Abstract
Study Objectives:
To determine if a home sleep apnea test (HSAT) using a type III portable monitor (PM), Nox-T3 (Nox Medical, Inc., Reykjavik, Iceland), detects obstructive sleep apnea in pregnant women.
Methods:
Ninety-two pregnant women (34.5 ± 4.3 years; gestational age 25.4 ± 8.9 weeks; body mass index 29.9 ± 4.7 kg/m2) with suspected obstructive sleep apnea underwent HSAT with the Nox-T3 PM followed by overnight polysomnography (PSG) and PM recording simultaneously in the laboratory within 1 week. PMs were scored automatically and manually using a 3% criteria and compared with PSGs scored by following guidelines.
Results:
Apnea-hypopnea indexes were 8.56 ± 10.42, 8.19 ± 13.79, and 8.71 ± 14.19 events/h on HSAT, in-laboratory PM recording, and PSG (P = .955), respectively. Bland-Altman analysis of the apnea-hypopnea index on PSG vs HSAT showed a mean difference (95% confidence interval) of −0.15 (−1.83, 1.53); limits of agreement (± 2 SD) were −16.26 to 16.56 events/h. Based on a threshold apnea-hypopnea index ≥ 5 events/h, HSAT had 91% sensitivity, 85% specificity, 84% positive-predictive value, and 92% negative-predictive value compared with PSG. When comparing the simultaneous recordings, closer agreement was observed. Automated vs manual analysis of PM showed no significant difference.
Conclusions:
A type III PM had an acceptable failure rate and high diagnostic performance operating as a reasonable alternative for in-laboratory PSG in pregnant women.
Citation:
Wang J, Zhang C, Xu L, et al. Home monitoring for clinically suspected obstructive sleep apnea in pregnancy. J Clin Sleep Med. 2023;19(11):1951–1960.
Keywords: pregnancy, home sleep apnea testing, obstructive sleep apnea, polysomnography
BRIEF SUMMARY
Current Knowledge/Study Rationale: Obstructive sleep apnea is a common health issue in pregnancy and associated with adverse maternal and fetal outcomes. However, polysomnography, the gold-standard diagnostic test, is relatively difficult to perform in pregnant women.
Study Impact: Based on a large sample size and with use of in-laboratory polysomnography as the reference, this study demonstrated that a type III portable monitor had high diagnostic performance and an acceptable failure rate, and could operate as an alternative for in-laboratory polysomnography in pregnant women. Additionally, this study revealed close agreement of auto scoring and technician scoring of the portable monitor.
INTRODUCTION
Obstructive sleep apnea (OSA) occurs in up to 15% of pregnancies worldwide.1 It is a serious health issue in pregnancy and may worsen as pregnancy progresses. OSA is not only associated with poor quality of life of the affected pregnant women but also with adverse perinatal outcomes, including gestational hypertension, gestational diabetes, preeclampsia, perinatal depression, and possibly fetal growth restriction.2–7 Moreover, accumulated evidence suggested that OSA may have long-term health consequences for both mothers and children.8–12 As a modifiable risk factor, OSA in pregnancy should be identified and treated promptly to prevent the potential development of adverse maternal and infant outcomes.13,14 Thus, timely and accurate diagnosis of OSA in pregnancy is an issue of public health priority.
However, the vast majority of OSA in pregnancy is likely underdiagnosed due to limited capacity and/or limited time-of-need. Polysomnography (PSG), the gold-standard diagnostic test, is labor-intensive, high-cost, requires a long waiting time for an appointment, and is relatively difficult to perform in pregnant women, especially for those in late gestation.
A home sleep apnea test (HSAT) is now widely accepted as an alternative medical test for detecting moderate to severe OSA in uncomplicated adults.15 The Nox-T3 monitor (Nox Medical, Inc., Reykjavik, Iceland), one of the widely used HSAT portable monitors (PMs), is well validated and being used in clinical practice in the nonpregnant population with or without cardiopulmonary diseases.16–18 Because common screening questionnaires for OSA perform poorly in this specific population,19 testing with an HSAT could be of special value in pregnant women. However, studies regarding the validation of different PMs in pregnancy are limited by relatively small sample sizes and lack of validation against in-laboratory PSG testing.20–22 The aim of the current study is to evaluate the performance of a type III PM Nox-T3 in detecting OSA during HSAT and concomitant with in-laboratory PSG in pregnant women with suspected OSA.
METHODS
Protocol
Pregnant women with suspected OSA were consecutively recruited from the obstetric clinic of Peking University People’s Hospital. Women were considered at risk of OSA when they met at least 1 of the following criteria: (1) snoring; (2) witnessed apneas; (3) suggestive physical examination findings (neck circumference > 40 cm, Mallampati class 3–4 airway, large tonsils, large/inflamed uvula); (4) chronic hypertension diagnosed prior to pregnancy, pregestational diabetes (type 1 or type 2); (5) obesity (prepregnancy body mass index [BMI] ≥ 27.5 kg/m2)23,24; and (6) prior history of preeclampsia.25,26 Individuals were excluded for the following reasons: complicated pregnancy with fetal anomalies, previous sleep testing or treatment for OSA, a clinically unstable medical condition, individuals with significant underlying pulmonary or cardiac comorbidities.
Participants were recruited from the early (weeks 9–15), middle (weeks 16–27), and late (weeks 28–40) trimesters of pregnancy. Demographic data including maternal and gestational age, prepregnancy and current BMI, and neck circumference were collected from each participant. Medical records included diagnosis of hypertensive disorders of pregnancy and hyperglycemia in pregnancy. This project was approved by the Institutional Review Board at Peking University People’s Hospital, and written informed consent was obtained from all participants in this study.
