Abstract
Study Objectives:
Insufficient sleep leads to overconsumption, but the factors contributing to this effect are poorly understood. Therefore, we assessed the influence of prolonged curtailment of sleep on free-living eating patterns linked with overconsumption and explored associations of these eating patterns with diet quality under different sleep conditions.
Methods:
Sixty-five adults (47 females) participated in outpatient randomized crossover studies with two 6-week conditions: adequate sleep (7–9 h/night) and sleep restriction (−1.5 h/night relative to screening). Food records were collected over 3 nonconsecutive days, from which we ascertained data on eating frequency, midpoint, and window and intakes of energy and nutrients. Linear mixed models were used to assess the impact of sleep condition on change in eating pattern (sleep × week interaction) and the relation between eating patterns and dietary intakes (sleep × eating pattern interaction).
Results:
Sleep condition impacted the change in eating frequency across weeks, with eating frequency increasing in sleep restriction relative to adequate sleep (β = 0.3 ± 0.1; P = .046). Across conditions, eating more frequently tended to relate to higher energy intakes (β = 60.5 ± 34.6; P = .082). Sleep also influenced the relation of variability in eating midpoint with intakes of saturated fat (β = 6.0 ± 2.1; P = .005), polyunsaturated fat (β = −3.9 ± 2.0; P = .051), and added sugar (β = 17.3 ± 6.2; P = .006), with greater midpoint variability associated with more adverse changes in these diet quality components in sleep restriction vs adequate sleep.
Conclusions:
Chronic short sleep increases eating frequency and adversely influences associations of variability in meal timing with components of diet quality. These findings help to explain how short sleep leads to overconsumption and obesity.
Clinical Trial Registration:
Registry: ClinicalTrials.gov; Name: Impact of Sleep Restriction in Women; URL: https://clinicaltrials.gov/ct2/show/NCT02835261; Identifier: NCT02835261 and Name: Impact of Sleep Restriction on Performance in Adults; URL: https://clinicaltrials.gov/ct2/show/NCT02960776; Identifier: NCT02960776.
Citation:
Barragán R, Zuraikat FM, Tam V, RoyChoudhury A, St-Onge M-P. Changes in eating patterns in response to chronic insufficient sleep and their associations with diet quality: a randomized trial. J Clin Sleep Med. 2023;19(11):1867–1875.
Keywords: sleep, eating frequency, eating window, eating midpoint, diet quality
BRIEF SUMMARY
Current Knowledge/Study Rationale: Short sleep leads to overeating and is associated with higher risk for obesity. Longer time awake provides greater opportunity to eat under conditions of short sleep.
Study Impact: Interventions for preventing obesity and other cardiometabolic diseases should incorporate information on sleep duration to make dietary counseling more effective. Reducing variability in meal frequency, meal timing, and/or eating window should be tested as potential countermeasures to the adverse dietary effects of short sleep.
INTRODUCTION
Over one-third of US adults fail to achieve the recommended 7 hours of sleep per night.1 This is of concern given consistent evidence of a role of suboptimal sleep in promoting adverse health outcomes, including obesity2 and other cardiometabolic diseases.3 While it is clear that a key driver of these associations is a shift toward higher energy intakes4 and poorer diet quality5–7 in response to short sleep, the mechanisms underlying these effects are poorly characterized. It has been postulated that these orexigenic effects of sleep loss could be due, in part, to prolonged eating window and increased opportunity to eat with less time spent asleep.8
The frequency and timing of eating, and their variability across days, were highlighted as emerging determinants of cardiometabolic health.9 Although there are mixed results across different populations, studies in US adults demonstrate a positive association between eating frequency and measures of abdominal obesity,10,11 and there is evidence of higher energy intakes among those eating more frequently.12,13 There is also growing support for adverse cardiometabolic effects of later mealtimes and longer eating windows. A small experiment from our lab showed that delaying mealtimes relative to a fixed sleep period increased 24-hour energy intake by > 400 kcal compared to earlier meals.14 Similarly, some studies find that restricting the eating window reduces caloric intakes among populations with metabolic diseases.15,16 Variability in these eating patterns may also be a determinant of obesity and cardiometabolic risk. We have shown that greater interdaily variability in time of first meal and nightly fasting duration, a proxy for eating window, tended to relate to higher waist circumference and body mass index (BMI), respectively, in a cross-sectional study of US women.17
Given the growing body of literature on the role of eating patterns and their day-to-day regularity in determining weight-related outcomes, it is plausible that these factors could explain shifts toward positive energy balance under conditions of short sleep. Indeed, there are studies to suggest that sleep curtailment could alter the frequency, timing, or window of eating in ways that adversely impact cardiometabolic health. Population-based data show that adults with short sleep have longer eating windows than their adequate sleeping counterparts.18,19 Moreover, we found that severe sleep restriction (SR) over 4 nights leads to greater meal frequency alongside higher energy and fat intakes,6 although we did not directly relate changes in eating frequency to diet quantity and quality. Given that much of the existing data linking sleep duration with eating patterns are observational, and intervention studies induce severe SR, longer-term intervention studies that more closely mimic real-life situations are needed. Such studies would allow us to test the underlying hypothesis that short sleep influences food intake via increased opportunity to eat.
In the current study, we exposed individuals to two 6-week sleep conditions in a random order and evaluated eating patterns and dietary intakes during each condition. The aims of this investigation were to test the effects of prolonged, mild SR on eating patterns (eating frequency, eating window length, and eating midpoint) and to assess associations of these eating patterns, and their regularity, with dietary outcomes predictive of cardiometabolic risk. In addition, we sought to explore whether associations of eating patterns with diet outcomes differed under various sleep duration conditions (SR vs adequate sleep [AS]).
