Abstract
Study Objectives:
Obstructive sleep apnea (OSA) and poor sleep quality are highly prevalent in children with obesity, but their individual associations with health-related quality of life (HRQOL) are unknown in this population. The primary objective was to describe the independent association of OSA and sleep quality with HRQOL in children with obesity.
Methods:
This was a cross-sectional study of children with obesity at 2 tertiary care centers. Sleep quality and HRQOL were measured with the Pittsburgh Sleep Quality Index and Pediatric Quality of Life Inventory questionnaires, respectively. Multivariable regression models were created to evaluate associations between OSA and sleep quality with HRQOL.
Results:
There were 98 children (median age 15.0 years, median body mass index z-score 3.8, 44% females). Among the study population, 49/98 (50%) children reported poor sleep quality, 41/98 (42%) children had OSA, and 52/98 (53%) children reported impaired HRQOL. Self-reported poor sleep quality was independently associated with reduced HRQOL, whereas the presence of OSA was not. Children with poor sleep quality had a reduced Pediatric Quality of Life Inventory score by 8.8 compared to children with good sleep quality (95% confidence interval, 2.6–14.9; P = .006), when adjusting for age, sex, body mass index z-score, attention-deficit/hyperactivity disorder, mood/anxiety disorder, and study site.
Conclusions:
In the current study of children with obesity, we found that HRQOL was more strongly associated with the self-reported experience of sleep than the presence of OSA. Clinicians should assess and optimize sleep quality as part of the evaluation for OSA in children with obesity.
Citation:
Xiao L, Voutsas G, Ryan CM, Katz SL, Narang I. The association between sleep quality and obstructive sleep apnea with health-related quality of life in children with obesity. J Clin Sleep Med. 2023;19(11):1877–1883.
Keywords: obstructive sleep apnea, insomnia, children, obesity, health-related quality of life
BRIEF SUMMARY
Current Knowledge/Study Rationale: Obstructive sleep apnea and poor sleep quality are highly prevalent in children with obesity, but their independent associations with health-related quality of life are unknown in this population. The primary objective was to describe the independent association of obstructive sleep apnea and sleep quality with health-related quality of life in children with obesity.
Study Impact: In the current study of children with obesity we found that health-related quality of life was more strongly related to the self-reported experience of sleep than the presence of obstructive sleep apnea. Clinicians should assess and optimize sleep quality as part of the evaluation for obstructive sleep apnea in children with obesity.
INTRODUCTION
Obstructive sleep apnea (OSA) is characterized by recurrent, partial or complete obstruction of the upper airway, predisposing to intermittent hypoxia and chronic sleep deprivation. Sleep quality is a multidimensional clinical construct including, but not limited to, sleep disturbance, difficulties falling asleep or maintaining sleep, and sleep duration. It is affected by many different factors, including sleep-disordered breathing symptoms, both of which are associated with the social determinants of health.1,2 While poor sleep quality is a defining feature of insomnia, it is a broader construct than insomnia severity.3
Poor sleep quality and OSA are highly prevalent in children,4,5 with OSA occurring in up to 60% of children with obesity6 and poor sleep quality, specifically insomnia symptoms, in 37% of healthy children.7–11 Both poor sleep quality and OSA are associated with reduced health-related quality of life (HRQOL), physical functioning, and psychosocial functioning.12–14 Children with obesity are expected to have increased rates of sleep-disordered breathing and poor sleep quality because obesity is a risk factor for OSA6 and may be related to poor sleep quality or insomnia.10,15–20 This is a population of interest because almost 1 in 7 children and adolescents in Canada are obese21 and increasingly large numbers are referred to pediatric sleep clinics for evaluation of sleep disorders. Furthermore, children with obesity report low HRQOL scores on average,22,23 with further impairments with comorbid OSA.13
Although both OSA and poor sleep quality have been associated with reduced HRQOL in children, the relative magnitude of associations for each condition with HRQOL has not been the focus of previous investigations. Elucidating the independent impact of each condition on HRQOL is vital to inform treatment approaches for sleep disorders in children with obesity. The objective of this study was therefore to describe the association between self-reported sleep quality and OSA with HRQOL in a clinical population of children with obesity. We hypothesized that self-reported sleep quality would have a stronger association with HRQOL compared to OSA.
METHODS
Study design and setting
This was a cross-sectional study of children with obesity from the Canadian Sleep and Circadian Network cohort who were referred to a tertiary sleep clinic for evaluation of suspected OSA. Children were prospectively recruited from 2015 to 2021 across 2 Canadian centers, The Hospital for Sick Children and Children’s Hospital of Eastern Ontario. Research ethics approval was obtained at both sites prior to study commencement and written consent was obtained from caregivers along with consent and/or assent from participants.