Each participant initially performed an overnight HSAT using the Nox-T3 PM, followed by in-laboratory PSG (Alice6, Philips Respironics, Inc., Murrysville, PA, United States) concurrent with a Nox-T3 recording within 1 week in the sleep center of Peking University People’s Hospital. The order of home and in-laboratory testing was fixed to evaluate the ability of individuals without previous experience in sleep testing to perform the HSAT successfully. For both the home and in-laboratory testing, participants were instructed to sleep in whatever position was comfortable for them and to take their regular medications.
Portable monitor and PSG recordings
Nasal pressure, snoring, rib cage, and abdominal movement by respiratory inductance plethysmography and activity, body position, and heart rate as well as oxygen saturation by pulse oximetry were recorded during the PM recordings. For the HSAT, trained sleep technicians instructed the participants on how to apply the sensors and perform the recording. Technologists confirmed that the participants could repeat the above procedure by themselves before leaving the center with the device. The participants reapplied the sensors at home before going to bed.
The success rate of performing the initial HSAT and the quality of the HSAT were used to evaluate the ability of participants to perform the HSAT. A successful HSAT was defined as (1) at least 3 hours of recording containing the oxygen saturation and (2) at least one of the respiratory signals (nasal pressure, rib cage movement, abdominal movement). If the initial HSAT failed, the participants were then asked to repeat the HSAT before the PSG. The HSAT was not repeated after the second unsuccessful attempt. The calculation of the success rate was modified based on the protocol of our previous studies.16–18
PSG tests were performed according to the recommendations of the American Academy of Sleep Medicine.27 The following standard signals were recorded: electroencephalogram (F3M2, F4M1, C3M2, C4M1, O1M2, O2M1), bilateral electrooculogram, chin muscle electromyogram, rib cage and abdominal movement, nasal pressure, oronasal thermistor, snoring, body position, bilateral anterior tibialis electromyograms, electrocardiogram (lead1), and heart rate and oxygen saturation by pulse oximetry. During in-laboratory testing, an experienced sleep technologist performed Nox-T3 PM recording and PSG simultaneously, using separate sensors.
Portable monitor and PSG scoring
The PM and PSG recordings were distributed randomly to experienced scorers in a blinded fashion to ensure that none of the scorers would know whether PM recordings were performed at home or in-laboratory, nor did they score PM recordings with knowledge of PSG results. Participants’ responses on the post-study questionnaire and the activity signal on the recording were analyzed to determine start time and stop time on the PM recordings. The PM recordings were manually edited after being automatically scored using the Noxturnal software from Nox-T3. The PSGs were scored manually with the aid of computer software.
Using American Academy of Sleep Medicine 2012 criteria28 for scoring PSGs and HSATs, apneas were defined as a ≥ 90% reduction in airflow from baseline for at least 10 seconds. Obstructive apneas were defined as an apnea associated with respiratory effort, and central apneas were defined as an apnea during which respiratory effort was absent. Mixed apneas were defined as an apnea during which respiratory effort was initially absent but appeared in the latter part of the event. Two separate, manually edited scorings were performed using different definitions for hypopnea on PM tests and PSG tests: ≥ 30% reduction in a respiratory signal for at least 10 seconds associated with an oxygen desaturation event of ≥ 3% on PM recordings and an oxygen desaturation event of ≥ 3% and/or an arousal on PSG. Flow signals derived from the respiratory inductance plethysmography signals were used to score respiratory events when the PM nasal pressure signals were absent or unable to be scored.29 Total analysis time on the Nox-T3 recordings and total sleep time on PSG were used to calculate the average number of apneas and hypopneas per hour (apnea-hypopnea index [AHI]) and oxygen desaturation events per hour (oxygen desaturation index).
Statistical analysis
Statistical analyses were performed using Stata/SE version 14.1 (StataCorp LP, College Station, TX, United States) and SAS version 9.4 (SAS Institute, Inc., Cary, NC, United States). A value of P < .05 was considered statistically significant. Continuous variables are summarized using means and standard deviations and categorical variables are summarized using counts and percentages. Repeated-measures analysis of variance was applied to compare respiratory parameters across the 3 monitoring methods (in-home Nox-T3, in-laboratory Nox-T3, and in-laboratory PSG), accounting for multiple observations for each participant. Paired t tests and methods described by Bland and Altman30,31 were used to evaluate the level of agreement between the monitoring methods. Primary agreement evaluation compared the ≥ 3% AHI on PSG with that obtained from in-home and in-laboratory PM recordings separately. Agreement between manual and automated AHI scoring within the in-home/in-laboratory monitors was also examined using a similar method.
The diagnostic characteristics of home- or laboratory-based PMs were assessed by calculating the sensitivity, specificity, positive-predictive value, and negative-predictive value at AHI thresholds of ≥ 5, ≥ 10, and ≥ 15 events/h. Results obtained from PSG were considered as the reference standard for the analysis.
RESULTS
Success rate of testing
A flowchart of patient selection for this study is shown in Figure 1. A total of 261 pregnant women with suspected OSA in the obstetric clinic were invited to perform the HSAT. Of these, 221 women took the HSAT using the Nox-T3 PM. The initial HSAT was successful in 208 of the 221 participants (94.1%). Among the remaining 13 participants, 5 refused to repeat the HSAT and 8 were willing to perform the second HSAT; 6 succeeded and 2 failed. In summary, final HSATs were successfully completed in 214 of 221 participants (96.8%).
Figure 1. Flowchart of patient selection.
HSAT = home sleep apnea test, OSA = obstructive sleep apnea, PM = portable monitor, PSG = polysomnography.
