol. Psychiatry. 10.1038/s41380-023-02038-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirois C. L., Bloom J. E., Fink J. J., Gorka D., Keller S., Germain N. D., et al. (2020). Abundance and localization of human UBE3A protein isoforms. Hum. Mol. Genet. 29 (18), 3021–3031. 10.1093/hmg/ddaa191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanurova J., Neureiter A., Hiber M., de Oliveira Kessler H., Stolp K., Goetzke R., et al. (2016). Angelman syndrome-derived neurons display late onset of paternal UBE3A silencing. Sci. Rep. 6, 30792. 10.1038/srep30792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H., Fan W., Coskun P. E., Vesa J., Gold J. A., Jiang Y. H., et al. (2011). Mitochondrial dysfunction in CA1 hippocampal neurons of the UBE3A deficient mouse model for Angelman syndrome. Neurosci. Lett. 487 (2), 129–133. 10.1016/j.neulet.2009.06.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun A. X., Yuan Q., Fukuda M., Yu W., Yan H., Lim G. G. Y., et al. (2019). Potassium channel dysfunction in human neuronal models of Angelman syndrome. Science 366 (6472), 1486–1492. 10.1126/science.aav5386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J., Liu Y., Moreno S., Baudry M., Bi X. (2015). Imbalanced mechanistic target of rapamycin C1 and C2 activity in the cerebellum of angelman syndrome mice impairs motor function. J. Neurosci. 35 (11), 4706–4718. 10.1523/JNEUROSCI.4276-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J., Liu Y., Tran J., O'Neal P., Baudry M., Bi X. (2016). mTORC1–S6K1 inhibition or mTORC2 activation improves hippocampal synaptic plasticity and learning in Angelman syndrome mice. Cell. Mol. Life Sci. 73 (22), 4303–4314. 10.1007/s00018-016-2269-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J., Zhu G., Liu Y., Standley S., Ji A., Tunuguntla R., et al. (2015). UBE3A regulates synaptic plasticity and learning and memory by controlling SK2 channel endocytosis. Cell. Rep. 12 (3), 449–461. 10.1016/j.celrep.2015.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K., et al. (2007). Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 131 (5), 861–872. 10.1016/j.cell.2007.11.019 [DOI] [PubMed] [Google Scholar]
- Takahashi K., Yamanaka S. (2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 126 (4), 663–676. 10.1016/j.cell.2006.07.024 [DOI] [PubMed] [Google Scholar]
- Takahashi Y., Wu J., Suzuki K., Martinez-Redondo P., Li M., Liao H. K., et al. (2017). Integration of CpG-free DNA induces de novo methylation of CpG islands in pluripotent stem cells. Science 356 (6337), 503–508. 10.1126/science.aag3260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan W. H., Bird L. M. (2016). Angelman syndrome: current and emerging therapies in 2016. Am. J. Med. Genet. Part C Seminars Med. Genet. 172 (4), 384–401. 10.1002/ajmg.c.31536 [DOI] [PubMed] [Google Scholar]
- Tang X., Jaenisch R., Sur M. (2021). The role of GABAergic signalling in neurodevelopmental disorders. Nat. Rev. Neurosci. 22 (5), 290–307. 10.1038/s41583-021-00443-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenreiro M. F., Branco M. A., Cotovio J. P., Cabral J. M. S., Fernandes T. G., Diogo M. M. (2023). Advancing organoid design through co-emergence, assembly, and bioengineering. Trends Biotechnol. 41 (7), 923–938. 10.1016/j.tibtech.2022.12.021 [DOI] [PubMed] [Google Scholar]
- Thomson J. A., Itskovitz-Eldor J., Shapiro S. S., Waknitz M. A., Swiergiel J. J., Marshall V. S., et al. (1998). Embryonic stem cell lines derived from human blastocysts. Science 282 (5391), 1145–1147. 10.1126/science.282.5391.1145 [DOI] [PubMed] [Google Scholar]
- Valente K. D., Varela M. C., Koiffmann C. P., Andrade J. Q., Grossmann R., Kok F., et al. (2013). Angelman syndrome caused by deletion: a genotype–phenotype correlation determined by breakpoint. Epilepsy Res. 105 (1–2), 234–239. 10.1016/j.eplepsyres.2012.12.005 [DOI] [PubMed] [Google Scholar]
