This systematic review and meta-analysis assesses the risk of all-cause mortality, major adverse cardiovascular events, and venous thromboembolism (VTE) with JAK inhibitors in patients with dermatologic conditions.
Key Points
Question
Are Janus kinase (JAK) inhibitors associated with higher risk of all-cause mortality, major adverse cardiovascular events (MACE), and venous thromboembolism (VTE) when used for a dermatologic indication?
Findings
In this systematic review and meta-analysis of 35 randomized clinical trials containing over 20 000 patients with dermatologic conditions, no significant difference was found between JAK inhibitors and placebo/active comparator in composite MACE and all-cause mortality or VTE.
Meaning
Short-term (<5 months) use of JAK inhibitors for a dermatologic indication likely is not associated with an increased risk of all-cause mortality, MACE, and VTE.
Abstract
Importance
Janus kinase (JAK) inhibitors are an effective treatment option for patients with certain skin-related conditions, such as atopic dermatitis, alopecia areata, and vitiligo, but there is a current US Food and Drug Administration (FDA) boxed warning label for oral and topical JAK inhibitors regarding increased risk of major adverse cardiovascular events (MACE), venous thromboembolism (VTE), serious infections, malignant neoplasm, and death. However, this boxed warning was precipitated by results of the Oral Rheumatoid Arthritis Trial (ORAL) Surveillance study, which only included patients with rheumatoid arthritis, and the same association may not be observed in dermatologic conditions.
Objective
To determine the risk of all-cause mortality, MACE, and VTE with JAK inhibitors in patients with dermatologic conditions.
Data Sources
PubMed and ClinicalTrials.gov were searched from database inception to April 1, 2023.
Study Selection
This review included phase 3 randomized clinical trials with a placebo/active comparator group of JAK inhibitors used for a dermatologic indication with FDA approval or pending approval or with European Union or Japanese approval. Studies without a comparison group, case reports, observational studies, and review articles were excluded.
Data Extraction and Synthesis
This study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines. Adverse events using odds ratios (ORs) and 95% CIs were calculated using a random-effects model and the DerSimonian-Laird method. Studies were screened, data abstracted, and quality assessed by 2 independent authors. The protocol was prospectively registered with PROSPERO.
Main Outcomes and Measures
Primary outcomes were a composite of adjudicated MACE and all-cause mortality, and VTE.
Results
The analysis included 35 randomized clinical trials with 20 651 patients (mean [SD] age, 38.5 [10.1] years; male, 54%) and a mean (SD) follow-up time of 4.9 (2.68) months. Findings did not show a significant difference between JAK inhibitors and placebo/active comparator in composite MACE and all-cause mortality (OR, 0.83; 95% CI, 0.44-1.57) or VTE (OR, 0.52; 95% CI, 0.26-1.04).
Conclusions and Relevance
In this systematic review and meta-analysis, use of JAK inhibitors was not associated with increased risk of all-cause mortality, MACE, and VTE compared to the placebo/active comparator groups. Additional trials with long-term follow-up are needed to better understand the safety risks of JAK inhibitors used for dermatologic indications.
Introduction
Oral and topical Janus kinase (JAK) inhibitors are increasingly used to treat chronic immune-mediated inflammatory diseases (IMIDs) of the skin.1 The US Food and Drug Administration (FDA) has approved their use in atopic dermatitis (upadacitinib, abrocitinib, ruxolitinib cream), vitiligo (ruxolitinib cream), and alopecia areata (baricitinib, ritlecitinib).
JAK inhibitors are effective in the treatment of IMIDs of the skin1; however, their safety profile is concerning for an increased risk of major adverse cardiovascular events (MACE), venous thromboembolic events (VTE), serious infections, malignant neoplasm, and death. In this study, we chose to focus on the cardiovascular and thromboembolic risks of JAK inhibitors. This is especially important because many IMIDs of the skin are associated with an increased risk of MACE and VTE.2,3,4,5,6 There is a current boxed warning label for topical and oral JAK inhibitors based primarily on studies evaluating older patient populations with rheumatoid arthritis (RA) with at least 1 cardiovascular risk factor, such as the Oral Rheumatoid Arthritis Trial (ORAL) Surveillance study.7 The study enrolled patients older than 50 years with RA who were receiving methotrexate and had at least 1 cardiovascular risk factor. Over 4 years of follow-up, the study found that patients treated with the higher dose of tofacitinib (10 mg) were at increased risk of VTE (pulmonary embolism, deep vein thrombosis), and those treated at either dose (5 mg or 10 mg) were at increased risk of MACE (nonfatal myocardial infarction, stroke, death).
