Abstract
Study Objectives:
We conducted this study to evaluate whether laboratory or home-based hypoglossal nerve stimulation (HNS) management would have equivalent objective and subjective obstructive sleep apnea outcomes 6 months after activation.
Methods:
Patients undergoing standard-of-care HNS implantation were randomly assigned in a prospective, multicenter clinical trial to either a 3-month postactivation in-laboratory titration polysomnography (tPSG) or an efficacy home sleep study (eHST) with tPSG by exception for eHST nonresponders at 5 months. Both groups underwent an eHST 6 months postactivation.
Results:
Sixty patients were randomly assigned. Patients experienced equivalent decreases in the apnea-hypopnea index (mean difference: −0.01 events/h [−8.75, 8.74]) across both groups with HNS; the selection of tPSG or eHST did not associate with therapy response rates (tPSG vs eHST: 63.2% vs 59.1%). The Epworth Sleepiness Scale (median of differences: 1 [−1, 3]) and device usage (median of differences: 0.0 hours [−1.3, 1.3]) outcomes were similar but did not meet a priori statistical equivalence criteria.
Conclusions:
This prospective, multicenter, randomized clinical trial demonstrated that patients undergoing HNS implantation experienced statistically equivalent improvements in objective obstructive sleep apnea outcomes and similar improvements in daytime sleepiness regardless of whether they underwent tPSG. HNS titration with tPSG may not be required for all postoperative patients.
Clinical Trial Registration:
Registry: ClinicalTrials.gov; Name: Inspire Home Study: Utilization of Home Monitoring During Therapy Optimization in Patients With an Inspire Upper Airway Stimulation System (Comparison of Home Sleep Testing vs. In-lab Polysomnography Testing) (HOME); URL: https://clinicaltrials.gov/ct2/show/NCT04416542; Identifier: NCT04416542.
Citation:
Kent D, Huyett P, Yu P, et al. Comparison of clinical pathways for hypoglossal nerve stimulation management: in-laboratory titration polysomnography vs home-based efficacy sleep testing. J Clin Sleep Med. 2023;19(11):1905–1912.
Keywords: obstructive sleep apnea, hypoglossal nerve stimulation, titration, in-laboratory polysomnography, home sleep study
BRIEF SUMMARY
Current Knowledge/Study Rationale: In-laboratory polysomnography for hypoglossal nerve stimulator titration has not previously been compared to patient-controlled, home-based device adjustment centered on subjective sleep experience to evaluate whether patients experience similar improvements in objective and subjective outcomes for obstructive sleep apnea. This prospective, multicenter, randomized clinical trial randomly assigned patients to 1 of 2 management pathways to evaluate whether patients experienced similar outcomes.
Study Impact: Home sleep apnea testing 6 months after activation demonstrated that patients experienced similar improvements in obstructive sleep apnea severity and daytime sleepiness regardless of whether they underwent device adjustment during in-laboratory polysomnography. Patient-controlled subjective adjustment of hypoglossal nerve stimulation with periodic home-based assessments of efficacy may be sufficient for many patients undergoing implantation.
INTRODUCTION
Hypoglossal nerve stimulation (HNS) therapy is an alternative treatment option for select patients with obstructive sleep apnea (OSA) who are intolerant to positive airway pressure therapy.1,2 The implanted neurostimulation device activates tongue protrusor muscles to dilate the pharyngeal airway and must be titrated over time to balance therapy efficacy and patient comfort during sleep. To optimize this balance, therapy adjustments are typically completed during in-laboratory titration polysomnography (tPSG) after approximately 3 months of patient HNS self-titration at home.3
The standard of care for HNS titration and efficacy assessment is tPSG, but high cost, geographic distance, resource limitations, and comfort with the laboratory environment all limit patients’ access to and tolerance of the examination.4–6 Home sleep apnea testing (HST) mitigates some of these issues with less-burdensome equipment, lower cost, and the ability for patients to sleep in their home environment.7,8 Although HST has become a commonly used tool for assessing HNS efficacy after a period of self-adjustment, it has never been directly compared to tPSG to determine whether one results in more effective therapy outcomes for patients.
This study was designed to evaluate whether tPSG or efficacy home sleep test (eHST) with tPSG by exception for eHST nonresponders would have equivalent outcomes as determined by the apnea-hypopnea index (AHI) and secondary end points of Epworth Sleepiness Scale (ESS), oxygen desaturation index (ODI), and therapy usage at 6 months after device activation.
METHODS
Study design
The HOME study (NCT04416542) was a prospective, randomized, nonblinded, multicenter trial across 5 centers in the United States evaluating 2 different care pathways of HNS titration with end points evaluated 6 months after therapy activation.