METHODS
Study participants
Adults from the New York City area who were 20–75 years old and had BMI 25–35, or 20–24.9 kg/m2 with ≥ 1 parent with obesity, were recruited to participate in clinical trials of prolonged mild sleep curtailment between 2016 and 2020. Eligible participants were free of neurological, psychiatric, metabolic, and cardiovascular diseases and had healthy habitual sleep, including good sleep quality (scored ≤ 5 on the Pittsburgh Sleep Quality Index20), low daytime sleepiness (< 10 on the Epworth Sleepiness Scale21), low risk for sleep apnea (Berlin Questionnaire22), and no other sleep disorders (Sleep Disorders Questionnaire23). Participants were excluded if they had delayed or advanced sleep phase (Morningness-Eveningness Questionnaire24), took daytime naps, worked nontraditional day hours or overnight, planned to travel across time zones over the course of the study, or had a history of smoking or substance abuse, excessive caffeine intake (> 300 mg/d), or recent weight change (± 2.5 kg in the previous 3 months). Females taking oral contraceptives or hormone replacement therapy and those who were pregnant or within 1 year postpartum were also excluded.
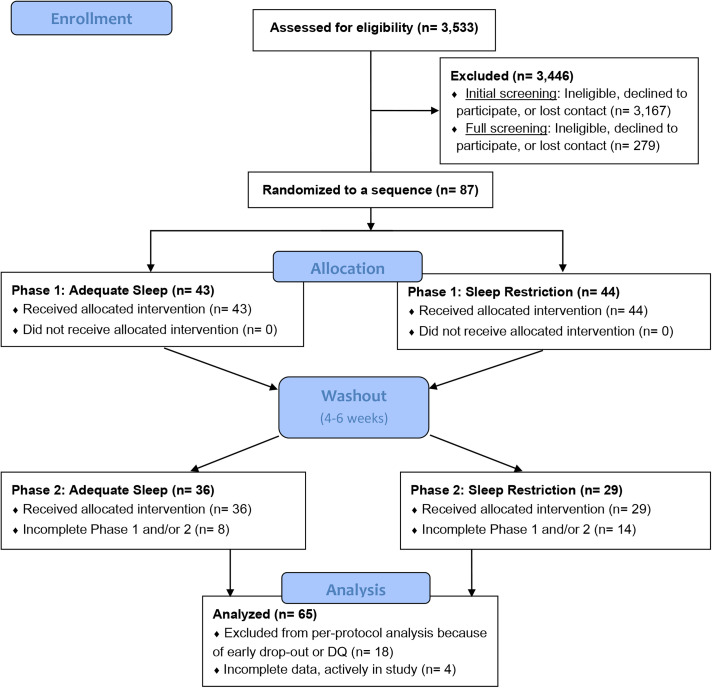
Participants meeting all predefined eligibility criteria underwent a 2-week sleep screening with wrist actigraphy (GT3×+, Actigraph LLC, Pensacola, FL) and nightly sleep diaries to determine habitual sleep. Those with AS, defined as achieving an average of ≥ 7 hours total sleep time over 14 nights with < 4 nights with sleep duration < 6 h/night, were invited to participate in the study. Data for this analysis were collected between August 2016 and March 2020, at which point research was paused due to the COVID-19 pandemic. The analytic sample comprised all participants who had completed the study prior to March 2020. The participant flow chart is provided in Figure 1. All research methods and procedures were performed in accordance with the Declaration of Helsinki for human studies and were approved by the institutional review board of Columbia University Irving Medical Center (New York, NY). Participants were given the opportunity to ask questions about the projects prior to providing informed consent. Along with informed consent, participants were required to certify that they would not operate a motor vehicle during the SR phase. The clinical trials are registered on clinicaltrials.gov (NCT02960776, NCT02835261).
Figure 1. CONSORT diagram for the analytic sample.
Sleep protocol
This evaluation combines data from 2 randomized crossover outpatient studies with identical sleep protocols. Namely, both studies were comprised of 2 sleep conditions of 6 weeks each: AS, consisting of scheduled sleep ≥ 7 h/night based on their screening data; and SR, consisting of scheduled sleep with a 1.5-hour delay in bedtimes relative to screening, with consistent wake time, to achieve a reduction of ∼90 minutes in total sleep time. Sleep was monitored by wrist actigraphy using the Actigraph GT3×+ and scored using the Cole-Kripke algorithm (ActiLife software version 6, ActiGraph, Pensacola, FL). Participants completed night sleep diaries to assist with scoring of nightly sleep episode. Sleep data were verified by research staff weekly for adherence. Participants were randomized to either SR or AS in phase 1 using a random digit generator; phase 2 was the alternate sleep condition. This was revealed by the research assistant at the completion of the baseline visit. Due to the nature of the intervention, blinding was not possible. Study phases were separated by a 4- to 6-week washout period.
Assessment of eating patterns and dietary intakes
At baseline, week 3 (n = 28), and endpoint of each study phase, participants completed 3-d food records on 2 nonconsecutive weekdays and 1 weekend day. At the baseline visit of each study phase, participants were given written and verbal instructions for completing the food records, including methods for estimating food amounts. Participants were also encouraged to provide images of the foods that they consumed with a standard-sized reference object in the image when possible (eg, ruler, US dollar bill) to aid in portion size estimation. They were also told to report the precise times when food and beverages were consumed.
Food records were entered into the Nutrition Data System for Research (University of Minnesota, Minneapolis, MN) for nutrient analysis. Prior to data entry, records were reviewed by research staff with the participant to verify unclear descriptions, errors, omissions, or potentially implausible entries. Data were exported from Nutrition Data System for Research and averaged over the 3-day collection period for each participant. Diet outcomes of interest were intakes of energy, macronutrients, including saturated fat (SFA), mono- (MUFA) and polyunsaturated (PUFA) fats, and dietary glycemic index given their roles in determining cardiometabolic outcomes.25–27
Information on eating patterns was also ascertained from food records. Participants labeled each eating occasion as breakfast, brunch, lunch, snack, dinner/supper, or beverage only. An eating occasion was defined as any eating/drinking episode providing at least 50 kcal (210 kJ) separated by > 15 minutes9 that occurred between wake time and bedtime and eating frequency was the sum of all eating occasions in a single day. The eating window was defined as the time elapsed between the first and last eating occasion of the day. The midpoint of the eating window was the clock time at the halfway point of the eating window. The standard deviation (SD) of eating frequency, eating window, and midpoint of eating over 3 days provided indices of variability of eating behaviors for each participant.