Among the 214 participants who successfully performed the HSAT, 94 completed simultaneous PSG and PM monitoring within 1 week of the successful HSAT. Five of the 94 participants undertook repeated simultaneous PSG and PM at different trimesters and generated 11 recordings, including 3 at both the middle and late trimester, 1 at both the early and late trimester, and 1 at all 3 trimesters. Thus, total 100 recordings that fulfilled the study protocol were generated, and 2 recordings from 2 individuals were excluded due to less than 3 hours of sleep on PSG. Therefore, the remaining 98 recordings from 92 women were included in the final analysis. Among them, 20, 34, and 44 recordings were completed at the early, middle, and late trimesters, respectively.
Demographic data are shown in Table 1. According to PSG results, 45.9% of recordings obtained in this study were diagnosed as OSA (defined as AHI ≥ 5 events/h). Compared with the non-OSA group, the OSA group had a greater neck circumference and BMI during both pre- and post-pregnancy (all P < .05). Higher rates of hypertension were observed in the OSA group (P < .05).
Table 1.
Demographic data of pregnant women recruited in the study.
| Total (n = 98) | OSA (n = 45)a | Non-OSA (n = 53) | P b | |
|---|---|---|---|---|
| Age, years | 34.5 ± 4.3 | 35.1 ± 4.6 | 34.1 ± 4.0 | .2632 |
| Gestational age, weeks | 25.4 ± 8.9 | 24.7 ± 10.3 | 26.0 ± 7.6 | .4821 |
| Prepregnancy weight, kg | 71.3 ± 15.0 | 73.5 ± 16.8 | 69.5 ± 13.1 | .2006 |
| Prepregnancy BMI, kg/m2 | 27.1 ± 5.8 | 28.3 ± 6.8 | 26.0 ± 4.7 | .0546 |
| Weight during pregnancy, kg | 78.8 ± 11.9 | 82.6 ± 11.6 | 76.0 ± 11.3 | .0036 |
| BMI during pregnancy, kg/m2 | 29.9 ± 4.7 | 31.8 ± 4.9 | 28.3 ± 3.9 | .0002 |
| Neck circumference, cm | 36.8 ± 2.7 | 38.0 ± 2.6 | 35.8 ± 2.4 | .0001 |
| GH, n (%) | 13 (13.3%) | 7 (15.6%) | 6 (11.3%) | .5380 |
| GDM, n (%) | 40 (40.8%) | 18 (40.0%) | 22 (41.5%) | .8796 |
| CH, n (%) | 46 (46.9%) | 28 (62.2%) | 18 (34.0%) | .0052 |
| PGDM, n (%) | 12 (12.2%) | 6 (13.3%) | 6 (11.3%) | .7620 |
| Preeclampsia, n (%) | 34 (34.7%) | 16 (35.6%) | 18 (34.0%) | .8688 |
| AHI by PSG | 8.7 ± 14.2 | 16.6 ± 18.0 | 2.0 ± 1.1 | <.0001 |
Continuous variables are presented as means ± standard deviations and evaluated by t test. Categorical variables are presented as n (%) and evaluated by chi-square test. aOSA was determined based on the 98 valid PSG recordings with the threshold of AHI ≥5 events/h. bP values from comparing between the OSA group and non-OSA group. AHI = apnea-hypopnea index, BMI = body mass index, CH = chronic hypertension, GDM = gestational diabetes mellitus, GH = gestational hypertension, ODI = oxygen desaturation index, OSA = obstructive sleep apnea, PGDM = pregestational diabetes mellitus, PSG = polysomnography.
Comparison of respiratory parameters across different monitoring methods
Comparisons of sleep parameters across PSG, in-laboratory PM, and HSATs are shown in Table 2. When compared with total sleep time on PSG, total analysis time was 110 minutes longer on both in-laboratory PM and HSAT recordings (P < .001). When examining respiratory indices, no significant difference was observed among PSG, in-laboratory PM recordings, and HSAT in the AHI, obstructive apnea index, and hypopnea index. However, there were differences in the number of events and per-hour indices of central mixed apneas. Compared with PSG, mean and minimum oxygen saturation was lower on both the HSAT and in-laboratory PM recording (all P < .05). Although there was no significant difference in oxygen desaturation index with events ≥ 3% between the HSAT and in-laboratory PM, this index on the HSAT and in-laboratory PM was significantly higher for both than that on PSG (all P < .05).
Table 2.
Comparison of sleep parameters observed in PSG, simultaneous in-laboratory portable monitor recording, and HSAT.