Study population
The inclusion criteria were children aged 8–17 years with obesity (defined as a body mass index [BMI] greater than the 95th percentile for age and sex) who were referred for evaluation of suspected sleep-disordered breathing. The participants included in this study underwent investigation for OSA with a baseline diagnostic polysomnogram (PSG), but not all participants met diagnostic criteria for OSA (obstructive apnea-hypopnea index [OAHI] < 5 events/h). The age range of 8–17 years reflects the obesity-related OSA phenotype. The exclusion criteria were (1) children with known chronic illness or underlying comorbidity associated with a higher risk for OSA (eg, Down syndrome), (2) obesity related to other diseases and syndromes (eg, Prader–Willi syndrome), or (3) children referred for an adenotonsillectomy or previously established on positive airway pressure therapy.
Measurements
HRQOL
The primary outcome was assessed using a self-reported pediatric HRQOL scale, the Pediatric Quality of Life Inventory (PedsQL generic version), which has previously been validated in children with chronic diseases.24 The PedsQL generates a total score as well as subscale scores for physical functioning and psychosocial functioning which is composed of emotional, social, and school functioning. The total and subscale scores range from 0 to 100, with lower scores corresponding to a lower HRQOL. A score below 69.7 is 1 standard deviation below the population mean and considered to be a meaningful threshold to identify individuals with impaired HRQOL.24
Sleep quality
Sleep quality was assessed using the self-reported Pittsburgh Sleep Quality Index (PSQI), a questionnaire with evidence of validity in an adolescent population25 to measure sleep dysfunction.26 The PSQI score ranges from 0 to 21, with greater values indicating poorer sleep quality.27,28 Children were classified as having poor sleep quality when the PSQI total score was greater than 5, as this is considered to be a meaningful threshold to identify individuals with sleep dysfunction including reduced sleep quality, duration, efficiency, prolonged sleep latency, sleep disturbance, daytime dysfunction, and use of sleeping medication.27,28 Questionnaires were administered at the same time as the baseline PSG, prior to treatment for sleep-disordered breathing.
Sleep studies
In-laboratory, attended PSGs were performed and scored according to American Academy of Sleep Medicine international guidelines.29 Baseline diagnostic PSGs on room air were analyzed. The PSG montage includes a 6-lead electroencephalogram, electrooculogram leads, submental and tibial electromyograms, an electrocardiogram lead, pulse oximetry, transcutaneous and end-tidal carbon dioxide, respiratory inductance plethysmography, and nasal pressure and thermal airflow signals. An obstructive apnea event was defined as a reduction in airflow more than 90% from baseline with chest and/or abdominal movement throughout the entire event. Hypopneas were defined as a decrease in airflow of at least 30% from baseline associated with a minimum 3% desaturation, arousal or awakening.29 A threshold OAHI of 5 events/h or greater was used as a cut-off point to determine OSA because this is considered to be the treatment threshold for OSA in children.30
Covariates
Age, sex, height, and weight were determined at baseline. BMI z-scores (BMIz) were calculated based on World Health Organization 2007 references.31,32 Participant study site was recorded. Medical history and current medication usage were extracted from medical records. A diagnosis of attention-deficit/hyperactivity disorder (ADHD) along with the use of ADHD medications was used to define ADHD in this study. A diagnosis of a mood/anxiety disorder along with the use of psychiatric medications was used to define a mood/anxiety disorder in this study.
Baseline measures
Annual household income and highest household education were collected through proxy- or self-reported questionnaires. Complex chronic conditions were defined according to Feudtner et al as “any medical condition that can be reasonably expected to last at least 12 months (unless death intervenes) and to involve either several different organ systems or 1 organ system severely enough to require specialty pediatric care probably some period of hospitalization in a tertiary care center.”33 Pubertal staging was assessed by pubic hair development reported on the self-reported Tanner stage questionnaire, a picture-based questionnaire based on 5 stages.34
Statistical analysis
Descriptive statistics were used to summarize the baseline demographic variables. Nonnormally distributed data were described with medians and interquartile ranges (IQR) and normally distributed data were described with means and standard deviations or 95% confidence intervals (CIs). Categorical variables were presented as frequency (percentage). To assess for demographic differences between children with poor sleep quality and children with good sleep quality, continuous variables were analyzed using Wilcoxon rank-sum tests and categorical variables were analyzed using Fisher’s exact tests.
Univariate linear regression was used to assess the association between HRQOL and baseline demographics as well as sleep parameters. A multivariable linear regression model was created to evaluate the association between HRQOL and OSA as well as sleep quality, with adjustment for covariates determined a priori including age, sex, BMIz, ADHD, mood/anxiety disorder, and study site to adjust for potential correlation. The same model was repeated for psychosocial and physical functioning subscale scores.
All analyses were performed using SAS OnDemand (SAS Institute Inc., Cary, North Carolina). Statistical significance was defined as P < .05, and 2-sided tests were performed throughout.