Statistical analysis
Baseline characteristics of the analytic sample are summarized as Mean ± SD for continuous variables and count (%) for categorical variables. Independent sample t tests were used to evaluate mean differences in descriptive characteristics between males and females for continuous variables. Linear mixed models were used to evaluate how the effect of eating pattern (eating frequency, window, midpoint, and their variability) on energy and nutrient intakes changes differentially over time for sleep condition (SR vs AS). In separate models, measures of food intake were used as outcome variables, and variables for eating pattern were used as independent variables. Further, sleeping condition (SR vs AS) and Week of measurement were used as independent variables as well. To evaluate how eating pattern and intake differentially change over time with sleep conditions (SR vs AS), we have also included interactions between sleep condition, week, and eating pattern in the model. We used sex (female vs male), age, and energy (kcal) as covariates in an initial model. Sex and age were dropped from the model if not found to be significant, but kcal was always kept in the final model. When energy (kcal) was the outcome variable, it was not used as a covariate. Subject was used as a random effect. Given limited power to detect interactions and our interest in assessing relations of eating patterns with diet outcomes under different sleep conditions, we also conducted linear model analyses evaluating associations of eating patterns with dietary intakes stratified by sleep condition. The R software version 3.6.1 was used for statistical analyses. Results are presented as β ± SE and were considered significant at P < .05 and approaching significance at P < .10.
RESULTS
Participant characteristics
Descriptive characteristics of the analytic sample are provided in Table 1. Of the 65 participants included in analyses, 47 were female (72.3%) and 44 identified as a racial and/or ethnic minority (67.7%). Average sleep duration at screening was 7 hours 37 minutes with a bedtime of 23:17. Participants reduced their total sleep time by 93.7 ± 4.1 min/d (relative to screening) during SR vs 15.1 ± 4.1 min/d during AS (sleep × week interaction P = .001). No differences between the sexes were observed for any measures except midpoint of sleep (P = .044) and eating (P = .026), which were both later in males compared to females.
Table 1.
Characteristics of the analytic sample at baseline.*
| Total (n = 65) | Male (n = 18) | Female (n = 47) | |
|---|---|---|---|
| Demographic and Health | |||
| Age (y) | 35.2 ± 13.0 | 32.4 ± 11.6 | 36.2 ± 13.5 |
| BMI (kg/m2) | 26.1 ± 3.6 | 26.8 ± 3.4 | 25.8 ± 3.6 |
| Race/ethnicity | |||
| Non-Hispanic White | 22 (34) | 6 (33) | 16 (34) |
| Non-White or Hispanic | 43 (66) | 12 (67) | 31 (66) |
| Education | |||
| ≥ College degree | 54 (83) | 13 (72) | 41 (87) |
| < College degree | 11 (17) | 5 (28) | 6 (13) |
| Sleep Behaviors | |||
| Sleep duration (min) | 457 ± 23 | 459 ± 26 | 441 ± 21 |
| Sleep efficiency (%) | 91.4 ± 2.9 | 90.6 ± 2.9 | 91.7 ± 2.9 |
| Midpoint of sleep (hh:mm) | 03:50 ± 01:02 | 04:14 ± 00:51a | 03:42 ± 01:02b |
| Bedtime (hh:mm) | 23:17 ± 02:57 | 22:52 ± 05:25 | 23:26 ± 01:03 |
| Eating Patterns | |||
| Eating frequency (#) | 4.5 ± 1.4 | 3.9 ± 1.2 | 4.7 ± 1.4 |
| Eating window (h) | 10.8 ± 1.8 | 10.9 ± 1.8 | 10.8 ± 1.9 |
| Eating midpoint (hh:mm) | 14:33 ± 01:04 | 15:02 ± 0:43a | 14:22 ± 01:08b |
| SD eating frequency (#) | 0.8 ± 0.5 | 0.7 ± 0.4 | 0.8 ± 0.5 |
| SD eating window (h) | 1.8 ± 1.2 | 1.7 ± 0.8 | 1.9 ± 1.3 |
| SD eating midpoint (hh:mm) | 0:48 ± 0:26 | 0:41 ± 0:19 | 0:51 ± 0:29 |
Values are presented as mean ± SD or n (%). *Row values with different letters differ significantly (P < .05) based on results of an independent samples t-test. BMI = body mass index, SD = standard deviation.
The effect of sleep condition on eating patterns
Participants increased their eating frequency (sleep condition × week: β = 0.3 ± 0.1; P = .046) and tended to increase the length of their eating window (β = 0.4 ± 0.2; P = .090) during SR relative to AS. The change in eating midpoint or variability of eating behaviors across weeks did not differ by sleep condition (all P > .10). Raw means for eating pattern outcomes at baseline and endpoint of each sleep condition are represented in Table S1 (110.5KB, pdf) in the supplemental material.
Associations between eating patterns and diet under different sleep conditions
Exposure: eating frequency
At baseline, in AS, eating more frequently tended to relate to higher intakes of energy (β = 60.5 ± 34.6; P = .082) and MUFA (β = 1.0 ± 0.6; P = .071). For week 0, MUFA intake is significantly less for SR than AS in participants with higher eating frequency (sleep condition × eating frequency: β = −2.2 ± 0.8; P = .004). However, we observed more MUFA intake increase over time for SR than AS, with increased eating frequency (sleep condition × week × eating frequency: β = 0.6 ± 0.2; P = .003). Results of stratified analyses demonstrated that, during SR, eating more frequently was associated with lower total intake of fat (β = −3.3 ± 1.2; P = .013) but higher intakes of carbohydrate (β = 9.2 ± 3.2; P = .006) and added sugars (β = 6.2 ± 1.9; P = .002) (Table 2). During AS, no association of eating frequency with nutrient intakes was detected.