| Mean ± SD | P a | |||
|---|---|---|---|---|
| PSG (n = 98) | Nox-T3lab (n = 92) | Nox-T3home (n = 98) | ||
| Total time,b minutes | 355.47 ± 62.42 | 468.02 ± 71.82 | 473.63 ± 68.08 | <.0001 |
| Respiratory indices, events/h | ||||
| AHI | 8.71 ± 14.19 | 8.19 ± 13.79 | 8.56 ± 10.42 | .9545 |
| OAI | 2.88 ± 9.87 | 2.96 ± 10.72 | 2.55 ± 7.19 | .9865 |
| CAI | 0.95 ± 5.78 | 0.09 ± 0.28 | 0.17 ± 0.52 | <.0001 |
| MAI | 0.13 ± 0.52 | 0.02 ± 0.07 | 0.03 ± 0.20 | <.0001 |
| HIc | 4.77 ± 4.60 | 5.11 ± 5.23 | 5.71 ± 6.12 | .5739 |
| No. of respiratory events | ||||
| Total apneas | 22.93 ± 80.9 | 21.91 ± 77.4 | 22.07 ± 64.77 | .9961 |
| Obstructive apneas | 18.07 ± 70.76 | 21.17 ± 76.51 | 20.44 ± 63.47 | .9919 |
| Central apneas | 4.90 ± 26.74 | 0.64 ± 1.82 | 1.38 ± 4.04 | <.0001 |
| Mixed apneas | 0.89 ± 3.21 | 0.14 ± 0.70d | 0.27 ± 1.46e | <.0001 |
| Hypopneasc | 27.74 ± 27.61 | 38.18 ± 37.22d | 44.04 ± 45.55e | .0362 |
| Oxygen saturation | ||||
| Mean SpO2, (%) | 96.00 ± 1.17 | 94.68 ± 1.21d | 94.63 ± 1.17e | <.0001 |
| Minimum SpO2, (%) | 87.80 ± 9.79 | 85.50 ± 5.91d | 85.17 ± 6.24e | .0127 |
| ODI 3%, (events/h) | 7.07 ± 12.38 | 10.54 ± 13.64d | 10.01 ± 10.91e | .2920 |
aP value from mixed-model analysis of variance comparing among methods. bTotal sleep time for PSG or total analysis time for Nox-T3lab and Nox-T3home. cScoring of hypopneas on PSG required an associated oxygen desaturation event of ≥ 3% and/or an arousal and scoring of hypopneas on the portable monitor recordings required an associated oxygen desaturation event ≥ 3%. dP < .05 from comparing between PSG and Nox-T3lab. eP < .05 from comparing between PSG and Nox-T3home. AHI = apnea-hypopnea index, CAI = central apnea index, HI = hypopnea index, HSAT = home sleep apnea testing, MAI = mixed apnea index, Nox-T3home = home sleep apnea testing using the Nox-T3 device, Nox-T3lab = in-laboratory portable monitor recording using the Nox-T3 device, OAI = obstructive apnea index, ODI = oxygen desaturation index, PSG = polysomnography, SD = standard deviation, SpO2 = oxygen saturation.
Figure 2 shows the percentage of participants with no OSA and mild, moderate, and severe OSA based on the AHI on PSG, HSAT, and in-laboratory PM recordings. Compared with PSG, a similar proportion of individuals were diagnosed with OSA (defined as AHI ≥ 5 events/h) and moderate or severe OSA (AHI ≥ 15 events/h), by both HSAT (P = .567 and .849) and in-laboratory PM (P = .971 and .674, respectively) (Table S2 (198.6KB, pdf) in the supplemental material). As mentioned above, 5 participants repeated this procedure at different trimesters. The tendency of AHI changes across trimesters measured by in-laboratory PM was similar to that measured by PSG (Figure S1 (198.6KB, pdf) ).
Figure 2. Percentage of patients falling into clinical OSA groupings.
This graph illustrates the percentage of patients falling into clinical OSA groupings of none (AHI < 5 events/h), mild (5 ≤ AHI < 15 events/h), moderate (15 ≤ AHI < 30 events/h), and severe (AHI ≥ 30 events/h) based on PSG, HSAT (NoxT3home), and in-laboratory portable monitor recording (Nox-T3lab). Scoring of hypopneas in all recordings required a ≥ 3% oxygen desaturation event. AHI = apnea-hypopnea index, HSAT = home sleep apnea testing, Nox-T3home = home sleep apnea testing using the Nox-T3 device, NoxT3lab = in-laboratory portable monitor recording using the Nox-T3 device, OSA = obstructive sleep apnea, PSG = polysomnogram.
Agreement between monitoring methods
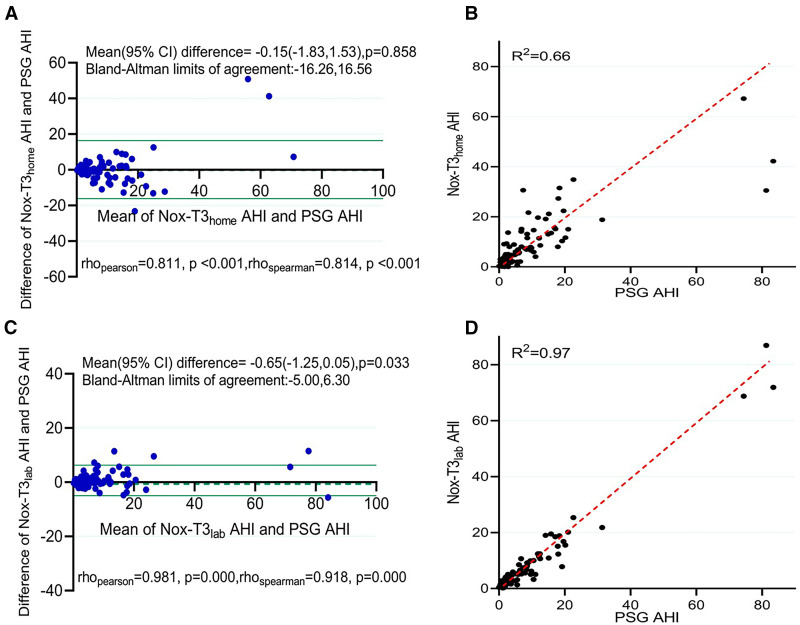
The AHI on PSG, HSAT, and in-laboratory PM was compared by Bland-Altman and identity plots, as shown in Figure 3. The Bland-Altman analysis of AHI on PSG vs HSAT showed a mean difference of −0.15 (95% confidence interval [CI]: −1.83, 1.53; P = .858), with limits of agreement ranging from −16.26 to 16.56 events/h. In contrast, in the Bland-Altman plot of AHI on PSG vs in-laboratory PM recordings, the mean difference was −0.65 (95% CI: −1.25, 0.05; P = .033), with narrower limits of agreement of −5.00 to 6.30 events/h. There was a closer relationship between PSG and simultaneous in-laboratory PM recordings than between PSG and HSAT, consistent with effects of differences in environment and night-to-night variability. For example, the AHI of 1 individual measured by the 3 methods varied from 30.5 events/h (HSAT) to 86.9 events/h (in-laboratory PM) and the percentage of sleep time in the supine position varied from 30.3% (HSAT) to 77.8% (in-laboratory PM).