- Vandereyken K., Sifrim A., Thienpont B., Voet T. (2023). Methods and applications for single-cell and spatial multi-omics. Nat. Rev. Genet. 24 (8), 494–515. 10.1038/s41576-023-00580-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanlerberghe C., Petit F., Malan V., Vincent-Delorme C., Bouquillon S., Boute O., et al. (2015). 15q11.2 microdeletion (BP1–BP2) and developmental delay, behaviour issues, epilepsy and congenital heart disease: a series of 52 patients. Eur. J. Med. Genet. 58 (3), 140–147. 10.1016/j.ejmg.2015.01.002 [DOI] [PubMed] [Google Scholar]
- Vu T. H., Hoffman A. R. (1997). Imprinting of the Angelman syndrome gene, UBE3A, is restricted to brain. Nat. Genet. 17 (1), 12–13. 10.1038/ng0997-12 [DOI] [PubMed] [Google Scholar]
- Wallace M. L., Burette A. C., Weinberg R. J., Philpot B. D. (2012). Maternal loss of Ube3a produces an excitatory/inhibitory imbalance through neuron type-specific synaptic defects. Neuron 74 (5), 793–800. 10.1016/j.neuron.2012.03.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston K. P., Gao X., Zhao J., Kim K. S., Maloney S. E., Gotoff J., et al. (2021). Identification of disease-linked hyperactivating mutations in UBE3A through large-scale functional variant analysis. Nat. Commun. 12 (1), 6809. 10.1038/s41467-021-27156-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams C. A., Beaudet A. L., Clayton-Smith J., Knoll J. H., Kyllerman M., Laan L. A., et al. (2006). Angelman syndrome 2005: updated consensus for diagnostic criteria. Am. J. Med. Genet. Part A 140A (5), 413–418. 10.1002/ajmg.a.31074 [DOI] [PubMed] [Google Scholar]
- Williams C. A., Driscoll D. J., Dagli A. I. (2010). Clinical and genetic aspects of Angelman syndrome. Genet. Med. 12 (7), 385–395. 10.1097/GIM.0b013e3181def138 [DOI] [PubMed] [Google Scholar]
- Williams C. A., Frias J. L., Opitz J. M. (1982). The angelman (“Happy puppet”) syndrome. Am. J. Med. Genet. 11 (4), 453–460. 10.1002/ajmg.1320110411 [DOI] [PubMed] [Google Scholar]
- Williams C. A., Zori R. T., Stone J. W., Gray B. A., Cantu E. S., Ostrer H. (1990). Maternal origin of 15q11-13 deletions in Angelman syndrome suggests a role for genomic imprinting. Am. J. Med. Genet. 35 (3), 350–353. 10.1002/ajmg.1320350308 [DOI] [PubMed] [Google Scholar]
- Wolter J. M., Mao H., Fragola G., Simon J. M., Krantz J. L., Bazick H. O., et al. (2020). Cas9 gene therapy for Angelman syndrome traps Ube3a-ATS long non-coding RNA. Nature 587 (7833), 281–284. 10.1038/s41586-020-2835-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Bolduc F. V., Bell K., Tully T., Fang Y., Sehgal A., et al. (2008). A Drosophila model for Angelman syndrome. Proc. Natl. Acad. Sci. 105 (34), 12399–12404. 10.1073/pnas.0805291105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing L., Simon J. M., Ptacek T. S., Yi J. J., Loo L., Mao H., et al. (2023). Autism-linked UBE3A gain-of-function mutation causes interneuron and behavioral phenotypes when inherited maternally or paternally in mice. Cell. Rep. 42 (7), 112706. 10.1016/j.celrep.2023.112706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Shu X., Mao S., Wang Y., Du X., Zou C. (2021). Genotype–phenotype correlations in angelman syndrome. Genes 12 (7), 987. 10.3390/genes12070987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yashiro K., Riday T. T., Condon K. H., Roberts A. C., Bernardo D. R., Prakash R., et al. (2009). Ube3a is required for experience-dependent maturation of the neocortex. Nat. Neurosci. 12 (6), 777–783. 10.1038/nn.2327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Korolj A., Lai B. F. L., Radisic M. (2018). Advances in organ-on-a-chip engineering. Nat. Rev. Mater. 3 (8), 257–278. 10.1038/s41578-018-0034-7 [DOI] [Google Scholar]
- Zhou Y., Song H., Ming G. (2023). Genetics of human brain development. Nat. Rev. Genet. 10.1038/s41576-023-00626-5 [DOI] [PMC free article] [PubMed] [Google Scholar]