RESULTS
Baseline demographics
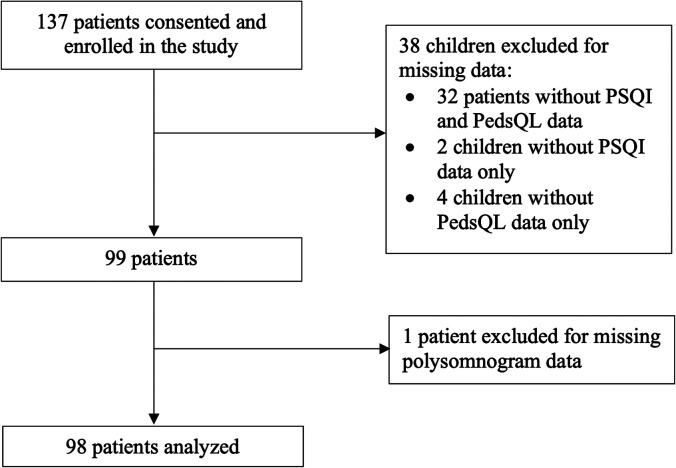
There was a total of 98 children in the analysis (Figure 1), including 43/98 (44%) females. The median (IQR) age at the time of the PSG was 15.0 (13.0–16.0) years and the median (IQR) BMIz was elevated at 3.8 (3.1–4.6) (Table 1). Among the study population, 49/98 (50%) children reported poor sleep quality and 41/98 (42%) children had OSA diagnosed on a baseline PSG (OAHI ≥ 5 events/h).
Figure 1. Flow of participants in the study of the association of comorbid insomnia and sleep apnea with health-related quality of life in children with obesity.
PedsQL = Pediatric Quality of Life Inventory, PSQI = Pittsburgh Sleep Quality Index.
Table 1.
Baseline demographics of children with obesity referred for evaluation of sleep-disordered breathing.
| Total Sample (n = 98) | Poor Sleep Quality (n = 49) | Good Sleep Quality (n = 49) | P | |
|---|---|---|---|---|
| Age (years), median (IQR) | 15.0 (13.0–16.0) | 15.0 (13.0–16.0) | 14.0 (12.0–16.0) | .17 |
| Female sex, n (%) | 43 (43.9) | 29 (59.2) | 14 (28.6) | .002 |
| BMI z-score, median (IQR) | 3.8 (3.1–4.6) | 3.8 (3.0–4.5) | 3.7 (3.3–4.6) | .71 |
| ADHD, n (%) | 14 (14.3) | 8 (16.3) | 6 (12.2) | .77 |
| Mood/anxiety disorder, n (%) | 10 (10.2) | 8 (16.3) | 2 (4.1) | .091 |
| Complex chronic conditions, n (%)a | 14 (14.3) | 4 (8.2) | 10 (20.4) | .15 |
| Annual household income, n (%)b | ||||
| ≥$68,700 | 34 (34.7) | 15 (41.7) | 19 (50) | .49 |
| Missing | 24 (24.5) | 13 (26.5) | 11 (22.4) | |
| Highest household education, n (%)c | ||||
| Low education | 4 (4.1) | 3 (7.5) | 1 (2.7) | .74 |
| Medium education | 27 (27.6) | 13 (32.5) | 14 (37.8) | |
| High education | 46 (46.9) | 24 (60.0) | 22 (59.5) | |
| Missing | 21 (21.4) | 9 (18.4) | 12 (24.5) | |
| Tanner stage, n (%) | ||||
| Early (1, 2) | 27 (27.6) | 13 (29.6) | 14 (32.6) | .82 |
| Late (3, 4, 5) | 60 (61.2) | 31 (70.5) | 29 (67.4) | |
| Missing | 11 (11.2) | 5 (10.2) | 6 (12.2) | |
| OAHI (events/h), median (IQR) | 3.7 (0.6–10.1) | 2.4 (0.5–7.7) | 6.4 (1.2–20.1) | .002 |
| Arousal index (events/h), median (IQR) | 11.7 (8.0–16.4) | 9.8 (7.4–14.5) | 13.0 (8.5–22.4) | .060 |
| Desaturation index (events/h), median (IQR) | 3.9 (1.6–11.3) | 3.6 (1.5–7.7) | 6.2 (2.1–12.3) | .064 |
The study population included children aged 8–17 years with obesity who were referred for evaluation of suspected sleep-disordered breathing. Not all participants met diagnostic criteria for obstructive sleep apnea. aPresence of 1 or more complex chronic conditions. bAnnual household income dichotomized into the lowest 3 quintiles and highest 2 quintiles. cSelf-reported household education based on the International Standard Classification of Education (ISCED) 2011 criteria. Levels of education were aggregated into low education (ISCED levels 0–2), medium education (ISCED levels 3–4), and high education (ISCED levels 5–8). ADHD = attention-deficit/hyperactivity disorder, BMI = body mass index, IQR = interquartile range, MV = missing value, OAHI = obstructive apnea-hypopnea index.
There was a significantly higher proportion of females reporting poor sleep quality than good sleep quality (59% in the poor sleep quality group vs 29% in the good sleep quality group; P = .002). There was also a significantly lower OAHI in the poor sleep quality group (median 2.4 events/h; IQR 0.5–7.7 events/h) than in the good sleep quality group (median 6.4 events/h; IQR 1.2–20.1 events/h; P = .002) (Table 1).