Figure 3. Comparison of manually edited AHI on PSG to HSAT and in-laboratory portable monitor recordings.
(A) Bland-Altman plot of manually edited AHI on PSG compared with HSAT (Nox-T3home). (B) Identity plot of manually edited AHI on PSG compared with HSAT (Nox-T3home). (C) Bland-Altman plot of manually edited AHI on PSG compared with in-laboratory portable monitor recording (Nox-T3lab). (D) Identity plot of manually edited AHI on PSG compared with in-laboratory portable monitor recording (Nox-T3lab). Dashed line is the line of identity. Scoring of hypopneas in all recordings required a ≥ 3% oxygen desaturation event. Limits of agreement on the Bland-Altman analysis denote the interval within which 95% of the differences in AHI were included. AHI = apnea-hypopnea index, CI = confidence interval, HSAT = home sleep apnea testing, Nox-T3home = home sleep apnea testing using the Nox-T3 device, Nox-T3lab = in-laboratory portable monitor recording using the Nox-T3 device, PSG = polysomnogram.
Table 3 shows the diagnostic characteristics for different cutoffs of manually edited scoring AHI from the HSAT and in-laboratory PM recording compared with PSG. With a threshold AHI ≥ 5 events/h, the HSAT had 91% sensitivity, 85% specificity, 84% positive-predictive value, and 92% negative-predictive value, and there was 88% agreement between the 2 methods with a high kappa coefficient of 0.76 (Table 4). At an AHI ≥ 10 and ≥ 15 events/h, the HSAT had 80% and 75% agreement for sensitivity and 90% and 94% for specificity, respectively. Simultaneous in-laboratory PM recording demonstrated higher consistency with PSG. Moreover, even when dividing AHI into three clinically meaningful groups (< 5 events/h, 5–15 events/h, and ≥ 15 events/h), moderate to high reliability was still observed, with kappa coefficients of 0.60 for PSG vs HSAT, 0.80 for PSG vs in-laboratory PM recording, and 0.59 for HSAT vs in-laboratory (Table 4).
Table 3.
Values for different cutoffs of manually edited AHI measured by Nox-T3home and Nox-T3lab vs PSG.
| AHI (events/h) | Prevalence | Sensitivity | Exact 95% CI | Specificity | Exact 95% CI | PPV | NPV | ||
|---|---|---|---|---|---|---|---|---|---|
| LB | UB | LB | UB | ||||||
| Nox-T3lab vs PSG | |||||||||
| ≥ 5 | 0.46 | 0.93 | 0.79 | 0.98 | 0.94 | 0.82 | 0.99 | 0.93 | 0.94 |
| ≥ 10 | 0.25 | 0.87 | 0.65 | 0.97 | 0.99 | 0.91 | 1.00 | 0.95 | 0.96 |
| ≥ 15 | 0.16 | 0.80 | 0.51 | 0.95 | 0.99 | 0.92 | 1.00 | 0.92 | 0.96 |
| Nox-T3home vs PSG | |||||||||
| ≥ 5 | 0.46 | 0.91 | 0.78 | 0.97 | 0.85 | 0.72 | 0.93 | 0.84 | 0.92 |
| ≥ 10 | 0.26 | 0.80 | 0.59 | 0.92 | 0.90 | 0.81 | 0.96 | 0.74 | 0.93 |
| ≥ 15 | 0.16 | 0.75 | 0.47 | 0.92 | 0.94 | 0.86 | 0.98 | 0.71 | 0.95 |
Prevalence, sensitivity, specificity, PPV, NPV for different cutoffs of manually edited AHI from Nox-T3home and Nox-T3lab vs the PSG. Scoring of hypopneas on all 3 types of sleep test required an associated oxygen desaturation event ≥ 3%. AHI = apnea-hypopnea index, CI = confidence interval, LB = lower bound, Nox-T3home = home sleep apnea testing using the Nox-T3 device, Nox-T3lab = in-laboratory portable monitor recording using the Nox-T3 device, NPV = negative-predictive value, PPV = positive-predictive value, PSG = polysomnography, UB = upper bound.
Table 4.
Comparison of the categorized data from manually edited AHIs derived from PSG, in-laboratory PM, and HSAT in the kappa coefficient of agreement.
| AHI Categorical | Nox-T3lab vs PSG | Nox-T3home vs PSG | Nox-T3home vs Nox-T3lab | |||
|---|---|---|---|---|---|---|
| Agreement | kappa | Agreement | kappa | Agreement | kappa | |
| < 5 events/h | 0.88 | 0.80 | 0.76 | 0.60 | 0.75 | 0.59 |
| ≥ 5 events/h | 0.93 | 0.87 | 0.88 | 0.76 | 0.87 | 0.74 |
| ≥ 10 events/h | 0.96 | 0.88 | 0.88 | 0.69 | 0.87 | 0.65 |
| ≥ 15 events/h | 0.96 | 0.83 | 0.91 | 0.67 | 0.90 | 0.63 |
AHI = apnea-hypopnea index, Nox-T3home = home testing using the Nox-T3 device, Nox-T3lab = in-laboratory portable monitor recording using the Nox-T3 device, PM = portable monitor, PSG = polysomnography.
Compared with manually edited scoring, the AHI on automatic scoring of the PM recording had good agreement (Figure 4). On HSAT, the mean difference of manual and automatic scored AHI was 1.04 (95% CI: 0.57, 1.52; P < .001) and associated limits of agreement ranged from −5.68 to 3.60 events/h. For the in-laboratory PM, there were similar results between the automatic and manual scoring (mean difference: 1.07; 95% CI: 0.58, 1.57; P < .001), with limits of agreement ranging from −5.78 to 3.63 events/h. As additional supporting evidence, near-perfect correlations were observed between automatic and manually scored AHI in identity plots for both HSAT (R2 = 0.96) and in-laboratory PM (R2 = 0.98).