Table 2.
Associations of eating patterns with dietary intakes under different sleep conditions.
| Exposure | Outcomea | β ± SE: AS and SRb | β ± SE: AS Onlyb | β ± SE: SR Onlyb |
|---|---|---|---|---|
| Eating frequency | Energy (kcal) | 60.5 ± 34.6# | 41.6 ± 36.7 | 68.5 ± 40.2# |
| Total fat | 0.7 ± 1.1 | 0.2 ± 1.1 | −3.3 ± 1.3** | |
| SFA | −0.5 ± 0.6 | −0.8 ± 0.7 | −1.2 ± 0.6 | |
| MUFA | 1.0 ± 0.6# | 0.9 ± 0.6 | −1.0 ± 0.6# | |
| PUFA | −0.0 ± 0.5 | 0.1 ± 0.6 | −1.0 ± 0.6# | |
| Protein | 1.1 ± 1.3 | 0.9 ± 1.5 | 0.5 ± 1.4 | |
| Carbohydrate | −1.4 ± 2.8 | 0.4 ± 2.9 | 9.2 ± 3.2** | |
| Fiber | 0.3 ± 0.5 | 0.3 ± 0.6 | 1.0 ± 0.6 | |
| Added sugar | −1.3 ± 1.7 | −0.7 ± 1.7 | 6.2 ± 1.9* | |
| Dietary GI (units) | −0.5 ± 0.4 | −0.2 ± 0.4 | −0.4 ± 0.5 | |
| Eating window | Energy (kcal) | −2.5 ± 20.5 | 4.6 ± 21.2 | 17.6 ± 22.3 |
| Total fat | 0.2 ± 0.6 | 0.4 ± 0.6 | −0.9 ± 0.7 | |
| SFA | −0.1 ± 0.3 | −0.1 ± 0.4 | −0.4 ± 0.3 | |
| MUFA | 0.2 ± 0.3 | 0.3 ± 0.3 | −0.4 ± 0.3 | |
| PUFA | 0.2 ± 0.3 | 0.3 ± 0.3 | 0.1 ± 0.3 | |
| Protein | −0.1 ± 0.7 | −0.5 ± 0.9 | 0.0 ± 0.7 | |
| Carbohydrate | 1.0 ± 1.6 | 1.2 ± 1.6 | 2.9 ± 1.7# | |
| Fiber | 0.3 ± 0.3 | 0.4 ± 0.3 | 0.3 ± 0.3 | |
| Added sugar | −0.8 ± 1.0 | −0.9 ± 1.0 | 1.6 ± 1.0 | |
| Dietary GI (units) | 1.7 ± 0.2 | 0.0 ± 0.2 | 0.2 ± 0.2 | |
| Eating midpoint | Energy (kcal) | 4.3 ± 43.6 | 8.9 ± 45.3 | 1.8 ± 42.4 |
| Total fat | 1.6 ± 1.4 | 1.2 ± 1.4 | 0.0 ± 1.3 | |
| SFA | 1.0 ± 7.2 | 0.9 ± 0.9 | −0.4 ± 0.6 | |
| MUFA | 0.9 ± 7.1 | 0.7 ± 0.7 | 0.2 ± 0.6 | |
| PUFA | −0.2 ± 0.7 | −0.3 ± 0.7 | 0.4 ± 0.6 | |
| Protein | −2.4 ± 1.6 | −2.9 ± 1.9 | −0.5 ± 3.6 | |
| Carbohydrate | −0.3 ± 3.4 | 0.9 ± 3.5 | 0.7 ± 3.3 | |
| Fiber | −0.2 ± 0.7 | −0.0 ± 0.7 | 1.1 ± 0.6# | |
| Added sugar | 1.7 ± 2.1 | 0.5 ± 2.1 | −4.0 ± 1.9* | |
| Dietary GI (units) | 0.1 ± 0.5 | −0.4 ± 0.5 | −0.4 ± 0.5 |
aUnits are grams (g) unless indicated otherwise in the table. bResults of multivariable linear regressions adjusted for age, sex, week, and total energy intake (except for the outcome of energy intake). #P < .10; *P < .05; **P < .01. AS = adequate sleep, GI = glycemic index, MUFA = monounsaturated fat, PUFA = polyunsaturated fat, SFA = saturated fat, SR = sleep restriction.
Exposure: eating window
At baseline, in AS, no associations were observed between length of eating window and dietary intakes (all P > .10). We observed more MUFA intake increase over time for SR than AS, with increased eating window (sleep condition × week × eating window: β = 0.4 ± 0.1; P = .001). Further, for week 0, sugar intake in SR trended higher for participants with longer eating window, as compared to AS (sleep condition × eating window: β = 2.3 ± 1.3; P = .081). In preplanned analyses stratified by sleep condition, no associations between duration of eating window and diet outcomes achieved statistical significance in either AS or SR (Table 2).
Exposure: eating midpoint
Across both sleep conditions, no significant associations were detected between timing of eating midpoint and dietary intakes, and there was no effect of sleep condition on the relation between eating midpoint and diet outcomes (Table 2). In analyses stratified by condition, later eating midpoint was associated with lower sugar intakes (β = −4.0 ± 1.9; P = .041) during SR. No associations between eating midpoint and dietary intakes were detected during AS (all P > .10).