There remains a knowledge gap regarding the risk of JAK inhibitor use and VTE and/or MACE in the dermatologic population. To our knowledge, there has not been a study specifically evaluating this risk for dermatologic indications only. Pooled safety studies suggest that the risk of MACE and VTE may be lower in patients treated with JAK inhibitors for a dermatologic indication than the risk observed in the ORAL Surveillance study,7 which may be related to the younger age and better health status of those enrolled in trials for dermatologic indications.8,9,10 In this meta-analysis of phase 3 dermatology randomized clinical trials (RCTs), we sought to evaluate the risk of MACE, VTE, and all-cause mortality with JAK inhibitors compared to placebo or active comparator in the treatment of IMIDs of the skin.
Methods
This combined systematic review and meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline.11 This analysis was not considered for institutional review board review because it was a trial-level meta-analysis of published clinical trial data. The study protocol was prospectively registered with PROSPERO (CRD42023439713).
Search Strategy and Selection Criteria
A time-limited search from database inception until April 1, 2023, restricted to human study participants, was conducted using PubMed. The following Medical Education Subject Headings (MeSH) terms were used for this search: “atopic dermatitis,” or “psoriasis,” or “psoriatic arthritis,” or “vitiligo,” or “alopecia areata,” and “JAK inhibitors,” or “tofacitinib,” or “abrocitinib,” or “baricitinib,” or “upadacitinib,” or “ritlecitinib,” or “delgocitinib cream,” or “ruxolitinib cream.” We also hand-searched ClinicalTrials.gov and the references of the eligible studies to identify further studies. The inclusion criteria were phase 3 RCTs of JAK inhibitors used for a dermatologic condition leading to US FDA approval or pending approval, European Union approval, or Japanese approval, such as abrocitinib, baricitinib, tofacitinib, upadacitinib, ritlecitinib, delgocitinib cream, and ruxolitinib cream. Studies without a comparison group, case reports, observational studies, and review articles were excluded.
We used PICO (Population, Intervention, Comparison, and Outcome) criteria for study selection.12 Population of interest was IMIDs of skin; intervention: JAK inhibitors; comparison: placebo or active comparator; and outcomes: primary safety outcomes were a composite of MACE (defined as stroke, nonfatal myocardial infarction, cardiovascular death) and all-cause mortality, and VTE (defined as deep vein thrombosis, pulmonary embolism, or retinal vein thrombosis).
Data Analysis
Data Extraction and Quality Assessment
The studies were screened, data abstracted, and quality assessed by 2 independent authors (M.S.G. and J.P.I.). Nonrelevant studies were excluded based on review of the abstract. Full-text studies were then screened for final selection based on the abovementioned, prespecified inclusion criteria. The methodological quality of included trials was assessed using the Cochrane Risk of Bias Tool, which assessed selection, allocation, performance, detection, attrition, and reporting bias (eTable in Supplement 1).13
Statistical Analysis
Continuous variables were reported as mean with standard deviation and categorical variables were expressed as frequency/percentage. Adverse events using odds ratio (ORs) and 95% CIs were calculated using a random-effects model and the DerSimonian-Laird method.14 Heterogeneity between studies was assessed using I2 statistic.15 An I2 value less than 25%, 25% to 50%, 50% to 75%, and greater than 75% indicated low, moderate, high, and extreme heterogeneity, respectively. Primary analysis included atopic dermatitis, alopecia areata, psoriasis, and vitiligo as IMIDs of the skin for each of the abovementioned outcomes. Secondary analysis included additional psoriatic arthritis RCTs to determine if the psoriatic arthritis phenotype was associated with the level of risk for VTE and MACE. Two subgroup analyses were conducted based on oral vs topical JAK inhibitors and various dermatologic conditions. A sensitivity analysis was done by including trials with adult populations (excluding pediatric trials). A funnel plot was used to assess publication bias. A P value less than .05 was considered statistically significant. Risk estimates and effect sizes, sensitivity analyses, and cumulative meta-analyses were calculated using Stata, version 18.0 (StataCorp LLC).