Figure 4. Comparison of automatically scored AHI to manually edited AHI on HSAT and in-laboratory portable monitor recording.
(A) Bland-Altman plot comparing automatically scored AHI with manually edited AHI on HSAT (Nox-T3home). (B) Identity plot comparing automatically scored AHI with manually edited AHI on HSAT (Nox-T3home). (C) Bland-Altman plot comparing automatically scored AHI with in-laboratory portable monitor recording (Nox-T3lab). (D) Identity plot comparing automatically scored AHI with in-laboratory portable monitor recording (Nox-T3lab). Dashed line is the line of identity. Scoring of hypopneas in all recordings required a ≥ 3% oxygen desaturation event. Limits of agreement on the Bland-Altman analysis denote the interval within which 95% of the differences in AHI were included. AHI = apnea-hypopnea index, CI = confidence interval, HSAT = home sleep apnea testing, Nox-T3home = home sleep apnea testing using the Nox-T3 device, Nox-T3lab = in-laboratory portable monitor recording using the Nox-T3 device.
DISCUSSION
Data in the current study demonstrated good performance of a type 3 PM Nox-T3 in detecting OSA in those pregnant women with a PSG. The sensitivity, specificity, positive-, and negative-predictive probabilities were high and simultaneous recordings of PM and PSG, with limits of agreement on Bland-Altman analysis of −5.00 and 6.30 events/h. The HSAT and PSG results showed good agreement at the different thresholds for AHI severity metrics. In our study, the prevalence of OSA among the enrolled participants was 45.9% determined by PSG, 50.0% determined by HSAT, and 45.7% determined by in-laboratory PM.
The failure rate of 5.9% on initial HSAT in the participants (3.2% after repeat testing) validated the feasibility of self-application of sensors by patients at home without supervision, consistent with our previous studies that validated HSAT in diagnosing OSA in Chinese adults with and without major comorbidities.16–18 An HSAT is cost-effective compared with in-laboratory PSG32 but can be performed in a more familiar sleeping environment. As described previously, pregnant women prefer HSAT, rather than stay overnight in sleep center.20 In our experience, nearly 50% of the pregnant women refused to do further in-laboratory PSG after HSAT, reflecting the perceived comfort of home study. In circumstances such as taking care of other children at home or exposure to hospital-based infections, an HSAT would address the need to confirm the clinical impression of OSA. In general, the results of HSAT and its low failure rate suggest beneficial effects for improving access, cutting costs, and guiding management.
The HSAT is more widely accepted as an alternative to PSG to detect OSA in the nonpregnant population, particularly in individuals with a high pretest likelihood of OSA.14 However, the majority of OSA severity identified in pregnancy is mild, even if the population is prone to be obesity.4,33,34 O’Brien et al20 performed the Watch-PAT-200 device (Itamar Medical Cesarea, Israel) concurrent with in-home PSG in 31 pregnant women at ≥ 28 weeks’ gestation. Sharkey et al21 examined 16 pregnant women with the Apnea Risk Evaluation System and in-laboratory PSG, measured simultaneously. Although these 2 studies reported reasonable to excellent sensitivity and specificity for the identification of OSA by different PMs, HSAT use was not performed at home.20,21 Additionally, the sample size was relatively small, and few pregnant women with AHI ≥ 30 events/h were included, leading to a severity bias. The current study advances these prior observations by comparing the PMs at home as well as simultaneously in the laboratory with a relatively larger sample size. Overall, our findings confirm their performance in prior studies20–22 and further validate the independent, home use of PMs.
The current study is also significant in validating the HSAT in Chinese pregnant women. Previous studies all targeted pregnant women at high risk of OSA and validation of type III PMs was performed in pregnant women (> 80% participants were Caucasian and African American) with a higher BMI.20–22 In contrast, the mean AHI in our study was higher and higher percentages of women were diagnosed with OSA at the criterion of AHI ≥ 5 events/h. One reason could be that Chinese patients with OSA who are less obese have a smaller retropalatal airway and more bony restriction compared with Icelandic patients of similar age, sex, and oxygen desaturation index.35 Ethnicity and craniofacial anatomic structures may interact to produce severity differences among pregnant populations. In addition, the physiological and hormonal changes in pregnancy can potentially lead to increased resistance36 and reduced cross-sectional area of the upper airway,37,38 accelerating the development of OSA. As in dominant male OSA with a lower BMI,18 HSAT by a type III PM Nox-T3 tool also works well in less obese, pregnant Chinese woman.
As in the study by Facco et al,22 close agreement between the automatic and manually edited scoring of the PM recording was also found here. Until now, there were few studies on how auto-scoring algorithms perform in pregnancy compared with both technician review of recordings of HSAT and in-laboratory PSG. Our study demonstrated the reliability of the automatic scoring algorithm of PM for pregnant women compared with manual scoring, with mean differences in AHI of 1.04 events/h for HSAT and 1.07 events/h for in-laboratory tests. Although manual scoring for HSAT is strongly recommended in the current guidelines, automatic scoring seems to be a reliable alternative in resource- and skill-set–limited communities.
The strengths of our study are the large sample size and using PSG as the reference when evaluating the reliability of PM during both HSAT and in-laboratory PM. Additionally, our study revealed close agreement of automatic scoring and technician scoring of the PM. A limitation of the current study is that more than 50% of participants who completed the HSAT refused further PSG tests, highlighting the need for home-based alternatives for evaluating OSA in this population, although the comparison of demographic data showed some differences between those with or without PSG. This is probably because the pregnant women with risk factors (increased age, higher BMI, with medical complications) tend to have a higher AHI according to HSAT results, and thus would choose to complete further testing with PSG (Table S1 (198.6KB, pdf) ). Another limitation is that the number of participants who repeated this procedure at different trimester for dynamic changes was relatively small. However, we still found a similar tendency of AHI changes across trimesters measured by in-laboratory PM and PSG (Figure S1 (198.6KB, pdf) ). Third, due to the small sample size at each trimester, we did not separate the data to show whether the diagnostic performance changes at different points in pregnancy.