HRQOL
The mean PedsQL score for the sample was 67.5 (standard deviation, 16.2). There were 52/98 (53%) children who reported impaired HRQOL.
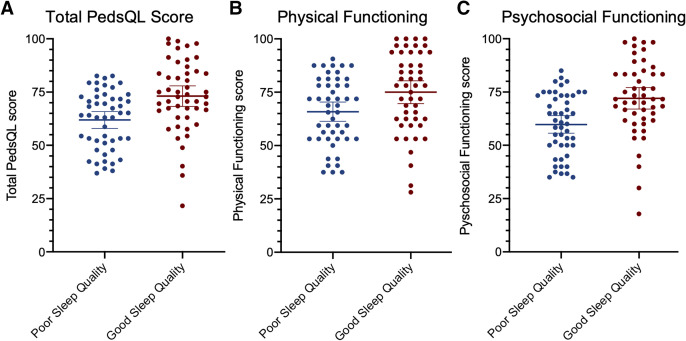
The mean PedsQL score was 61.9 (standard deviation, 13.7) among children with poor sleep quality and 73.1 (standard deviation, 16.8) among children with good sleep quality (Figure 2). Poor sleep quality was independently associated with total PedsQL score and psychosocial functioning when adjusting for age, sex, OSA, ADHD, mood/anxiety disorder, and study site (Table 2). Poor sleep quality was associated with a decrease in total PedsQL score by 8.8 (95% CI, 2.6–14.9; P = .006) and psychosocial functioning score by 10.4 (95% CI, 3.9–16.9; P = .002). In contrast, the presence of OSA was not associated with total PedsQL score, psychosocial functioning, or physical functioning.
Figure 2. Health-related quality of life score by sleep quality.
The means and 95% confidence intervals are denoted by the horizontal bars. PedsQL = Pediatric Quality of Life Inventory.
Table 2.
Associations with health-related quality of life scores.
| Pediatrics Quality of Life Total Score | Physical Score | Psychosocial Score | ||||
|---|---|---|---|---|---|---|
| Beta Coefficient (95% CI) | P | Beta Coefficient (95% CI) | P | Beta Coefficient (95% CI) | P | |
| OSA | ||||||
| OSA present (unadjusted) | 3.8 (−2.9, 10.3) | .26 | 3.1 (−4.1, 10.4) | .39 | 4.0 (−2.9, 11.0) | .25 |
| OSA present (adjusted) | 2.7 (−4.1, 9.6) | .43 | 2.7 (−5.0, 10.3) | .50 | 2.7 (−4.6, 10.0) | .46 |
| Sleep quality | ||||||
| Poor sleep quality (unadjusted) | −11.2 (−17.3, −5.0) | <.001 | -9.1 (−16.0, −2.2) | .010 | −12.3 (−18.7, −5.8) | <.001 |
| Poor sleep quality (adjusted) | −8.8 (−14.9, −2.6) | .006 | −5.7 (−12.5, 1.1) | .10 | −10.4 (−16.9, −3.9) | .002 |
| BMI z-score (per z-score) | ||||||
| Unadjusted | −2.8 (−5.9, 0.3) | .078 | −3.9 (−7.3, −0.5) | .026 | −2.3 (−5.6, 1.1) | .18 |
| Adjusted | −3.2 (−6.3, −0.2) | .040 | −4.0 (−7.4, −0.5) | .024 | −2.8 (−6.1, 0.4) | .090 |
| ADHD | ||||||
| Present (unadjusted) | −14.0 (−22.9, −5.1) | .002 | −12.4 (−22.3, −2.5) | .015 | −14.9 (−24.2, −5.5) | .002 |
| Present (adjusted) | −9.5 (−18.4, −0.7) | .035 | −6.0 (−15.9, 3.8) | .23 | −11.4 (−20.8, −2.0) | .018 |
| Mood/anxiety disorder | ||||||
| Present (unadjusted) | −20.2 (−30.2, −10.2) | <.001 | −24.1 (−34.9, −13.4) | <.001 | −18.1 (−28.9, −7.3) | .001 |
| Present (adjusted) | −10.6 (−21.5, 0.3) | .057 | −18.0 (−30.1, −5.9) | .004 | −6.6 (−18.1, 5.0) | .26 |
| Annual household income | .66 | .19 | .92 | |||
| <$68,700 (unadjusted) | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | |||
| ≥$68,700 (unadjusted) | 1.7 (−5.8, 9.2) | 5.5 (−2.8, 13.8) | −0.4 (−8.2, 7.4) | |||
| Highest household education | ||||||
| Low (unadjusted) | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | |||
| Medium (unadjusted) | 8.1 (−8.9, 25.2) | .34 | 10.1 (−8.8, 29.0) | .30 | 7.0 (−10.9, 25.0) | .43 |
| High (unadjusted) | 3.9 (−12.7, 20.4) | .64 | 6.9 (−11.5, 25.3) | .46 | 2.2 (−15.3, 19.7) | .80 |
Bold P values indicate statistical significance. The multivariable linear regression model includes the presence of obstructive sleep apnea (OSA), sleep quality, age, sex, body mass index z-score, attention-deficit/hyperactivity disorder (ADHD), mood/anxiety disorder, and study site. The same model was repeated for psychosocial and physical functioning subscale scores. BMI = body mass index, CI = confidence interval.