Associations between eating pattern variability and diet under different sleep conditions
Exposure: variability in eating patterns
Across both sleep conditions, greater variability in eating frequency (β = −7.0 ± 3.0; P = .021) and eating window duration (β = −3.4 ± 1.5; P = .020) across days were associated with lower protein intakes (Table 3). Higher variability in eating midpoint was associated with higher PUFA intakes (β = 3.2 ± 1.5; P = .031). When considering an effect of sleep condition on the relation between eating pattern variability and diet outcome across weeks, a 3-way interaction was detected for sugar intakes. We observed higher sugar intake (indicating increase in added sugar consumed) over time for SR than AS, with increased eating frequency variability (sleep condition × week × eating frequency variability: β = 3.7 ± 1.5; P = .014).
Table 3.
Associations of eating pattern variability with dietary intakes under different sleep conditions.
| Exposure | Outcomea | β ± SE: AS and SRb | β ± SE: AS onlyb | β ± SE: SR onlyb |
|---|---|---|---|---|
| SD eating frequency | Energy (kcal) | 12.1 ± 85.5 | 51.9 ± 91.8 | 140.4 ± 104.9 |
| Total fat | 0.1 ± 2.7 | 0.9 ± 2.7 | 2.7 ± 3.3 | |
| SFA | −1.5 ± 1.4 | −1.6 ± 1.7 | −0.3 ± 1.6 | |
| MUFA | 1.8 ± 1.4 | 1.8 ± 1.4 | 2.4 ± 1.6 | |
| PUFA | 0.1 ± 1.3 | 1.0 ± 1.4 | 0.9 ± 1.5 | |
| Protein | −7.0 ± 3.0* | −6.3 ± 3.7# | −5.0 ± 3.6 | |
| Carbohydrate | 2.0 ± 6.7 | 0.2 ± 7.0 | −3.9 ± 8.4 | |
| Fiber | 1.1 ± 1.3 | −0.1 ± 1.4 | −1.8 ± 1.6 | |
| Added sugar | 0.8 ± 4.1 | 4.3 ± 4.2 | −7.4 ± 5.0 | |
| Dietary GI (units) | −0.4 ± 1.0 | 0.0 ± 1.0 | −1.1 ± 1.2 | |
| SD eating window | Energy (kcal) | −70.8 ± 40.9# | −83.6 ± 43.7# | −52.1 ± 41.1 |
| Total fat | 1.4 ± 1.3 | 1.5 ± 1.3 | 1.1 ± 1.3 | |
| SFA | −0.0 ± 0.7 | −0.3 ± 0.9 | 0.7 ± 0.6 | |
| MUFA | 0.9 ± 0.7 | 0.6 ± 0.7 | 0.7 ± 0.6 | |
| PUFA | 0.6 ± 0.6 | 1.0 ± 0.7 | −0.3 ± 0.6 | |
| Protein | −3.4 ± 1.5* | −2.4 ± 1.8 | −2.7 ± 1.4# | |
| Carbohydrate | −1.0 ± 3.2 | −2.1 ± 3.4 | −2.9 ± 3.3 | |
| Fiber | −0.0 ± 0.6 | −0.8 ± 0.7 | −0.7 ± 0.6 | |
| Added sugar | −0.6 ± 2.0 | −1.0 ± 2.1 | 2.2 ± 2.0 | |
| Dietary GI (units) | 0.0 ± 0.5 | 0.2 ± 0.5 | −0.2 ± 0.5 | |
| SD eating midpoint | Energy (kcal) | −43.0 ± 95.2 | −74.7 ± 100.4 | −59.5 ± 102.1 |
| Total fat | 1.9 ± 3.0 | 1.8 ± 3.0 | 1.6 ± 3.1 | |
| SFA | −2.7 ± 1.6# | −3.3 ± 1.9# | 2.5 ± 1.5# | |
| MUFA | 1.9 ± 1.5 | 1.7 ± 1.6 | −0.5 ± 1.5 | |
| PUFA | 3.2 ± 1.5* | 3.9 ± 1.5* | −0.3 ± 1.4 | |
| Protein | −0.2 ± 3.4 | 1.3 ± 4.1 | −5.7 ± 3.3# | |
| Carbohydrate | −6.0 ± 7.4 | −7.8 ± 7.7 | −2.1 ± 8.0 | |
| Fiber | −0.4 ± 1.4 | −1.5 ± 1.5 | −2.4 ± 1.5 | |
| Added sugar | −5.8 ± 4.6 | −6.5 ± 4.7 | 10.9 ± 4.7* | |
| Dietary GI (units) | −0.7 ± 1.1 | 0.2 ± 1.1 | 0.3 ± 1.1 |
aUnits are grams (g) unless indicated otherwise in the table. bResults of multivariable linear regressions adjusted for age, sex, week, and total energy intake (except for the outcome of energy intake). #P < .10; *P < .05; **P < .01. AS = adequate sleep, GI = glycemic index, MUFA = monounsaturated fat, PUFA = polyunsaturated fat, SFA = saturated fat, SR = sleep restriction.
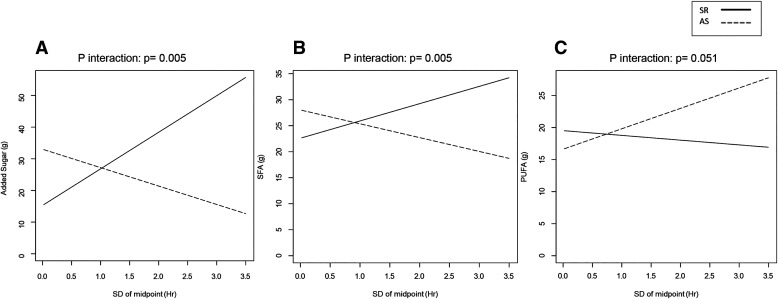
There was a sleep condition × eating midpoint variability interaction on intakes of added sugars (β = 17.3 ± 6.2; P = .006) (Figure 2A) and SFA (β = 6.0 ± 2.1; P = .005) (Figure 2B), and a trend for PUFA (β = −3.9 ± 2.0; P = .051) (Figure 2C). Namely, greater midpoint variability was associated with increased sugar intakes in SR but not in AS, and SFA intake tended to increase in SR and decrease in AS. Similarly, PUFA intake increased in AS but not in SR (Table 2). No additional associations between eating pattern variability and dietary intakes were observed in analyses stratified by sleep condition.