Study groups for postoperative HNS management
Enrolled patients underwent unilateral HNS device (Inspire Medical Systems, Inc.; Golden Valley, Minnesota) implantation as part of regular clinical care and were randomly assigned on a 1:1 basis between 2 postimplant management groups at the 1-month postoperative activation visit. Patients in both groups were permitted ad libitum clinical visits during the study for therapy assessment and reprogramming per the discretion of their treating study physician. The control group underwent tPSG at 3 months postactivation as per current standard of care. The 3-month HNS responder status was determined using the Sher15 responder criteria (AHI < 15 events/h and ≥ 50% reduction from baseline).9 Therapeutic AHI from tPSG was not defined a priori and was determined exclusively by study-site physicians based on their assessment of tPSG data. Control group nonresponders could also undergo tPSG between the 3- and 6-month visits but were not required to do so per protocol. The home monitoring group underwent a 2-night eHST at the 3-month postactivation visit. If eHST results did not meet Sher15 responder criteria, patients were required to attend a tPSG at 5 months postactivation. Both groups underwent a 2-night eHST at 6 months postactivation.
Postoperative sleep study methodology
A third-party service (SleepTest.com; Laguna Niguel, California) was used for eHST (ApneaLink Air; ResMed, San Diego, California) delivery and interpretation. The AHI and ODI values from both nights of the eHST were averaged for each study visit. The tPSG studies were completed and interpreted by a board-certified sleep physician at each local study site. Hypopneas for both study types were scored using the American Academy of Sleep Medicine 2012 rule 1B scoring criteria requiring an associated ≥ 4% decrease in oxygen saturation.10
Data collection
The primary dependent variable was the postoperative AHI. Independent variables included preoperative demographic, anthropomorphic, and polysomnographic data, including age, sex, ethnicity, body mass index, preoperative AHI, and preoperative ESS scores. Preoperative AHI was collected from a standard clinical polysomnogram (PSG) or HST completed prior to enrollment, scored using either the 1A or 1B American Academy of Sleep Medicine 2012 hypopnea scoring criteria.10 Secondary dependent variables included postoperative ODI, ESS scores, and HNS device amplitude and usage data. ESS scores were collected monthly, and HNS device data were collected at each postoperative visit. Full-night AHI and ODI were collected from eHST postoperative sleep studies, and titrated AHI and full-night ODI were collected from the tPSG postoperative sleep studies. Titration AHI represented at least a 30-minute portion of the tPSG night where HNS settings were determined to be optimally adjusted by the interpreting physician. HNS amplitude settings were collected from the electronic medical record or the HNS device manufacturer’s clinical monitoring database (Inspire SleepSync). Inadequate eHST studies (defined as any night recording missing or < 2.5 hours) were excluded from analyses.
Data analysis
Data analyses were designed to test the primary hypothesis that tPSG and eHST at 3 months postactivation would yield equivalent AHI outcomes at 6 months after following the previously described protocol for HNS responders and nonresponders. The primary end point was equivalence in 6-month AHI and change in AHI from baseline between the study groups, with an equivalence margin of ± 15 events/h for both outcomes. A recruitment sample size of 60 patients was estimated for random assignment on a 1:1 basis based on an a priori power analysis of the primary outcomes using an α of 0.05 and power of 80% using 12-month AHI information from the manufacturer’s ADHERE database registry tracking postoperative outcomes in thousands of patients undergoing HNS implantation,3 yielding 17 patients per group. Recruitment targets were increased to 30 patients per group to account for possible attrition.
Secondary end points included 6-month equivalence in ESS (equivalence margin of ±2 points), ODI (equivalence margin of ± 15 events/h), and nightly HNS device usage (equivalence margin of ± 0.5 h/night). Additional analyses included comparisons of baseline characteristics, AHI outcomes using either the Sher15 or Sher20 criteria (AHI < 15 or 20 events/h, respectively, and ≥ 50% reduction from baseline), and HNS device amplitude settings by randomization group.