In summary, this larger, more powerful study demonstrates the ability to use the Nox-T3 PM for HSAT to diagnose OSA in pregnant women with suspected OSA. Comparison results suggest that close agreement was present between PSG and HSAT, and even closer agreement was observed on the simultaneous in-laboratory PSG and PM recordings. Close agreement was also observed between automatic software scoring and manually edited scoring. The low failure rate, reliable self-application, and good performance suggest that PMs could be available tools for the pregnant population to detect OSA, particularly in worldwide communities where access to antenatal care is challenging.
Taken together, the results of this study indicate that a PM is a reasonable substitute for in-laboratory PSG in pregnancy with suspected OSA. Automatic scoring could be considered as a reliable alternative in clinical practice when resources for manual scoring are insufficient.
DISCLOSURE STATEMENT
The research was supported by National Natural Science Foundation of China (82020108001) to F.H. The authors report no conflict of interest.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- BMI
body mass index
- CI
confidence interval
- HSAT
home sleep apnea test
- OSA
obstructive sleep apnea
- PM
portable monitor
- PSG
polysomnography
REFERENCES
- 1. Liu L , Su G , Wang S , Zhu B . The prevalence of obstructive sleep apnea and its association with pregnancy-related health outcomes: a systematic review and meta-analysis . Sleep Breath. 2019. ; 23 ( 2 ): 399 – 412 . [DOI] [PubMed] [Google Scholar]
- 2. Phan K , Pamidi S , Gomez YH , et al . Sleep-disordered breathing in high-risk pregnancies is associated with elevated arterial stiffness and increased risk for preeclampsia . Am J Obstet Gynecol. 2022. ; 226 ( 6 ): 833.e1 – 833.e20 . [DOI] [PubMed] [Google Scholar]
- 3. Bourjeily G , Danilack VA , Bublitz MH , et al . Obstructive sleep apnea in pregnancy is associated with adverse maternal outcomes: a national cohort . Sleep Med. 2017. ; 38 : 50 – 57 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Facco FL , Parker CB , Reddy UM , et al . Association between sleep-disordered breathing and hypertensive disorders of pregnancy and gestational diabetes mellitus . Obstet Gynecol. 2017. ; 129 ( 1 ): 31 – 41 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pamidi S , Marc I , Simoneau G , et al . Maternal sleep-disordered breathing and the risk of delivering small for gestational age infants: a prospective cohort study . Thorax. 2016. ; 71 ( 8 ): 719 – 725 . [DOI] [PubMed] [Google Scholar]
- 6. Redhead K , Walsh J , Galbally M , Newnham JP , Watson SJ , Eastwood P . Obstructive sleep apnea is associated with depressive symptoms in pregnancy . Sleep. 2020. ; 43 ( 5 ): zsz270 . [DOI] [PubMed] [Google Scholar]
- 7. Facco FL , Ouyang DW , Zee PC , et al . Implications of sleep-disordered breathing in pregnancy . Am J Obstet Gynecol. 2014. ; 210 ( 6 ): 559.e1 – 559.e6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bellamy L , Casas JP , Hingorani AD , Williams D . Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis . Lancet. 2009. ; 373 ( 9677 ): 1773 – 1779 . [DOI] [PubMed] [Google Scholar]
- 9. Morrakotkhiew W , Chirdkiatgumchai V , Tantrakul V , Thampratankul L . Early developmental outcome in children born to mothers with obstructive sleep apnea . Sleep Med. 2021. ; 88 : 90 – 95 . [DOI] [PubMed] [Google Scholar]
- 10. Kramer CK , Campbell S , Retnakaran R . Gestational diabetes and the risk of cardiovascular disease in women: a systematic review and meta-analysis . Diabetologia. 2019. ; 62 ( 6 ): 905 – 914 . [DOI] [PubMed] [Google Scholar]
- 11. Heida KY , Franx A , van Rijn BB , et al . Earlier age of onset of chronic hypertension and type 2 diabetes mellitus after a hypertensive disorder of pregnancy or gestational diabetes mellitus . Hypertension. 2015. ; 66 ( 6 ): 1116 – 1122 . [DOI] [PubMed] [Google Scholar]
- 12. Facco FL , Redline S , Hunter SM , et al . Sleep-disordered breathing in pregnancy and after delivery: associations with cardiometabolic health . Am J Respir Crit Care Med. 2022. ; 205 ( 10 ): 1202 – 1213 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Blyton DM , Skilton MR , Edwards N , Hennessy A , Celermajer DS , Sullivan CE . Treatment of sleep disordered breathing reverses low fetal activity levels in preeclampsia . Sleep. 2013. ; 36 ( 1 ): 15 – 21 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guilleminault C , Palombini L , Poyares D , Takaoka S , Huynh NT , El-Sayed Y . Pre-eclampsia and nasal CPAP: part 1. Early intervention with nasal CPAP in pregnant women with risk-factors for pre-eclampsia: preliminary findings . Sleep Med. 2007. ; 9 ( 1 ): 9 – 14 . [DOI] [PubMed] [Google Scholar]
- 15. Collop NA , Anderson WM , Boehlecke B , et al. Portable Monitoring Task Force of the American Academy of Sleep Medicine . Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients . J Clin Sleep Med. 2007. ; 3 ( 7 ): 737 – 747 . [PMC free article] [PubMed] [Google Scholar]