Figure 2. Sleep condition influenced the relationship between variability in eating midpoint and intake of added sugars (β = 17.3 ± 6.2; P interaction < .01), SFA (P interaction < .01), and PUFA (P interaction = .05 [trend]).
This figure illustrates the relationship between the standard deviation of eating midpoint and intakes of added sugar (A), SFA (B), and PUFA (C) under conditions of SR and AS. The regression lines between exposure and outcome under SR and AS are depicted using solid and dashed lines, respectively. AR = adequate sleep, PUFA = polyunsaturated fat, SFA = saturated fat, SR = sleep restriction.
DISCUSSION
This is the longest study to date that simulates real-life short sleep conditions to assess the influence of insufficient sleep on eating patterns linked with overconsumption and cardiometabolic disease risk. As has been suggested,8 we find that prolonged mild curtailment of sleep increased eating frequency and tended to prolong the eating window. These eating patterns were generally associated with higher energy intakes, which tended to be more pronounced during SR. Moreover, specifically in the context of SR, higher variability in eating midpoint was associated with lower PUFA along with higher SFA and sugar intakes, representing a dietary pattern associated with poor cardiometabolic health profile.28
Our results support observational findings of associations between short sleep duration and longer eating window18,19 and provide causal validation for the postulation that prolonged time awake increases eating opportunities leading to higher energy intake and poorer diet quality.8 Furthermore, we confirm data from a model of acute, severe SR demonstrating increased eating frequency under conditions of short sleep.6 Taken together, our data provide further support for a key role of the nonhomeostatic drive to eat as a key driver of observed effects of insufficient sleep on shifts to positive energy balance.
Prior research relating eating frequency with dietary profiles has provided mixed findings. Some studies report favorable dietary patterns with higher eating frequency,29–31 rationalized by increased likelihood of active lifestyle and greater energy expenditure in those with more frequent eating occasions.32 In contrast, other studies report that greater meal frequency relates to higher energy intakes,13 and, in particular, higher snacking frequency is consistently associated with lower diet quality.30,33 Notably, none of those studies have considered a potential influence of sleep duration on these relations. This is important because we note more pronounced increase in energy intakes along with reductions in healthful fats and increases in added sugars with higher eating frequency specifically under conditions of SR while dietary quality was not altered with greater eating frequency during AS.
Day-to-day variability in eating patterns had mild associations with diet quality in this study with sleep impacting these relations. Greater variability in eating frequency and duration of the eating window was associated with reduced protein intakes with similar associations in both conditions. Greater variability in timing of the eating window (eating midpoint) had opposite associations in AS and SR with regards to added sugar, SFA, and PUFA intakes. In AS, having a more variable timing of eating was associated with higher PUFA intakes (desirable dietary fat intakes) while intakes of added sugars were increased in SR. Studies on day-to-day variability in eating behaviors are limited. To our knowledge, there is only one observational study that examined eating pattern regularity and nutrient intakes.34 That observational study showed that the percentage of participants with inadequate intake of energy, vitamins, and proteins was higher in those reporting irregular meal patterns than those reporting regular meal patterns.34 Additional work is needed to firmly establish the associations between variability in eating patterns and dietary quality and how sleep duration influences these relations.
Our clinical trial is not without its limitations. First, our sample population consisted of adequate sleepers at baseline and cannot be generalized to habitual short sleepers. However, this is the most robust way of assessing causal effects of insufficient sleep on behaviors. Furthermore, our sleep restriction protocol led to sleep durations that reflect those of short sleepers in the general population. Our measures of dietary intake are limited by the subjectivity of self-reported data. However, our study utilized multiday food records collected in real time and was conducted in a crossover design, thereby limiting the likelihood of systematic bias and interindividual variability. However, we cannot dismiss the possibility of greater misreporting during SR, when participants experienced more fatigue. Although this study included a large sample size for a clinical intervention, there was limited power relative to observational studies, which could contribute to type 2 errors, particularly with assessment of interactions between sleep conditions and eating patterns. Moreover, our findings may be sex-specific since most of our sample are women (72%). Further extension in men is needed to replicate the results observed in this work.
Some strengths of our intervention include mild SR imposed over a sustained period, which is representative of the typical short sleeping adult. An additional strength is the objective assessment of sleep by actigraphy that was applied continuously throughout the 6 weeks of each phase, allowing for careful assessment of adherence and increasing the strength of findings. Multiple dietary records were obtained from each participant with each record providing 3 days of data, which enabled us to examine variability in eating patterns. Nevertheless, future studies should obtain longer periods of consecutive recording of dietary intakes to obtain more robust information on variability. Furthermore, we were able to test a causal impact of short sleep on eating patterns and dietary quality, which provide further evidence for a role of sleep duration on cardiometabolic health via changes in eating patterns and diet quantity and quality.