Equivalence in AHI change from baseline was assessed using a 2 1-sided tests procedure for independent t tests as it was normally distributed. Other equivalence end points were assessed using nonparametric Hodges–Lehmann tests. For each test, statistical equivalence was observed if the 95% confidence interval of the median of differences (Hodges–Lehmann test) or mean difference (2 1-sided tests equivalence test) between the 2 study groups was entirely contained within the respective equivalence margin. For the additional analyses, Student’s t test, Fisher’s exact test, and the Wilcoxon rank-sum test were used for comparison of normally and nonnormally distributed variables. Statistical significance was inferred at a P-value threshold of < .05. Statistical analyses were performed in R (version 4.2.1; R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
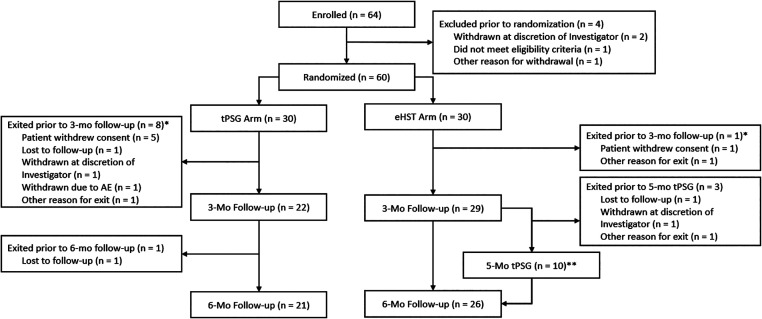
Sixty-four patients were enrolled in the study, and 60 patients completed random assignment at the HNS activation visit. Patients were similar between the 2 groups, composed of predominantly older, male Caucasians with severe OSA diagnosed primarily via HST (Table 1). Nine patients exited the study prior to the 3-month assessment; 8 were in the tPSG group. Four further patients exited the study prior to completion, with 1 in the tPSG group and 3 in the eHST group exiting prior to the 5-month tPSG. Ultimately, 70% completed the tPSG group, and 87% completed the eHST group (Figure 1).
Table 1.
Baseline characteristics (mean ± SD) by randomization group.
| Patient Characteristics | tPSG | eHST | All Patients | P |
|---|---|---|---|---|
| n | 30 | 30 | 60 | |
| Age (years) | 58.07 ± 10.11, n = 30 | 54.23 ± 10.89, n = 30 | 56.15 ± 10.6, n = 60 | .16 |
| Sex | ||||
| Male, % | 53.3% | 76.7% | 65.0% | .10 |
| Female, % | 46.7% | 23.3% | 35.0% | |
| White, % | 96.7% | 100.0% | 98.3% | >.99 |
| BMI (kg/m2) | 28.69 ± 3.12, n = 30 | 29.2 ± 3.78, n = 30 | 28.95 ± 3.45, n = 60 | .57 |
| Baseline | ||||
| AHI (events/h) | 35.13 ± 19.14, n = 30 | 35.02 ± 14.46, n = 30 | 35.08 ± 16.81, n = 60 | .98 |
| ODI (events/h) | 29.95 ± 25.18, n = 24 | 30.65 ± 20.08, n = 22 | 30.29 ± 22.64, n = 46 | .92 |
| ESS | 9.96 ± 5.58, n = 25 | 9.75 ± 6.58, n = 28 | 9.85 ± 6.07, n = 53 | .90 |
| Baseline sleep test | ||||
| HST, % | 56.7% | 80.0% | 68.3% | .09 |
| PSG, % | 43.3% | 20.0% | 31.7% | |
| Baseline comorbidities | ||||
| Sleep (other than OSA) | 10.0% | 16.7% | 13.3% | .71 |
| Cardiovascular | 46.7% | 36.7% | 41.7% | .60 |
| Neurologic | 13.3% | 6.7% | 10.0% | .67 |
| Psychiatric | 36.7% | 46.7% | 41.7% | .60 |
| Endocrine | 23.3% | 26.7% | 25.0% | 1.00 |
| Other conditions | 40.0% | 30.0% | 35.0% | .59 |
See supplemental material for diagnoses matching baseline comorbidity categories. AHI = apnea-hypopnea index; BMI = body mass index, eHST = efficacy home sleep apnea test, ESS = Epworth Sleepiness Scale, ODI = oxygen desaturation index, OSA = obstructive sleep apnea, PSG = polysomnography, tPSG = in-laboratory titration polysomnography.
Figure 1. Study enrollment, randomization, and attrition.
*Patients may have had more than one reason for study exit. **One patient missed the 5-month tPSG but completed the 6-month follow-up visit. AE = adverse event, eHST = efficacy home sleep apnea test, tPSG = in-laboratory titration polysomnography.
Using an equivalence margin of ± 15 events/h, the 6-month AHI was statistically equivalent between the 2 groups, with a median of differences of −0.23 events/h [−6.2, 4.2] (Table 2). The mean 6-month postactivation AHI with HNS therapy was 13.5 [7.8, 19.2] events/h in the tPSG group and 14.6 [8.4, 20.8] in the eHST group. The mean change in AHI was −21.9 [−28.3, −15.5] and −21.9 [−29.8, −14.0] events/h for the tPSG and eHST groups, respectively, with a mean difference of −0.01 events/h [−8.8, 8.7], again demonstrating statistical equivalence.
Table 2.