Results
Literature Search
The initial search yielded 1027 reports in PubMed and 9 trials in ClinicalTrials.gov, which were screened by reviewing the abstract. A total of 35 RCTs (6 abrocitinib, 8 baricitinib, 2 delgocitinib cream, 4 ruxolitinib cream, 7 tofacitinib, 1 ritlecitinib, and 7 upadacitinib) were included in the final meta-analysis (eFigure 1 in Supplement 1).16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44
Baseline Characteristics
The trials enrolled a total of 20 651 patients (13 597 randomized to JAK inhibitors and 7054 to placebo/active comparator) with atopic dermatitis (21 trials), alopecia areata (3 trials), psoriasis (including psoriatic arthritis) (9 trials), and vitiligo (2 trials), with a mean (SD) follow-up of 4.9 (2.68) months. The 35 included trials were published between 2015 and 2023 with an almost equal proportion of female and male participants (male, 54%); mean (SD) age was 38.5 (10.1) years. The baseline characteristics of the study populations are provided in the Table.16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44
Table. Baseline Characteristics of Included Randomized Clinical Trials.
| Trial name | Reference | JAKI No. | PBO/AC No. | Total | Disease condition | JAKI | Approval | Mean age, y | Male, % | Follow-up, mo | PBO, AC, or both | AC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| JADE MONO-1 | Simpson et al,16 2020 | 310 | 77 | 387 | Atopic dermatitis | Abrocitinib | FDA and EU | 54.8 | 32.5 | 3 | PBO | NA |
| JADE-MONO-2 | Silverberg et al,17 2020 | 313 | 78 | 391 | Atopic dermatitis | Abrocitinib | FDA and EU | 35.1 | 58.6 | 3 | PBO | NA |
| JADE Teen | Eichenfield et al,18 2021 | 96 | 189 | 285 | Atopic dermatitis | Abrocitinib | FDA and EU | 15.0 | 50.9 | 12 | PBO | NA |
| JADE DARE | Riech et al,19 2022 | 365 | 362 | 727 | Atopic dermatitis | Abrocitinib | FDA and EU | 36.6 | 53.0 | 6.5 | Both | Dupilumab |
| JADE REGIMEN | Blauvelt et al,20 2021 | 531 | 267 | 798 | Atopic dermatitis | Abrocitinib | FDA and EU | 28.7 | 55.0 | 3 | PBO | NA |
| JADE Compare | Bieber et al,21 2021 | 464 | 374 | 838 | Atopic dermatitis | Abrocitinib | FDA and EU | 37.9 | 50.4 | 4 | Both | Dupilumab |
| Heads Up | Blauvelt et al,22 2021 | 348 | 344 | 692 | Atopic dermatitis | Upadacitinib | FDA and EU | 36.7 | 54.5 | 4 | AC | Dupilumab |
| Measure Up 1 | Guttman-Yassky et al,23 2021 | 566 | 281 | 847 | Atopic dermatitis | Upadacitinib | FDA and EU | 34.0 | 54.0 | 4 | PBO | NA |
| Measure Up 2 | Guttman-Yassky et al,23 2021 | 558 | 278 | 836 | Atopic dermatitis | Upadacitinib | FDA and EU | 33.6 | 56.0 | 4 | PBO | NA |
| AD Up | Reich et al,24 2021 | 597 | 304 | 901 | Atopic dermatitis | Upadacitinib | FDA and EU | 34.0 | 62.0 | 4 | PBO | NA |
| Rising Up | Katoh et al,25 2023 | 182 | 90 | 272 | Atopic dermatitis | Upadacitinib | FDA and EU | 35.6 | 75.8 | 6 | PBO | NA |
| BREEZE-AD1 | Simpson et al,26 2020 | 375 | 249 | 624 | Atopic dermatitis | Baricitinib | EU | 35.8 | 62.7 | 4 | PBO | NA |