Overall, our findings suggest that eating frequency is increased in response to insufficient sleep, and the associations of eating patterns and their regularity with diet outcomes are modified by sleep duration, with adverse associations particularly under conditions of short sleep. These results may help to explain the shift toward obesogenic eating behaviors observed when sleep duration is curtailed.35,36 Replication of our findings from other clinical intervention studies, and with systematic investigation of potential individual differences, is warranted. Nevertheless, with the insights provided by this investigation of sleep curtailment, eating patterns, and diet quality, we suggest that interventions to improve dietary patterns and counter overeating should consider the role of sleep duration and target this modifiable behavior. Most notably, health providers counseling patients for weight management should highlight the need to achieve adequate sleep duration as a potential means to improve eating behaviors. Furthermore, clinical trials should be designed to evaluate whether limiting eating frequency and/or increasing regularity of eating patterns under conditions of short sleep can counter the adverse effects of insufficient sleep on eating behaviors.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. This work was performed at Columbia University Irving Medical Center. This work was supported in part by National Institutes of Health Grants R01HL128226 and R35HL155670 and American Heart Association Grant 16SFRN27950012 (M.P.S.O.), T32 HL007343 (F.M.Z.), as well as the National Center for Advancing Translational Sciences, National Institutes of Health Grant UL1TR001873. R.B. has been supported by Generalitat Valencia and Fondo Social Europeo (fellow APOSTD/2019/136). F.M.Z. is a Berrie Fellow in Diabetes and Obesity Research. The authors have no relevant conflicts of interest to disclose.
ACKNOWLEDGMENTS
The authors thank the participants for taking part in this study as well as trainees and research assistants who assisted with data collection. Author contributions are as follows: M.P.S.O. conceived, designed, and supervised the study; V.T. collected the data, and M.P.S.O. and F.M.Z. oversaw data collection and compilation; R.B. and A.R. designed and conducted statistical analyses; M.P.S.O., R.B., and F.M.Z. prepared the tables and interpreted results; R.B., F.M.Z., and M.P.S.O. wrote the paper; M.P.S.O. was responsible for final content. All authors provided input and final approval of the submitted and published versions.
Data sharing: Deidentified data described in the manuscript, code book, and analytic code will be made available upon reasonable request to the corresponding author.
ABBREVIATIONS
- AS
adequate sleep
- BMI
body mass index
- MUFA
monounsaturated fat
- PUFA
polyunsaturated fat
- SFA
saturated fat
- SR
sleep restriction
REFERENCES
- 1. Liu Y , Wheaton AG , Chapman DP , Cunningham TJ , Lu H , Croft JB . Prevalence of healthy sleep duration among adults—United States, 2014 . MMWR Morb Mortal Wkly Rep. 2016. ; 65 ( 6 ): 137 – 141 . [DOI] [PubMed] [Google Scholar]
- 2. Wu Y , Zhai L , Zhang D . Sleep duration and obesity among adults: a meta-analysis of prospective studies . Sleep Med. 2014. ; 15 ( 12 ): 1456 – 1462 . [DOI] [PubMed] [Google Scholar]
- 3. St-Onge M-P , Grandner MA , Brown D , Conroy MB , Jean-Louis G , Coons M , Bhatt DL ; American Heart Association Obesity, Behavior Change, Diabetes, and Nutrition Committees of the Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular Disease in the Young; Council on Clinical Cardiology; and Stroke Council . Sleep duration and quality: impact on lifestyle behaviors and cardiometabolic health: a scientific statement from the American Heart Association . Circulation. 2016. ; 134 ( 18 ): e367 – e386 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Al Khatib HK , Harding SV , Darzi J , Pot GK . The effects of partial sleep deprivation on energy balance: a systematic review and meta-analysis . Eur J Clin Nutr. 2017. ; 71 ( 5 ): 614 – 624 . [DOI] [PubMed] [Google Scholar]
- 5. Nedeltcheva AV , Kilkus JM , Imperial J , Kasza K , Schoeller DA , Penev PD . Sleep curtailment is accompanied by increased intake of calories from snacks . Am J Clin Nutr. 2009. ; 89 ( 1 ): 126 – 133 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. St-Onge MP , Roberts AL , Chen J , Kelleman M , O’Keeffe M , RoyChoudhury A , Jones PJ . Short sleep duration increases energy intakes but does not change energy expenditure in normal-weight individuals . Am J Clin Nutr. 2011. ; 94 ( 2 ): 410 – 416 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Beebe DW , Simon S , Summer S , Hemmer S , Strotman D , Dolan LM . Dietary intake following experimentally restricted sleep in adolescents . Sleep. 2013. ; 36 ( 6 ): 827 – 834 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chaput JP , St-Onge MP . Increased food intake by insufficient sleep in humans: are we jumping the gun on the hormonal explanation? Front Endocrinol (Lausanne). 2014. ; 5 : 116 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. St-Onge MP , Ard J , Baskin ML , Chiuve SE , Johnson HM , Kris-Etherton P , Varady K ; American Heart Association Obesity Committee of the Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular Disease in the Young; Council on Clinical Cardiology; and Stroke Council . Meal timing and frequency: implications for cardiovascular disease prevention: a scientific statement from the American Heart Association . Circulation. 2017. ; 135 ( 9 ): e96 – e121 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Murakami K , Livingstone MBE . Eating frequency is positively associated with overweight and central obesity in U.S. adults . J Nutr. 2015. ; 145 ( 12 ): 2715 – 2724 . [DOI] [PubMed] [Google Scholar]
- 11. Tamez M , Rodriguez-Orengo JF , Mattei J . Higher eating frequency, but not skipping breakfast, is associated with higher odds of abdominal obesity in adults living in Puerto Rico . Nutr Res. 2020. ; 73 : 75 – 82 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McCrory MA , Howarth NC , Roberts SB , Huang TT-K . Eating frequency and energy regulation in free-living adults consuming self-selected diets . J Nutr. 2011. ; 141 ( 1 ): 148 – 153 . [DOI] [PubMed] [Google Scholar]