Equivalence in 6-month apnea-hypopnea indices (AHI) and change in AHI from baseline between randomization groups.
| End point | tPSG | eHST | Estimate | 95% CI | Equivalence Margin |
|---|---|---|---|---|---|
| 6-month AHI | 13.49 [7.75, 19.23], n = 19 | 14.62 [8.41, 20.83], n = 22 | −0.23 | −6.2, 4.2 | −15, 15 |
| 6-month change in AHI | −21.89 [−28.33, −15.45], n = 19 | −21.89 [−29.75, −14.03], n = 22 | −0.01 | −8.75, 8.74 | −15, 15 |
Data presented as mean [95% CI]. For 6-month AHI, the estimate (median of differences or location shift) and confidence interval were derived using nonparametric Hodges–Lehmann test and for the change in AHI, the estimate (mean difference) and confidence interval were derived using the 2 1-sided tests procedure for independent t tests. CI = confidence interval, eHST = efficacy home sleep apnea test, tPSG = in-laboratory titration polysomnography.
There were no significant differences (P = > .99) between the tPSG and eHST groups by Sher15 (63.2% vs 59.1%) or Sher20 (68.4% vs 63.6%) responder rates at 6 months (Table 3). In the eHST group, 8/11 (72.7%) of 3-month nonresponders successfully completed the 5-month tPSG. Only 2/8 (25%) eHST group 3-month nonresponders who completed the 6-month HST successfully converted to responder status following amplitude adjustments. One patient had no amplitude adjustments between the 2 studies, and 1 patient had a clinician-programmed decrease in amplitude at a clinic visit between the 5-month PSG (where they were a responder) and the 6-month HST. Four patients that were nonresponders at month 3 had advanced programming changes before month 6, but none of them flipped responder status. Three patients underwent electrode configuration changes in the tPSG group. Adjustments to HNS pulse width were made during the 5-month tPSG in the remaining patient in the eHST group, but that value was adjusted back to default by the time of the 6-month eHST.
Table 3.
Six-month responder rate per Sher15 and Sher20 criteria by randomization group.
| Variable | tPSG | eHST | P |
|---|---|---|---|
| n | 19 | 22 | |
| Sher15 criteria | |||
| Responder | 63.2% (12) | 59.1% (13) | >.99 |
| Nonresponder | 36.8% (7) | 40.9% (9) | |
| Sher20 criteria | |||
| Responder | 68.4% (13) | 63.6% (14) | >.99 |
| Nonresponder | 31.6% (6) | 36.4% (8) | |
Responder rates were compared using Fisher’s exact test. eHST = efficacy home sleep apnea test, tPSG = in-laboratory titration polysomnography.
Five therapy responders at 3 months (2 from the tPSG group and 3 from the eHST group) were nonresponders on the 6-month eHST. Three of these patients had no therapy amplitude change between months 3 and 6, and 2 of them had a reduction in amplitude.
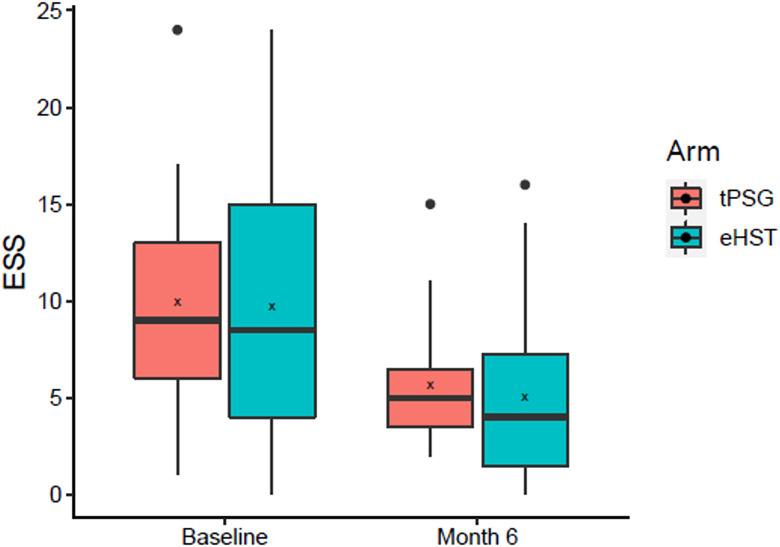
Six-month ESS was 5.7 [4.2, 7.1] for the tPSG group and 5.0 [2.9, 7.1] for the eHST group, but statistical equivalence was not met because the confidence interval of the median of differences (1 [−1, 3]) crossed the 2-point equivalence margin (Figure 2 and Table 4). ODI was 11.6 [7.4, 15.8] events/h vs 13.6 [8.8, 18.3] events/h (tPSG vs eHST) between the 2 groups and was statistically equivalent (−0.9 [−7.4, 4.4]).
Figure 2. Baseline and 6-month Epworth Sleepiness Scale (ESS) by randomization group.
Box = interquartile range; whiskers = outer quartiles; line = median; x = mean; dots = outliers. eHST = efficacy home sleep apnea test, tPSG = in-laboratory titration polysomnography.
Table 4.