| BREEZE-AD2 | Simpson et al,26 2020 | 370 | 244 | 614 | Atopic dermatitis | Baricitinib | EU | 34.5 | 62.0 | 4 | PBO | NA |
| BREEZE-AD4 | Bieber et al,27 2022 | 370 | 93 | 463 | Atopic dermatitis | Baricitinib | EU | 38.4 | 62.5 | 4 | PBO | NA |
| BREEZE AD5 | Simpson et al,28 2021 | 292 | 146 | 438 | Atopic dermatitis | Baricitinib | EU | 40.0 | 49.0 | 4 | PBO | NA |
| BREEZE-AD7 | Reich et al,43 2020 | 220 | 108 | 328 | Atopic dermatitis | Baricitinib | EU | 33.8 | 66.0 | 4 | PBO | NA |
| BREEZE-AD-PEDS | Torrelo et al,29 2023 | 361 | 122 | 483 | Atopic dermatitis | Baricitinib | EU | 12.0 | 50.7 | 4 | PBO | NA |
| TRuE-AD1 | Papp et al,30 2021 | 505 | 126 | 631 | Atopic dermatitis | Ruxolitinib cream | FDA | 32.0 | 38.0 | 2 | PBO | NA |
| TRuE-AD2 | Papp et al,30 2021 | 494 | 124 | 618 | Atopic dermatitis | Ruxolitinib cream | FDA | 33.0 | 38.5 | 2 | PBO | NA |
| BRAVE-AA1 | King et al,31 2022 | 465 | 189 | 654 | Alopecia areata | Baricitinib | FDA and EU | 37.0 | 39.3 | 9 | PBO | NA |
| BRAVE-AA2 | King et al,31 2022 | 390 | 156 | 546 | Alopecia areata | Baricitinib | FDA and EU | 37.0 | 39.3 | 9 | PBO | NA |
| NA | Bachelez et al,44 2015 | 659 | 442 | 1101 | Psoriasis | Tofacitinib | EU | 44.3 | 71.1 | 3 | Both | Etanercept |
| OPT Pivotal 1 | Papp et al,32 2015 | 723 | 177 | 900 | Psoriasis | Tofacitinib | EU | 45.8 | 71.4 | 4 | PBO | NA |
| OPT Pivotal 2 | Papp et al,32 2015 | 763 | 196 | 959 | Psoriasis | Tofacitinib | EU | 45.4 | 67.6 | 4 | PBO | NA |
| NA | Zhang et al,33 2017 | 178 | 88 | 266 | Psoriasis | Tofacitinib | EU | 41.1 | 72.9 | 12 | PBO | NA |
| SELECT-PSA 1 | McInnes et al,34 2021 | 852 | 852 | 1704 | Psoriatic arthritis | Upadacitinib | FDA and EU | 50.6 | 46.3 | 3 | Both | Adalimumab |
| SELECT-PSA 2 | Mease et al,35 2021 | 429 | 212 | 641 | Psoriatic arthritis | Upadacitinib | FDA and EU | 53.4 | 54.3 | 6 | PBO | NA |
| Opal Broaden | Mease et al,36 2017 | 211 | 211 | 422 | Psoriatic arthritis | Tofacitinib | EU | 48.0 | 44.6 | 12 | Both | Adalimumab |
| OPAL Beyond | Gladman et al,37 2017 | 263 | 131 | 394 | Psoriatic arthritis | Tofacitinib | EU | 49.9 | 44.7 | 6 | PBO | NA |
| NA | Leng et al,38 2023 | 136 | 68 | 204 | Psoriatic arthritis | Tofacitinib | EU | 44.8 | 59.3 | 6 | PBO | NA |
| TRuE-V1 | Rosmarin et al,39 2022 | 221 | 109 | 330 | Vitiligo | Ruxolitinib cream | FDA and EU | 40.2 | 43.5 | 6 | PBO | NA |
| TRuE-V2 | Rosmarin et al,39 2022 | 228 | 115 | 344 | Vitiligo | Ruxolitinib cream | FDA and EU | 38.9 | 49.9 | 6 | PBO | NA |
| ALLEGRO-2b/3 | King et al,42 2023 | 587 | 131 | 718 | Alopecia areata | Ritlecitinib | FDA and Japan | 33.8 | 37.9 | 12 | PBO | NA |
| Peds: Part 1 | Nakagawa et al,40 2021 | 69 | 68 | 137 | Atopic dermatitis | Delgocitinib cream | Japan | 8.3 | 51.1 | 4 | PBO | NA |
| Adults: QBA4-1 | Nakagawa et al,41 2020 | 106 | 52 | 158 | Atopic dermatitis | Delgocitinib cream | Japan | 31.7 | 62.0 | 4 | PBO | NA |
Abbreviations: AC, active comparator; EU, European Union; FDA, US Food and Drug Administration; JAKI, Janus kinase inhibitor; NA, not applicable; PBO, placebo.