- 13. Hunter SR , Mattes RD . The Role of Eating Frequency and Snacking on Energy Intake and BMI . In: Meiselman HL , ed. Handbook of Eating and Drinking: Interdisciplinary Perspectives. Cham, Switzerland: : Springer International Publishing; ; 2020. : 659 – 678 . [Google Scholar]
- 14. St-Onge MP , Pizinger T , Kovtun K , RoyChoudhury A . Sleep and meal timing influence food intake and its hormonal regulation in healthy adults with overweight/obesity . Eur J Clin Nutr. 2019. ; 72 ( Suppl 1) : 76 – 82 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cienfuegos S , Gabel K , Kalam F , et al . Effects of 4- and 6-h time-restricted feeding on weight and cardiometabolic health: a randomized controlled trial in adults with obesity . Cell Metab. 2020. ; 32 ( 3 ): 366 – 378.e3 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wilkinson MJ , Manoogian ENC , Zadourian A , et al . Ten-hour time-restricted eating reduces weight, blood pressure, and atherogenic lipids in patients with metabolic syndrome . Cell Metab. 2020. ; 31 ( 1 ): 92 – 104.e5 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Makarem N , Sears DD , St-Onge MP , et al . Variability in daily eating patterns and eating jetlag are associated with worsened cardiometabolic risk profiles in the American Heart Association go red for women strategically focused research network . J Am Heart Assoc. 2021. ; 10 ( 18 ): e022024 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kant AK , Graubard BI . Association of self-reported sleep duration with eating behaviors of American adults: NHANES 2005-2010 . Am J Clin Nutr. 2014. ; 100 ( 3 ): 938 – 947 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Castro MA , Garcez MR , Pereira JL , Fisberg RM . Eating behaviours and dietary intake associations with self-reported sleep duration of free-living Brazilian adults . Appetite. 2019. ; 137 : 207 – 217 . [DOI] [PubMed] [Google Scholar]
- 20. Buysse DJ , Reynolds CF 3rd , Monk TH , Berman SR , Kupfer DJ . The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research . Psychiatry Res. 1989. ; 28 ( 2 ): 193 – 213 . [DOI] [PubMed] [Google Scholar]
- 21. Johns MW . A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale . Sleep. 1991. ; 14 ( 6 ): 540 – 545 . [DOI] [PubMed] [Google Scholar]
- 22. Netzer NC , Stoohs RA , Netzer CM , Clark K , Strohl KP . Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome . Ann Intern Med. 1999. ; 131 ( 7 ): 485 – 491 . [DOI] [PubMed] [Google Scholar]
- 23. Douglass AB , Bornstein R , Nino-Murcia G , et al . The Sleep Disorders Questionnaire. I: creation and multivariate structure of SDQ . Sleep. 1994. ; 17 ( 2 ): 160 – 167 . [DOI] [PubMed] [Google Scholar]
- 24. Horne JA , Ostberg O . A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms . Int J Chronobiol. 1976. ; 4 ( 2 ): 97 – 110 . [PubMed] [Google Scholar]
- 25. Hooper L , Martin N , Jimoh OF , Kirk C , Foster E , Abdelhamid AS . Reduction in saturated fat intake for cardiovascular disease . Cochrane Database Syst Rev. 2020. ; 8 ( 8 ): CD011737 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sacks FM , Lichtenstein AH , Wu JHY , et al. American Heart Association . Dietary fats and cardiovascular disease: a presidential advisory from the American Heart Association . Circulation. 2017. ; 136 ( 3 ): e1 – e23 . [DOI] [PubMed] [Google Scholar]
- 27. Zhang J-Y , Jiang Y-T , Liu Y-S , Chang Q , Zhao Y-H , Wu Q-J . The association between glycemic index, glycemic load, and metabolic syndrome: a systematic review and dose-response meta-analysis of observational studies . Eur J Nutr. 2020. ; 59 ( 2 ): 451 – 463 . [DOI] [PubMed] [Google Scholar]
- 28. Mozaffarian D . Dietary and policy priorities for cardiovascular disease, diabetes, and obesity: a comprehensive review . Circulation. 2016. ; 133 ( 2 ): 187 – 225 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Murakami K , Livingstone MB . Associations between meal and snack frequency and diet quality in US adults: National Health and Nutrition Examination Survey 2003-2012 . J Acad Nutr Diet. 2016. ; 116 ( 7 ): 1101 – 1113 . [DOI] [PubMed] [Google Scholar]
- 30. Leech RM , Livingstone KM , Worsley A , Timperio A , McNaughton SA . Meal frequency but not snack frequency is associated with micronutrient intakes and overall diet quality in Australian men and women . J Nutr. 2016. ; 146 ( 10 ): 2027 – 2034 . [DOI] [PubMed] [Google Scholar]
- 31. Garcidueñas-Fimbres TE , Paz-Graniel I , Nishi SK , Salas-Salvadó J , Babio N . Eating speed, eating frequency, and their relationships with diet quality, adiposity, and metabolic syndrome, or its components . Nutrients. 2021. ; 13 ( 5 ): 1687 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang YQ , Zhang YQ , Zhang F , Zhang YW , Li R , Chen GX . Increased eating frequency is associated with lower obesity risk, but higher energy intake in adults: a meta-analysis . Int J Environ Res Public Health. 2016. ; 13 ( 6 ): 603 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Barrington WE , Beresford SAA . Eating occasions, obesity and related behaviors in working adults: does it matter when you snack? Nutrients. 2019. ; 11 ( 10 ): 2320 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yoon SR , Choi M , Kim OY . Effect of breakfast consumption and meal time regularity on nutrient intake and cardiometabolic health in Korean adults . J Lipid Atheroscler. 2021. ; 10 ( 2 ): 240 – 250 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhu B , Shi C , Park CG , Zhao X , Reutrakul S . Effects of sleep restriction on metabolism-related parameters in healthy adults: a comprehensive review and meta-analysis of randomized controlled trials . Sleep Med Rev. 2019. ; 45 : 18 – 30 . [DOI] [PubMed] [Google Scholar]
- 36. St-Onge MP . Sleep-obesity relation: underlying mechanisms and consequences for treatment . Obesity Rev. 2017. ; 18 ( Suppl 1 ): 34 – 39 . [DOI] [PubMed] [Google Scholar]