Equivalence in 6-month Epworth Sleepiness Scale (ESS), oxygen desaturation index (ODI), and therapy usage between randomization groups.
| End Point | tPSG | eHST | Median of Differences | Confidence Interval | Equivalence Margin |
|---|---|---|---|---|---|
| ESS equivalence | 5.68 [4.23, 7.13], n = 19 | 5 [2.87, 7.13], n = 20 | 1.00 | −1, 3 | −2, 2 |
| ODI equivalence | 11.57 [7.35, 15.79] n = 19 | 13.57 [8.81, 18.33] n = 22 | −0.85 | −7.4, 4.4 | −15, 15 |
| Therapy usage (h/night) equivalence | 6.1 [5.09, 7.11] n = 21 | 6.1 [5.35, 6.85] n = 26 | 0.00 | −1.29, 1.29 | −0.5, 0.5 |
Data presented as mean [95% confidence interval]. Median of differences (location shift) and confidence interval derived using nonparametric Hodges–Lehmann test. eHST = efficacy home sleep apnea test, tPSG = in-laboratory titration polysomnography.
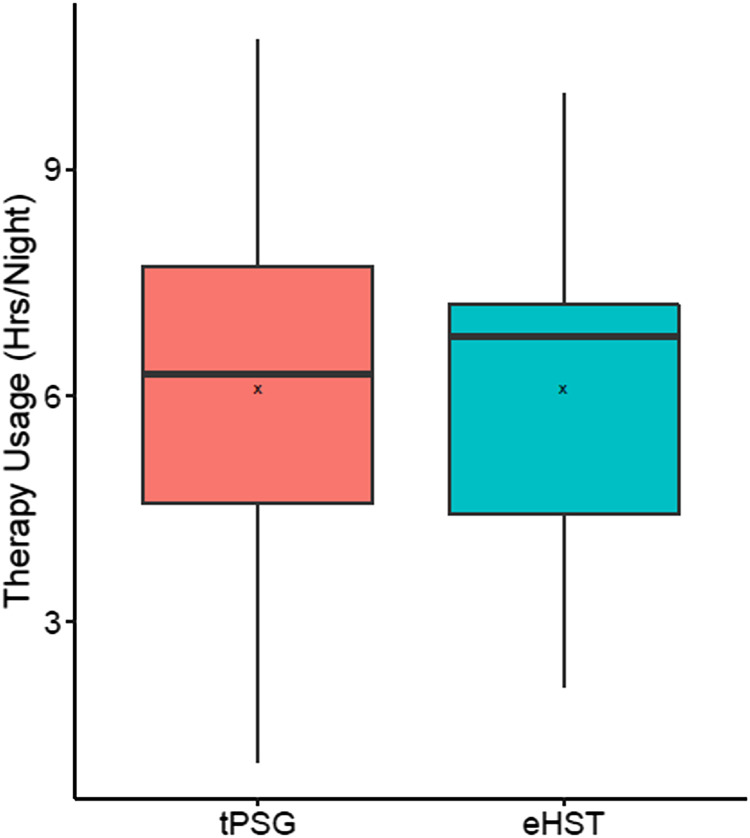
Therapy usage at 6 months was similar between the 2 groups (6.1 hours/night each), but the confidence intervals of the median of differences did not meet the equivalence margin of ± 0.5 h/night (0.0 [−1.3, 1.3]; Figure 3). The tPSG group had a mean activation amplitude of 1.4 [1.1, 1.7] V that increased to 2.2 [1.9, 2.5] V at 3 months (Table 5), whereas the eHST group had a mean activation amplitude of 1.1 [0.9, 1.3] V that increased to 2.0 [1.6, 2.3] V at 3 months. There was no significant difference in amplitudes between groups at each visit (P > .1), and the differences in 3- and 6-month amplitudes for each group were not significantly different, although it should be noted that voltage values were not scaled to account for changes in electrode configuration that were programmed for a few patients. The 6-month therapy response rates and change in AHI were not significantly different (P > .05) between patients with preoperative sleep studies scored using the American Academy of Sleep Medicine 1A rule vs those scored with 1B.10
Figure 3. Six-month therapy usage by randomization group.
Box = interquartile range; whiskers = outer quartiles; line = median; x = mean. eHST = efficacy home sleep apnea test, tPSG = in-laboratory titration polysomnography.
Table 5.
Comparison of mean therapy amplitudes by randomization group.
| Time Point | tPSG | eHST | P |
|---|---|---|---|
| Activation | 1.41 [1.13, 1.69] n = 29 | 1.07 [0.86, 1.28] n = 29 | .13 |
| Month 3 | 2.23 [1.94, 2.52] n = 22 | 1.95 [1.62, 2.28] n = 26 | .22 |
| Month 6 | 2.19 [1.85, 2.53] n = 21 | 1.97 [1.64, 2.3] n = 26 | .36 |
| Change from month 3 to month 6 | −0.1 [−0.31, 0.11] n = 21 | 0 [−0.14, 0.14] n = 25 | .82 |
Data presented as mean [95% confidence interval] and are inclusive of patients with alternative electrode configurations. Follow-up amplitude data were collected from the Inspire Cloud database when available and otherwise were collected from remote control interrogation. eHST = efficacy home sleep apnea test, tPSG = in-laboratory titration polysomnography.