Primary Analysis
Composite MACE and All-Cause Mortality
There was no significant difference in composite MACE and all-cause mortality between JAK inhibitors and placebo/active comparator using a random-effects DerSimonian-Laird method in the primary analysis (OR, 0.83; 95% CI, 0.44-1.57; I2 = 0%; 30 trials) (Figure 1). Subgroup analyses based on oral vs topical JAK inhibitors (heterogeneity P = .84) showed a similar association without significant heterogeneity (supporting data in Figure 1) or evidence of publication bias (eFigure 2A in Supplement 1). Sensitivity analysis by exclusion of pediatric trials (mean age <18 years) showed nonsignificant outcomes (OR, 0.83; 95% CI, 0.43-1.61; I2 = 0%; 27 trials) (eFigure 2B-C in Supplement 1). Subgroup analyses (of the sensitivity analysis) based on oral vs topical JAK inhibitors (heterogeneity P = .80) and dermatologic conditions (heterogeneity P = .93) showed a similar association without significant heterogeneity (supporting data in eFigure 2B-C in Supplement 1).
Figure 1. Primary Analysis: Composite of Major Adverse Cardiac Events (MACE) and All-Cause Mortality With Janus Kinase (JAK) Inhibitors Excluding Psoriatic Arthritis Trials.
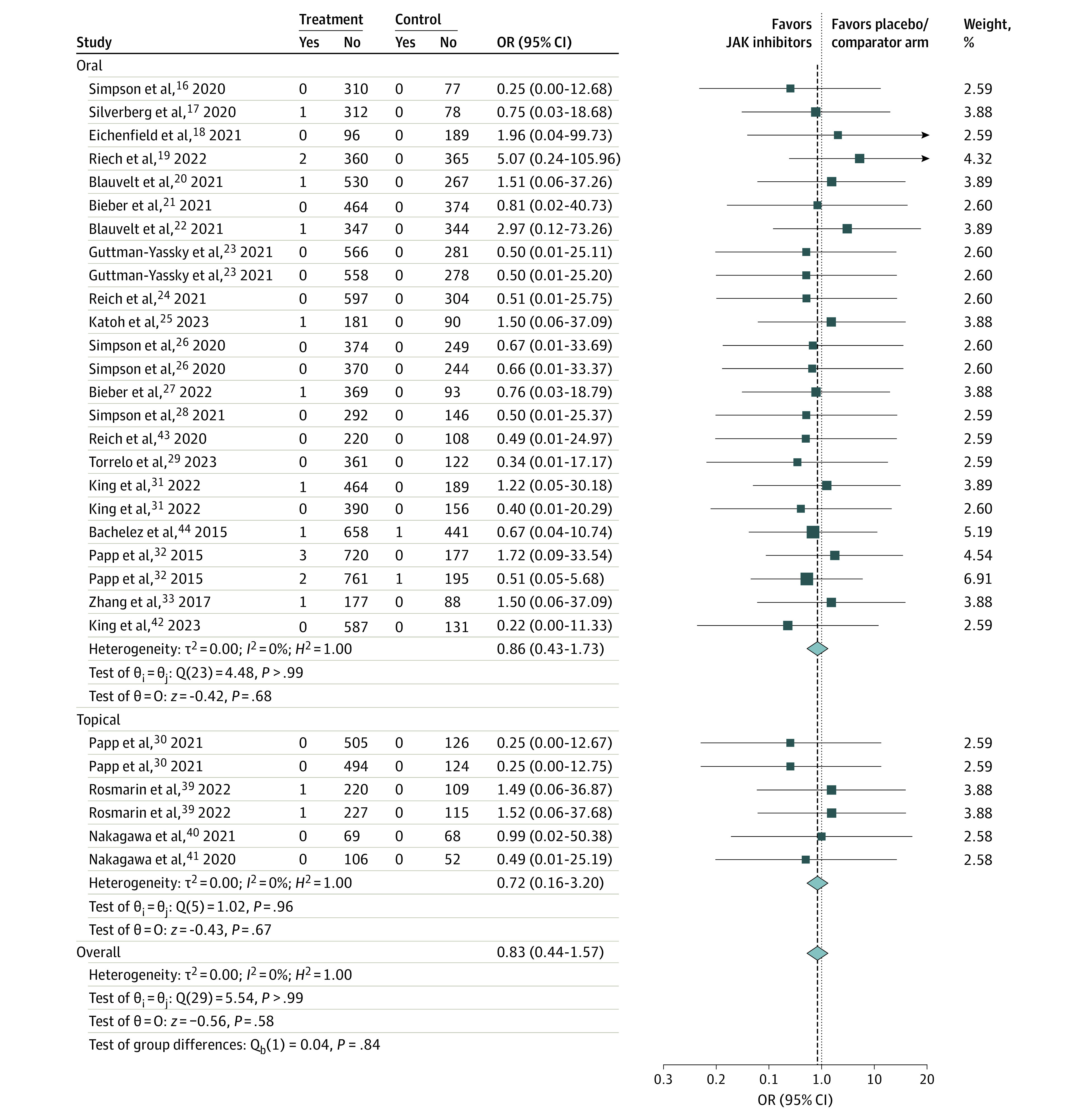
OR indicates odds ratio.