On univariate analysis, an increase in BMIz was associated with reduced physical functioning scores and this association remained significant with adjustment for age, sex, OSA, sleep quality, ADHD, mood/anxiety disorder, and study site. On multivariable analysis, a 1-point increase in BMIz was associated with a 3.2-point decrease on physical functioning scores (95% CI, 0.2–6.3; P = .040) (Table 2).
The presence of ADHD was associated with a reduced total PedsQL score on univariate and multivariable analyses, whereby there was a 9.5-point decrease in total PedsQL scores with the presence of ADHD (95% CI, 0.7–18.5; P = .035). Mood/anxiety disorders were associated with reduced total PedsQL scores on univariate analysis, and this relationship approached statistical significance on multivariable analysis. The presence of a mood/anxiety disorder was associated with a 10.6-point change in total PedsQL score (95% CI, −21.5 to 0.3; P = .057). On univariate and multivariable analyses, ADHD was associated with reduced psychosocial functioning, whereas mood/anxiety disorders were associated with reduced physical functioning.
DISCUSSION
In our study focused on children with obesity referred for evaluation of sleep-disordered breathing we observed that self-reported sleep quality was independently associated with HRQOL, whereas the presence of OSA was not associated with HRQOL. These findings suggest that HRQOL is more strongly associated with self-reported sleep quality as compared to OSA. Whether the treatment of poor sleep quality results in improved HRQOL in children remains to be elucidated.
We hypothesize that the lack of association between OSA and HRQOL may be related to several factors. First, there may be a disconnect between objective airway obstruction measured on a PSG and symptoms of OSA that would be expected to mediate the relationship between OSA and HRQOL. Although sleep quality may be hypothesized to act as a mediator in the pathway between OSA and HRQOL, we surprisingly found a significantly higher median OAHI in the good-sleep-quality group compared with the poor-sleep-quality group. Our data suggest that OSA may not affect perceived sleep quality for many individuals, and this is a similar finding to previous studies in adult populations that showed no association between OSA and sleep quality.35,36 Similarly, children and adolescents with OSA may not experience other symptoms of OSA, such as daytime sleepiness, that would be expected to mediate the pathway between OSA and HRQOL. Formal mediation analyses to further elucidate these complex relationships may be of interest in the future for children and adolescents. Second, the use of a clinically referred population may limit detectable differences between those with and without OSA. Finally, HRQOL is a broad construct that is influenced by multiple factors. As such, the magnitude of association between HRQOL and sleep, which is one of many factors expected to be associated with HRQOL, may be limited. Nonetheless, sleep quality was found to have a significant relationship with HRQOL.
Sleep quality is a complex phenomenon that is difficult to objectively define. Subsequently, recent sleep literature has focused more specifically on insomnia, a disorder defined by both the Diagnostic and Statistical Manual of Mental Disorders37 as well as the International Classification of Sleep Disorders.38 While insomnia and OSA may occur independently, there is also evidence for a bidirectional relationship between these entities.39 Data from adult literature as well as emerging data in pediatric literature40 show that OSA and insomnia, commonly associated with poor sleep quality, may coexist. This entity, referred to as comorbid insomnia and sleep apnea,40 is highly prevalent in adult populations, occurring in 39–55% of those with OSA.41 The coexistence of these conditions is associated with greater morbidity than either condition alone, including reduced HRQOL and increased daytime functional impairment.39 Furthermore, the treatment options for insomnia and OSA differ, with emerging evidence that comorbid insomnia and sleep apnea is more difficult to treat compared to either condition alone. Our research study highlights the association between sleep quality and HRQOL in children with obesity who were referred for evaluation of OSA. However, we were unable to specifically assess for the presence of insomnia. Therefore, future research exploring the relationship between insomnia and OSA could have implications for targeted treatment strategies for children with sleep disorders.
The impact of poor sleep quality on health outcomes in children with obesity is a topic of interest. While the association between short sleep duration and obesity is not consistent across studies,42–45 insomnia disorder with short sleep duration has been associated with a higher risk of hypertension,42,43 hyperglycemia,43 and hyperlipidemia43 in adult populations. It has also been found that individuals with snoring and insomnia have the highest risk for cardiovascular disease compared to either condition alone.46 Given the high prevalence of insomnia, poor sleep quality, and OSA in children with obesity, their impact on health outcomes, including metabolic syndrome, is an important area of future research.