DISCUSSION
This prospective, multicenter, randomized clinical trial demonstrated that patients self-titrating HNS therapy with periodic home-based efficacy assessments experienced equivalent decreases in objective measures of OSA burden to patients undergoing active laboratory-based adjustments. Moreover, the eHST patients experienced similar overall response rates to therapy and experienced similar improvements in ODI. Taken together, our results suggest that most patients can effectively adjust HNS therapy in the home setting according to subjective experience and comfort to achieve substantive objective decreases in OSA burden.
Home sleep testing is less expensive than PSG and has been previously shown to be effective for diagnosing OSA and initiating continuous positive airway pressure treatment.11 Several studies report that patients generally prefer its convenience and less-intensive nature.12,13 In this study, the majority of attrition occurred in the tPSG group following initial randomization, with 5 patients specifically citing an aversion to PSG as their reason for withdrawing consent.
HST is often criticized for being less sensitive than PSG for detection of respiratory events using the 2012 1A hypopnea rule (≥ 3% decrease in oxygen saturation or neurologic arousal) because most HST units do not include electroencephalography.14 Although this study utilized the 1B scoring rule for postoperative PSG and HST studies, we nevertheless observed that 6-month therapy response rates and change in AHI were not significantly different between patients with preoperative sleep studies scored using the 1A rule vs those scored with 1B. Further research is required to assess whether the difference in hypopnea definition substantially alters HNS responder rate classification.
Active adjustment of HNS therapy with tPSG is the current standard of care, but the utility of this approach over patient self-titration of therapy with periodic eHST assessment remains unclear. The most recent device tPSG protocol from the manufacturer recommends exploring amplitudes higher than previously trialed at home if required and as long as the patient tolerates them (personal communication). In this study, 72.7% of the 6-month responders subjectively titrated HNS at home to their 6-month amplitude prior to the 3-month sleep study. In the eHST group, only 25% of 3-month nonresponders were converted to responder status on the 6-month eHST, but no changes were made at or after the 5-month tPSG to convert them to responder status. Both study groups additionally had similar mean amplitude levels at the 3- and 6-month visits. These findings indicate that tPSGs may not provide substantial benefit over eHST monitoring during the initial HNS titration process, because most responders achieved success through straightforward increases in amplitude. Furthermore, nonresponders proved difficult to rescue regardless of the utilized management pathway, with only 6/17 (35.3%) of 3-month nonresponders in the study ultimately achieving 6-month responder status. Whereas 2 of these responders increased therapy amplitude between 3 and 6 months, 2 maintained their 3-month settings and 2 patients decreased therapy amplitude, suggesting that night-to-night variation in sleep apnea severity may confound therapy efficacy assessments. Additionally, electrode configuration and pulse-width changes in 4 of the 3-month nonresponders failed to convert them to therapy responders at 6 months. The Sher20 responder rates in both groups of this study agree closely with several other large cohorts of HNS patients,2,15–17 implying that therapy responsiveness is largely a predetermined outcome of judicious preoperative patient selection. It is important to acknowledge that although the mean ESS and therapy usage outcomes between the two groups were quite similar the observed variances did not fit within the prespecified equivalence intervals. The sample size for this study was not powered for secondary outcomes and although it is possible that a larger sample size might result in narrower equivalence intervals there may also be true subtle differences in the titration experience between the 2 study groups that requires further exploration. Ultimately, the role of tPSG for elucidating more complex phenomena contributing to HNS therapy nonresponse (such as first-night effect of sleeping in a new location, amplitude overtitration, or the need for more complex programming parameter adjustments) remains poorly understood.18,19 Further research is needed to determine what role tPSG or other clinical interventions can play in rescuing patients struggling to benefit from HNS therapy.