There was no significant difference in composite MACE and all-cause mortality between JAK inhibitors and placebo/active comparator when including trials with psoriatic arthritis (OR, 0.74; 95% CI, 0.42-1.31; I2 = 0%; 35 trials) (eFigure 6A in Supplement 1). Subgroup analyses based on IMID conditions (heterogeneity P = .87) showed a similar association without significant heterogeneity (supporting data in eFigure 6A in Supplement 1). There was no evidence of publication bias assessed by funnel plot (eFigure 4 in Supplement 1).
Venous Thromboembolism
There was no significant difference in VTE between JAK inhibitors and placebo/active comparator (OR, 0.52; 95% CI, 0.26-1.04; I2 = 0%; 30 trials) (Figure 2). Subgroup analyses based on oral vs topical JAK inhibitors (heterogeneity P = .83) showed similar association without significant heterogeneity (supporting data in Figure 2). There was no evidence of publication bias assessed by funnel plot (eFigure 3A in Supplement 1).
Figure 2. Primary Analysis: Composite of Venous Thromboembolism (VTE) With Janus Kinase (JAK) Inhibitors Excluding Psoriatic Arthritis Trials.
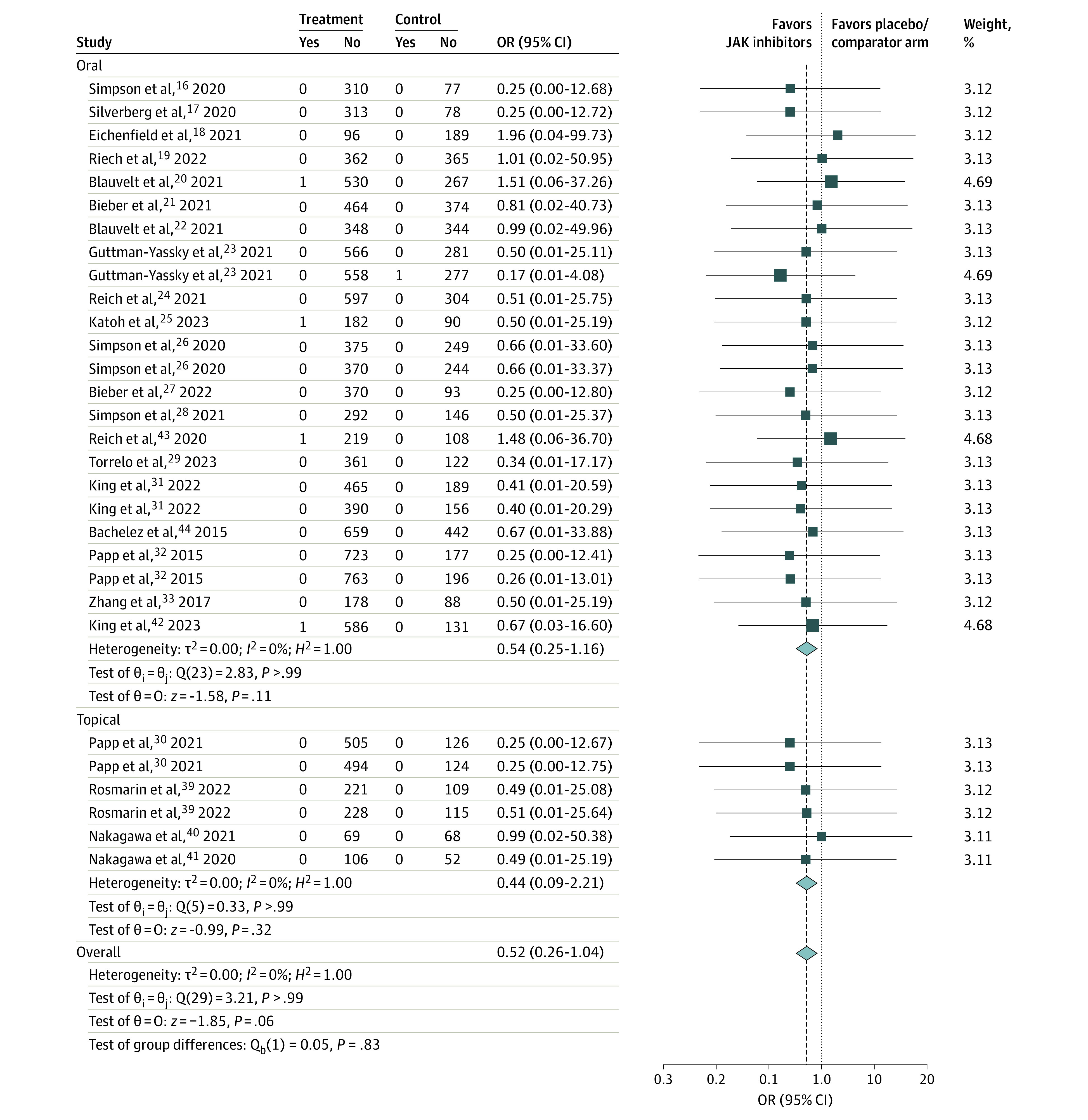
OR indicates odds ratio.