The current study is among the first to describe the relative magnitude of association between self-reported sleep quality and OSA with HRQOL. However, there are several limitations that must be addressed. First, the study sample is limited to a group of children with obesity referred to tertiary care academic centers for the evaluation of sleep-disordered breathing and may have more medical comorbidities than a community-based sample. Additionally, there was a clinical concern for OSA in all participants secondary to a combination of symptoms and risk factors, which may have limited the detectable differences between those with and without OSA. As such, this may limit the generalizability of findings. Second, socioeconomic variables were not included in the multivariable regression model due to a high rate of missing data and modest study sample size. Reassuringly, socioeconomic variables were not associated with PedsQL scores in the univariate analysis. The inclusion of socioeconomic variables as a covariate in multivariable models would be important to consider in future research studies. Third, we were limited by the assessment of sleep symptoms over a 1-month period using the PSQI, and therefore we could not assess for chronic poor sleep quality over a longer period of time. Nonetheless, the PSQI is a widely used questionnaire with evidence of validity in children and adolescents. Finally, we were limited by an overall small sample size due to our strict eligibility criteria and the effect of the COVID-19 pandemic on recruitment. Future larger studies would further support our findings.
CONCLUSIONS
In the current study of children with obesity we found that HRQOL was more strongly associated with the self-reported experience of sleep than the presence of OSA. Clinicians should consider assessing and optimizing sleep quality when evaluating sleep-disordered breathing in children with obesity. Longitudinal studies delineating the aspects of sleep quality that influence HRQOL and health outcomes in children may inform targeted management strategies for this population.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. Work for this study was performed at The Hospital for Sick Children and Children’s Hospital of Eastern Ontario. This work was supported by the Canadian Institutes of Health Research (grant number CDT-142656), the Hospital for Sick Children Research Institute, and the Children’s Hospital of Eastern Ontario Research Institute. The funding sources had no involvement in study design, collection, analysis, or interpretation of data; in the writing of the report; or in the decision to submit this article for publication. The authors report no conflicts of interest.
ACKNOWLEDGMENTS
The authors thank the children and their families for their participation. The study team gratefully acknowledges the support of the Canadian Sleep and Circadian Network, as well as the funding support from the Canadian Institutes of Health Research, the Hospital for Sick Children Research Institute, and the Children’s Hospital of Eastern Ontario Research Institute. The members of the Canadian Sleep and Circadian Network include Drs. Najib Ayas, Julie Carrier, Patrick Hanly, John Peever, Sachin Pendharkar, Roberto Skomro, and Indra Narang.
ABBREVIATIONS
- ADHD
attention-deficit/hyperactivity disorder
- BMIz
body mass index z-score
- CI
confidence interval
- HRQOL
health-related quality of life
- IQR
interquartile range
- OAHI
obstructive apnea-hypopnea index
- OSA
obstructive sleep apnea
- PedsQL
Pediatric Quality of Life Inventory
- PSG
polysomnography
- PSQI
Pittsburgh Sleep Quality Index
Contributor Information
on behalf of the Canadian Sleep and Circadian Network:
Najib Ayas, Julie Carrier, Patrick Hanly, John Peever, Sachin Pendharkar, Roberto Skomro, and Indra Narang
REFERENCES
- 1. Ersu R , Chen ML , Ehsan Z , Ishman SL , Redline S , Narang I . Persistent obstructive sleep apnoea in children: treatment options and management considerations [published online ahead of print 2022. Sept 23]. Lancet Respir Med . [DOI] [PubMed] [Google Scholar]
- 2. Billings ME , Cohen RT , Baldwin CM , et al . Disparities in sleep health and potential intervention models: a focused review . Chest. 2021. ; 159 ( 3 ): 1232 – 1240 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Morin CM , Belleville G , Bélanger L , Ivers H . The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response . Sleep. 2011. ; 34 ( 5 ): 601 – 608 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. de Zambotti M , Goldstone A , Colrain IM , Baker FC . Insomnia disorder in adolescence: diagnosis, impact, and treatment . Sleep Med Rev. 2018. ; 39 : 12 – 24 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lumeng JC , Chervin RD . Epidemiology of pediatric obstructive sleep apnea . Proc Am Thorac Soc. 2008. ; 5 ( 2 ): 242 – 252 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Verhulst SL , Van Gaal L , De Backer W , Desager K . The prevalence, anatomical correlates and treatment of sleep-disordered breathing in obese children and adolescents . Sleep Med Rev. 2008. ; 12 ( 5 ): 339 – 346 . [DOI] [PubMed] [Google Scholar]
- 7. Falch-Madsen J , Wichstrøm L , Pallesen S , et al . Predictors of diagnostically defined insomnia in child and adolescent community samples: a literature review . Sleep Med. 2021. ; 87 : 241 – 249 . [DOI] [PubMed] [Google Scholar]