Several limitations of this study should be acknowledged. First, as observed above, the use of both 2012 hypopnea scoring rules in the preoperative sleep studies complicates assessments of change in AHI with HNS therapy. In this study the absolute and relative changes in AHI were similar between both groups, but the 2-rule criteria remain a pervasive challenge for clinical studies of OSA outcomes. We additionally acknowledge that the prespecified equivalence margin of ± 15 events/h for the primary outcomes may be too broad for some clinicians, although the marginal mean differences in AHI and confidence intervals within 9 events/h suggest that any true difference is likely not clinically relevant in this population. This HNS cohort was similar to others in that it was predominantly White and of low body mass index,3 and our results may not generalize to other populations. Second, the primary end point of this study occurred 6 months after therapy activation instead of the more commonly observed 12-month end point.1,2 Some of the 6-month nonresponders may have eventually achieved responder status; prior published data from the Stimulation Therapy for Apnea Reduction trial suggest that titration AHI decreased in participants between 6 and 12 months.2 Third, this trial assigned 3-month eHST nonresponders to tPSG at 5 months for further management, whereas the tPSG group nonresponders were not required to undergo tPSG. While changes in programming settings for the 2 eHST patients that converted to responder status at 6 months did not originate from the 5-month tPSG, the design of this trial prevents us from concluding that nonresponders can be rescued with clinic-based programming changes and eHST assessments alone, or whether a 5-month tPSG can contribute to 6-month secondary outcomes. Further research is required to ascertain what patients with HNS are suitable for exclusively home-based sleep apnea testing. Fourth, this study utilized titration AHI for determination of responder status at the 3- and 5-month PSG because it is widely used in the literature for HNS responder assessment. Nevertheless, titration AHI is a contentious metric,20 and it is unknown whether some patients classified as responders at 3 or 5 months in this study would have been managed differently if the definition of titration AHI were standardized, or if whole-night AHI assessments had been utilized. Finally, almost a third of the patients assigned to tPSG exited the study prior to the 3-month in-laboratory assessment. This study was conducted during the Covid-19 pandemic, which may have affected patients’ willingness to attend laboratory and clinic-based visits in both study groups. Future studies will need to focus on reducing barriers to tPSG completion to further evaluate this management pathway.
CONCLUSIONS
This prospective, multicenter, randomized clinical trial was designed to evaluate whether laboratory or home-based HNS management would have equivalent objective and subjective sleep outcomes at 6 months. Patients in the 2 groups experienced equivalent improvements in AHI, ODI, and therapy response rates and had similar final programming settings. Mean ESS and hours of device usage were similar but did not demonstrate statistical equivalence based on the prespecified equivalence margins. The 5-month tPSG ultimately played no role in rescuing HNS nonresponders after the 3-month eHST. Further research is needed to determine what role tPSG plays in rescuing patients struggling to benefit from HNS therapy, and whether patients struggling with initial HNS response can be rescued with clinic-based device reprogramming changes and eHST assessments without the use of tPSG.
DISCLOSURE STATEMENT
All authors have read and approved the manuscript. Financial and statistical support was provided by Inspire Medical Systems, Inc. David Kent reports research support from Inspire Medical Systems, Inc., Invicta Medical, Inc., and Nyxoah SA; consulting for Invicta Medical, Inc.; and being a scientific advisory board member of and holding intellectual property interests in Nyxoah SA. Phillip Huyett reports research support from Inspire Medical Systems, Inc. and Nyxoah SA and educational consulting for Inspire Medical Systems, Inc. Asim Roy reports consulting for Jazz Pharmaceuticals, Suven Pharmaceuticals, Inspire Medical Systems, Inc., and Avadel Pharmaceuticals; research support from Jazz Pharmaceuticals, Suven Pharmaceuticals, Inspire Medical Systems, Inc., Nyxoah SA, LivaNova, and Avadel Pharmaceuticals; and being on the speaker’s bureau for Jazz Pharmaceuticals, Eisai Pharmaceuticals, and Harmony Biosciences. Reena Mehra reports receiving honorarium from the American Academy of Sleep Medicine, funds for service on the American Board of Internal Medicine Writing Committee and Associate Editor for the American Journal of Respiratory and Critical Care Medicine, National Institutes of Health funding, and royalties from Up to Date. Shalini Manchanda reports being on the Physician Advisory Council for Inspire Medical Systems, Inc. The other authors report no conflicts of interest.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- eHST
single setting effectiveness home sleep apnea test