Sensitivity Analysis
Sensitivity analysis by the exclusion of pediatric trials (mean age <18 years) showed nonsignificant outcomes (OR, 0.38; 95% CI, 0.07-2.19; I2 = 0%; 27 trials) (eFigure 3B-C in Supplement 1). Subgroup analyses (of the sensitivity analysis) based on oral vs topical JAK inhibitors (heterogeneity P = .75) and dermatologic conditions (heterogeneity P = .99) showed a similar association without significant heterogeneity (supporting data in eFigure 3B-C in Supplement 1).
There was no significant difference in VTE between JAK inhibitors and placebo/active comparator when including trials with psoriatic arthritis (OR, 0.54; 95% CI, 0.29-1.02; I2 = 0%; 35 trials) (eFigure 6B in Supplement 1). Subgroup analyses based on dermatologic conditions (heterogeneity P = .99) showed a similar association without significant heterogeneity (supporting data in eFigure 6B in Supplement 1). There was no evidence of publication bias assessed by funnel plot (eFigure 5 in Supplement 1).
Discussion
We performed a meta-analysis of 35 RCTs with over 20 000 patients to determine the cardiovascular and VTE risk of JAK inhibitors used for atopic dermatitis, alopecia areata, psoriasis (including psoriatic arthritis), and vitiligo. In our primary analysis, we did not find a significant difference in composite MACE and all-cause mortality or in VTE between JAK inhibitors and the placebo/active comparator. In our secondary analysis, which included additional psoriatic arthritis RCTs, we similarly found no significant differences between the treatment and placebo/active comparator groups. Subgroup analysis of oral vs topical JAK inhibitors and a sensitivity analysis excluding pediatric trials failed to demonstrate significant differences between those exposed to JAK inhibitors and those not exposed.
This study addresses an important literature gap and, to our knowledge, is the first comprehensive meta-analysis specifically evaluating the risk of all-cause mortality, VTE, and MACE with JAK inhibitors used for a primary dermatologic indication. A previous meta-analysis specifically explored the association between VTE (but not MACE or all-cause mortality) and JAK inhibitors in atopic dermatitis and did not find an increased risk of VTE with their use (hazard ratio, 0.95; 95% CI, 0.62-1.45).45 JAK inhibitors have demonstrated high efficacy in treating inflammatory skin diseases, owing to their ability to target multiple cytokines simultaneously. IMIDs of the skin may be recalcitrant to traditional therapies, and JAK inhibitor therapy increases the treatment options for these patients.46,47 Fewer than half of patients treated with dupilumab, an approved first-line biologic for moderate to severe atopic dermatitis, achieved clear or nearly clear skin after 16 weeks of therapy.22 Furthermore, some patients may experience a reduction in their response over time.48 In patients with severe alopecia areata, baricitinib and the newly approved JAK inhibitor ritlecitinib are the only FDA-approved therapies.