- 8. Calhoun SL , Fernandez-Mendoza J , Vgontzas AN , Liao D , Bixler EO . Prevalence of insomnia symptoms in a general population sample of young children and preadolescents: gender effects . Sleep Med. 2014. ; 15 ( 1 ): 91 – 95 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gradisar M , Gardner G , Dohnt H . Recent worldwide sleep patterns and problems during adolescence: a review and meta-analysis of age, region, and sleep . Sleep Med. 2011. ; 12 ( 2 ): 110 – 118 . [DOI] [PubMed] [Google Scholar]
- 10. Fernandez-Mendoza J , Bourchtein E , Calhoun S , et al . Natural history of insomnia symptoms in the transition from childhood to adolescence: population rates, health disparities, and risk factors . Sleep. 2021. ; 44 ( 3 ): zsaa187 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li SX , Chan NY , Man Yu MW , et al . Eveningness chronotype, insomnia symptoms, and emotional and behavioural problems in adolescents . Sleep Med. 2018. ; 47 : 93 – 99 . [DOI] [PubMed] [Google Scholar]
- 12. Combs D , Goodwin JL , Quan SF , Morgan WJ , Shetty S , Parthasarathy S . Insomnia, health-related quality of life and health outcomes in children: a seven year longitudinal cohort . Sci Rep. 2016. ; 6 ( 1 ): 27921 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Katz SL , MacLean JE , Barrowman N , et al . Long-term impact of sleep-disordered breathing on quality of life in children with obesity . J Clin Sleep Med. 2018. ; 14 ( 3 ): 451 – 458 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee S , Kim JH , Chung JH . The association between sleep quality and quality of life: a population-based study . Sleep Med. 2021. ; 84 : 121 – 126 . [DOI] [PubMed] [Google Scholar]
- 15. Krueger PM , Reither EN , Peppard PE , Burger AE , Hale L . Cumulative exposure to short sleep and body mass outcomes: a prospective study . J Sleep Res. 2015. ; 24 ( 6 ): 629 – 638 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jarrin DC , McGrath JJ , Drake CL . Beyond sleep duration: distinct sleep dimensions are associated with obesity in children and adolescents . Int J Obes Lond. 2013. ; 37 ( 4 ): 552 – 558 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Duraccio KM , Simmons DM , Beebe DW , Byars KC . Relationship of overweight and obesity to insomnia severity, sleep quality, and insomnia improvement in a clinically referred pediatric sample . J Clin Sleep Med. 2022. ; 18 ( 4 ): 1083 – 1091 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Beebe DW , Lewin D , Zeller M , et al . Sleep in overweight adolescents: shorter sleep, poorer sleep quality, sleepiness, and sleep-disordered breathing . J Pediatr Psychol. 2007. ; 32 ( 1 ): 69 – 79 . [DOI] [PubMed] [Google Scholar]
- 19. Fatima Y , Doi SAR , Mamun AA . Sleep quality and obesity in young subjects: a meta-analysis . Obes Rev. 2016. ; 17 ( 11 ): 1154 – 1166 . [DOI] [PubMed] [Google Scholar]
- 20. Krietsch KN , Chardon ML , Beebe DW , Janicke DM . Sleep and weight-related factors in youth: a systematic review of recent studies . Sleep Med Rev. 2019. ; 46 : 87 – 96 . [DOI] [PubMed] [Google Scholar]
- 21. Rao DP , Kropac E , Do MT , Roberts KC , Jayaraman GC . Childhood overweight and obesity trends in Canada . Health Promot Chronic Dis Prev Can. 2016. ; 36 ( 9 ): 194 – 198 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Williams J , Wake M , Hesketh K , Maher E , Waters E . Health-related quality of life of overweight and obese children . JAMA. 2005. ; 293 ( 1 ): 70 – 76 . [DOI] [PubMed] [Google Scholar]
- 23. Schwimmer JB , Burwinkle TM , Varni JW . Health-related quality of life of severely obese children and adolescents . JAMA. 2003. ; 289 ( 14 ): 1813 – 1819 . [DOI] [PubMed] [Google Scholar]
- 24. Varni JW , Burwinkle TM , Seid M , Skarr D . The PedsQL 4.0 as a pediatric population health measure: feasibility, reliability, and validity . Ambul Pediatr. 2003. ; 3 ( 6 ): 329 – 341 . [DOI] [PubMed] [Google Scholar]
- 25. Raniti MB , Waloszek JM , Schwartz O , Allen NB , Trinder J . Factor structure and psychometric properties of the Pittsburgh Sleep Quality Index in community-based adolescents . Sleep. 2018. ; 41 ( 6 ): zsy066 . [DOI] [PubMed] [Google Scholar]
- 26. Buysse DJ , Reynolds CF 3rd , Monk TH , Berman SR , Kupfer DJ . The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research . Psychiatry Res. 1989. ; 28 ( 2 ): 193 – 213 . [DOI] [PubMed] [Google Scholar]
- 27. Mollayeva T , Thurairajah P , Burton K , Mollayeva S , Shapiro CM , Colantonio A . The Pittsburgh sleep quality index as a screening tool for sleep dysfunction in clinical and non-clinical samples: a systematic review and meta-analysis . Sleep Med Rev. 2016. ; 25 : 52 – 73 . [DOI] [PubMed] [Google Scholar]
- 28. Narang I , Manlhiot C , Davies-Shaw J , et al . Sleep disturbance and cardiovascular risk in adolescents . CMAJ. 2012. ; 184 ( 17 ): E913 – E920 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Berry RB , Quan SF , Abreu AR , et al. for the American Academy of Sleep Medicine . The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Version 2.6 . Darien, IL: : American Academy of Sleep Medicine; ; 2020. . [Google Scholar]
- 30. Kaditis AG , Alonso Alvarez ML , Boudewyns A , et al . Obstructive sleep disordered breathing in 2- to 18-year-old children: diagnosis and management . Eur Respir J. 2016. ; 47 ( 1 ): 69 – 94 . [DOI] [PubMed] [Google Scholar]
- 31. World Health Organization . Growth Reference Data for 5-19 Years. https://www.who.int/tools/growth-reference-data-for-5to19-years/application-tools . Accessed April 22, 2022.