- ESS
Epworth Sleepiness Scale
- HNS
hypoglossal nerve stimulation
- HSAT
home sleep apnea test
- ODI
oxygen desaturation index
- OSA
obstructive sleep apnea
- PSG
polysomnography
- tPSG
titration polysomnography
REFERENCES
- 1. Strollo PJ Jr , Soose RJ , Maurer JT , et al. STAR Trial Group . Upper-airway stimulation for obstructive sleep apnea . N Engl J Med. 2014. ; 370 ( 2 ): 139 – 149 . [DOI] [PubMed] [Google Scholar]
- 2. Woodson BT , Strohl KP , Soose RJ , et al . Upper airway stimulation for obstructive sleep apnea: 5-year outcomes . Otolaryngol Head Neck Surg. 2018. ; 159 ( 1 ): 194 – 202 . [DOI] [PubMed] [Google Scholar]
- 3. Boon M , Huntley C , Steffen A , et al. ADHERE Registry Investigators . Upper airway stimulation for obstructive sleep apnea: results from the ADHERE registry . Otolaryngol Head Neck Surg. 2018. ; 159 ( 2 ): 379 – 385 . [DOI] [PubMed] [Google Scholar]
- 4. Edinger JD , Fins AI , Sullivan RJ Jr , et al . Sleep in the laboratory and sleep at home: comparisons of older insomniacs and normal sleepers . Sleep. 1997. ; 20 ( 12 ): 1119 – 1126 . [DOI] [PubMed] [Google Scholar]
- 5. Iber C , Redline S , Kaplan Gilpin AM , et al . Polysomnography performed in the unattended home versus the attended laboratory setting--Sleep Heart Health Study methodology . Sleep. 2004. ; 27 ( 3 ): 536 – 540 . [DOI] [PubMed] [Google Scholar]
- 6. Newell J , Mairesse O , Verbanck P , Neu D . Is a one-night stay in the lab really enough to conclude? First-night effect and night-to-night variability in polysomnographic recordings among different clinical population samples . Psychiatry Res. 2012. ; 200 ( 2-3 ): 795 – 801 . [DOI] [PubMed] [Google Scholar]
- 7. Bruyneel M , Sanida C , Art G , et al . Sleep efficiency during sleep studies: results of a prospective study comparing home-based and in-hospital polysomnography . J Sleep Res. 2011. 20 ( 1 Pt 2 ): 201 – 206 . [DOI] [PubMed] [Google Scholar]
- 8. Bruyneel M , Libert W , Ameye L , Ninane V . Comparison between home and hospital set-up for unattended home-based polysomnography: a prospective randomized study . Sleep Med. 2015. ; 16 ( 11 ): 1434 – 1438 . [DOI] [PubMed] [Google Scholar]
- 9. Sher AE , Schechtman KB , Piccirillo JF . The efficacy of surgical modifications of the upper airway in adults with obstructive sleep apnea syndrome . Sleep. 1996. ; 19 ( 2 ): 156 – 177 . [DOI] [PubMed] [Google Scholar]
- 10. Berry RB , Budhiraja R , Gottlieb DJ , et al . Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine . J Clin Sleep Med. 2012. ; 8 ( 5 ): 597 – 619 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rosen CL , Auckley D , Benca R , et al . A multisite randomized trial of portable sleep studies and positive airway pressure autotitration versus laboratory-based polysomnography for the diagnosis and treatment of obstructive sleep apnea: the HomePAP study . Sleep. 2012. ; 35 ( 6 ): 757 – 767 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Skomro RP , Gjevre J , Reid J , et al . Outcomes of home-based diagnosis and treatment of obstructive sleep apnea . Chest. 2010. ; 138 ( 2 ): 257 – 263 . [DOI] [PubMed] [Google Scholar]
- 13. Garg N , Rolle AJ , Lee TA , Prasad B . Home-based diagnosis of obstructive sleep apnea in an urban population . J Clin Sleep Med. 2014. ; 10 ( 8 ): 879 – 885 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kapur VK , Auckley DH , Chowdhuri S , et al . Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline . J Clin Sleep Med. 2017. ; 13 ( 3 ): 479 – 504 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Suurna MV , Steffen A , Boon M , et al. ADHERE Registry Investigators . Impact of body mass index and discomfort on upper airway stimulation: ADHERE registry 2020 update . Laryngoscope. 2021. ; 131 ( 11 ): 2616 – 2624 . [DOI] [PubMed] [Google Scholar]
- 16. Kent DT , Carden KA , Wang L , Lindsell CJ , Ishman SL . Evaluation of hypoglossal nerve stimulation treatment in obstructive sleep apnea . JAMA Otolaryngol Head Neck Surg. 2019. ; 145 ( 11 ): 1044 – 1052 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bosschieter PFN , de Vries N , Mehra R , et al. ADHERE Registry Investigators . Similar effect of hypoglossal nerve stimulation for obstructive sleep apnea in 5 disease severity categories . J Clin Sleep Med. 2022. ; 18 ( 6 ): 1657 – 1665 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Suurna MV , Jacobowitz O , Chang J , et al . Improving outcomes of hypoglossal nerve stimulation therapy: current practice, future directions, and research gaps. Proceedings of the 2019 International Sleep Surgery Society Research Forum . J Clin Sleep Med. 2021. ; 17 ( 12 ): 2477 – 2487 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Soose RJ , Faber K , Greenberg H , Boon M , Woodson T , Strollo P . Post-implant care pathway: lessons learned and recommendations after 5 years of clinical implementation of hypoglossal nerve stimulation therapy . Sleep. 2021. ; 44 ( Suppl 1 ): S4 – S10 . [DOI] [PubMed] [Google Scholar]
- 20. Dedhia RC , Woodson BT . Standardized reporting for hypoglossal nerve stimulation outcomes . J Clin Sleep Med. 2018. ; 14 ( 11 ): 1835 – 1836 . [DOI] [PMC free article] [PubMed] [Google Scholar]