- 16. Li S , Xu L , Dong X , et al . Home sleep apnea testing of adults with chronic heart failure . J Clin Sleep Med. 2021. ; 17 ( 7 ): 1453 – 1463 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chang Y , Xu L , Han F , et al . Validation of the Nox-T3 portable monitor for diagnosis of obstructive sleep apnea in patients with chronic obstructive pulmonary disease . J Clin Sleep Med. 2019. ; 15 ( 4 ): 587 – 596 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xu L , Han F , Keenan BT , et al . Validation of the Nox-T3 portable monitor for diagnosis of obstructive sleep apnea in Chinese adults . J Clin Sleep Med. 2017. ; 13 ( 5 ): 675 – 683 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tantrakul V , Numthavaj P , Guilleminault C , et al . Performance of screening questionnaires for obstructive sleep apnea during pregnancy: a systematic review and meta-analysis . Sleep Med Rev. 2017. ; 36 : 96 – 106 . [DOI] [PubMed] [Google Scholar]
- 20. O’Brien LM , Bullough AS , Shelgikar AV , Chames MC , Armitage R , Chervin RD . Validation of Watch-PAT-200 against polysomnography during pregnancy . J Clin Sleep Med. 2012. ; 8 ( 3 ): 287 – 294 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sharkey KM , Waters K , Millman RP , Moore R , Martin SM , Bourjeily G . Validation of the Apnea Risk Evaluation System (ARES) device against laboratory polysomnography in pregnant women at risk for obstructive sleep apnea syndrome . J Clin Sleep Med. 2014. ; 10 ( 5 ): 497 – 502 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Facco FL , Lopata V , Wolsk JM , Patel S , Wisniewski SR . Can we use home sleep testing for the evaluation of sleep apnea in obese pregnant women? Sleep Disord. 2019. ; 2019 : 3827579 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shiwaku K , Anuurad E , Enkhmaa B , Kitajima K , Yamane Y . Appropriate BMI for Asian populations . Lancet. 2004. ; 363 ( 9414 ): 1077 . [DOI] [PubMed] [Google Scholar]
- 24. Rasmussen KM , Abrams B , Bodnar LM , Butte NF , Catalano PM , Maria Siega-Riz A . Recommendations for weight gain during pregnancy in the context of the obesity epidemic . Obstet Gynecol. 2010. ; 116 ( 5 ): 1191 – 1195 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Facco FL , Ouyang DW , Zee PC , Grobman WA . Development of a pregnancy-specific screening tool for sleep apnea . J Clin Sleep Med. 2012. ; 8 ( 4 ): 389 – 394 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dominguez JE , Krystal AD , Habib AS . Obstructive sleep apnea in pregnant women: a review of pregnancy outcomes and an approach to management . Anesth Analg. 2018. ; 127 ( 5 ): 1167 – 1177 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kushida CA , Littner MR , Morgenthaler T , et al . Practice parameters for the indications for polysomnography and related procedures: an update for 2005 . Sleep. 2005. ; 28 ( 4 ): 499 – 521 . [DOI] [PubMed] [Google Scholar]
- 28. Berry RB , Budhiraja R , Gottlieb DJ , et al. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine . Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events . J Clin Sleep Med. 2012. ; 8 ( 5 ): 597 – 619 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Eberhard A , Calabrese P , Baconnier P , Benchetrit G . Comparison between the respiratory inductance plethysmography signal derivative and the airflow signal . Adv Exp Med Biol. 2001. ; 499 : 489 – 494 . [DOI] [PubMed] [Google Scholar]
- 30. Bland JM , Altman DG . Statistical methods for assessing agreement between two methods of clinical measurement . Lancet. 1986. ; 1 ( 8476 ): 307 – 310 . [PubMed] [Google Scholar]
- 31. Bland JM , Altman DG . Comparing methods of measurement: why plotting difference against standard method is misleading . Lancet. 1995. ; 346 ( 8982 ): 1085 – 1087 . [DOI] [PubMed] [Google Scholar]
- 32. Louis J , Auckley D , Miladinovic B , et al . Perinatal outcomes associated with obstructive sleep apnea in obese pregnant women . Obstet Gynecol. 2012. ; 120 ( 5 ): 1085 – 1092 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Facco FL , Ouyang DW , Zee PC , Grobman WA . Sleep disordered breathing in a high-risk cohort prevalence and severity across pregnancy . Am J Perinatol. 2014. ; 31 ( 10 ): 899 – 904 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kim RD , Kapur VK , Redline-Bruch J , et al . An economic evaluation of home versus laboratory-based diagnosis of obstructive sleep apnea . Sleep. 2015. ; 38 ( 7 ): 1027 – 1037 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Xu L , Keenan BT , Wiemken AS , et al . Differences in three-dimensional upper airway anatomy between Asian and European patients with obstructive sleep apnea . Sleep. 2020. ; 43 ( 5 ): zsz273 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Franklin KA , Holmgren PA , Jönsson F , Poromaa N , Stenlund H , Svanborg E . Snoring, pregnancy-induced hypertension, and growth retardation of the fetus . Chest. 2000. ; 117 ( 1 ): 137 – 141 . [DOI] [PubMed] [Google Scholar]
- 37. Izci B , Riha RL , Martin SE , et al . The upper airway in pregnancy and pre-eclampsia . Am J Respir Crit Care Med. 2003. ; 167 ( 2 ): 137 – 140 . [DOI] [PubMed] [Google Scholar]
- 38. Izci B , Vennelle M , Liston WA , Dundas KC , Calder AA , Douglas NJ . Sleep-disordered breathing and upper airway size in pregnancy and post-partum . Eur Respir J. 2006. ; 27 ( 2 ): 321 – 327 . [DOI] [PubMed] [Google Scholar]