- 32. de Onis M , Onyango AW , Borghi E , Siyam A , Nishida C , Siekmann J . Development of a WHO growth reference for school-aged children and adolescents . Bull World Health Organ. 2007. ; 85 ( 9 ): 660 – 667 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Feudtner C , Feinstein JA , Zhong W , Hall M , Dai D . Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation . BMC Pediatr. 2014. ; 14 ( 1 ): 199 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Taylor SJ , Whincup PH , Hindmarsh PC , Lampe F , Odoki K , Cook DG . Performance of a new pubertal self-assessment questionnaire: a preliminary study . Paediatr Perinat Epidemiol. 2001. ; 15 ( 1 ): 88 – 94 . [DOI] [PubMed] [Google Scholar]
- 35. Nishiyama T , Mizuno T , Kojima M , et al . Criterion validity of the Pittsburgh Sleep Quality Index and Epworth Sleepiness Scale for the diagnosis of sleep disorders . Sleep Med. 2014. ; 15 ( 4 ): 422 – 429 . [DOI] [PubMed] [Google Scholar]
- 36. Kezirian EJ , Harrison SL , Ancoli-Israel S , et al. Study of Osteoporotic Fractures Research Group . Behavioral correlates of sleep-disordered breathing in older women . Sleep. 2007. ; 30 ( 9 ): 1181 – 1188 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: : American Psychiatric Association Publishing; ; 2013. . [Google Scholar]
- 38. American Academy of Sleep Medicine . International Classification of Sleep Disorders. 3rd ed . Darien, IL: : American Academy of Sleep Medicine; ; 2014. . [Google Scholar]
- 39. Sweetman A , Lack L , McEvoy RD , et al . Bi-directional relationships between co-morbid insomnia and sleep apnea (COMISA) . Sleep Med Rev. 2021. ; 60 : 101519 . [DOI] [PubMed] [Google Scholar]
- 40. Meira E Cruz M , Salles C , Seixas L , D Elia C , Rocha I , Gozal D . Comorbid insomnia and sleep apnea in children: a preliminary explorative study . J Sleep Res. 2023. ; 32 ( 1 ): e13705 . [DOI] [PubMed] [Google Scholar]
- 41. Meira E Cruz M , Kryger MH , Morin CM , Palombini L , Salles C , Gozal D . Comorbid insomnia and sleep apnea: mechanisms and implications of an underrecognized and misinterpreted sleep disorder . Sleep Med. 2021. ; 84 : 283 – 288 . [DOI] [PubMed] [Google Scholar]
- 42. Johnson KA , Gordon CJ , Chapman JL , et al . The association of insomnia disorder characterised by objective short sleep duration with hypertension, diabetes and body mass index: a systematic review and meta-analysis . Sleep Med Rev. 2021. ; 59 : 101456 . [DOI] [PubMed] [Google Scholar]
- 43. Zhang Y , Jiang X , Liu J , Lang Y , Liu Y . The association between insomnia and the risk of metabolic syndrome: a systematic review and meta-analysis . J Clin Neurosci. 2021. ; 89 : 430 – 436 . [DOI] [PubMed] [Google Scholar]
- 44. Chan WS , Levsen MP , McCrae CS . A meta-analysis of associations between obesity and insomnia diagnosis and symptoms . Sleep Med Rev. 2018. ; 40 : 170 – 182 . [DOI] [PubMed] [Google Scholar]
- 45. Tse LA , Wang C , Rangarajan S , et al . Timing and length of nocturnal sleep and daytime napping and associations with obesity types in high-, middle-, and low-income countries . JAMA Netw Open. 2021. ; 4 ( 6 ): e2113775 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hägg SA , Ilieva E , Ljunggren M , et al . The negative health effects of having a combination of snoring and insomnia . J Clin Sleep Med. 2022. ; 18 ( 4 ): 973 – 981 . [DOI] [PMC free article] [PubMed] [Google Scholar]