With the use of JAK inhibitors rising, understanding their safety profile in the target population is important. JAK inhibitors are less specific than biologics due to the inherent redundancy of the JAK/STAT pathway and therefore may display an increase in off-target effects, which may be related to the association of increased MACE and VTE observed in patients with RA.1 The ORAL Surveillance study7 highlighted safety concerns of JAK inhibitors and found an increased risk of MACE, VTE, all-cause mortality, serious infections, and malignant neoplasm with their use. However, this study only included patients with RA with at least 1 cardiovascular risk factor, and it is well established that RA, similar to psoriasis, independently increases the risk of MACE and VTE.49,50,51 The mechanism by which JAK inhibitors increased the risk of VTE and MACE is unclear and highlights the need for a future mechanistic study.
There are key differences between the dermatology RCTs included in our meta-analysis and the ORAL Surveillance study.7 The patients with dermatologic conditions were younger (mean age, 38.5 years) than the patients with RA (mean age, 61 years), which could explain the low overall incidence of MACE, all-cause mortality, and VTE among patients included in dermatology clinical trials. Additionally, the patients in ORAL Surveillance had higher cardiovascular risk than the patients with dermatologic conditions, as the study only enrolled patients with RA with at least 1 cardiovascular risk factor. Also, the patients with RA studied were receiving concomitant methotrexate and some were also receiving systemic corticosteroids, unlike the patients included in dermatology clinical trials, who were receiving JAK inhibitor monotherapy. Another difference between studies was the study duration, which was 4 years in ORAL Surveillance vs 4.9 months on average in this meta-analysis. Also, the majority of JAK inhibitors tested in dermatology clinical trials are selective for JAK1/2, which differs from the selectivity of tofacitinib, which predominantly inhibits JAK1/3. Finally, the control group in ORAL Surveillance consisted of patients with RA receiving tumor necrosis factor inhibitors. The majority of RCTs in this study had a placebo group rather than an active comparator group. In summary, the different IMIDs studied, age of participants, number of cardiovascular risk factors, concomitant immunosuppressive therapy, differential JAK selectivity, and study durations are critical differences between the dermatologic RCTs and ORAL Surveillance and may help to explain our dissimilar findings.
Our results differ from those found in a recent, expanded meta-analysis52 of 66 RCTs of JAK inhibitors across IMIDs. The study found a numerically higher rate of VTE with JAK inhibitors compared to active comparator groups, but not the placebo group, and a nonsignificant, numerically higher rate of MACE in those treated with JAK inhibitors compared to the placebo/active comparator groups. The majority of patients included in this study were being treated for a rheumatologic rather than a dermatologic condition. Only 16 of 66 trials (not including psoriatic arthritis) included in the meta-analysis were for dermatologic indications. In contrast, our study focused only on dermatologic conditions and included 30 dermatology-specific RCTs. This meta-analysis52 also found that the risk of MACE and VTE may occur in a time-dependent manner (≥12 months of drug exposure). Similarly, ORAL Surveillance7 included 4 years of safety data in its analysis. While both of these studies primarily focused on the rheumatology cohort, the results of our study may not be generalizable to patients receiving JAK inhibitor therapy for dermatologic conditions for extended time periods. We only analyzed short-term safety data because we chose to include RCTs with an active comparator or placebo group, and many of the extension trials had a crossover design without a comparator arm. Whether an association between JAK inhibitor use and MACE and/or VTE would be observed with longer-term safety data in the population with dermatologic conditions is not known.
An evolving area of research is trying to understand how systemic inflammatory conditions are associated with cardiovascular risk factors. IMIDs, including those of the skin, have been associated with an increased risk of cardiovascular disease.5 Post hoc analyses of ORAL Surveillance found that rates of MACE with JAK inhibitors primarily occurred in patients with preexisting atherosclerotic cardiovascular disease and that the risk of VTE and MACE was higher for those with active RA.53,54 Another study55 found that the incidence rates of MACE and VTE with JAK inhibitors were lower for patients with fewer cardiovascular risk factors across conditions, including RA. It is likely that the patients included in the dermatology RCTs had fewer cardiovascular comorbidities than the patients enrolled in the rheumatology RCTs. The risks of MACE and VTE with JAK inhibitors likely increase in patients with elevated cardiovascular comorbidities. Therefore, the results of our study may not be translatable to patients with dermatologic conditions at higher cardiovascular risk. An individualized approach to cardiovascular risk management in patients who may benefit from JAK inhibitor therapy is likely appropriate.56
Limitations
This study should be interpreted in light of several limitations. First, we did not have access to patient-level data. Second, the duration of follow-up time varied from short-term to long-term follow-up; the majority of studies only included short-term follow-up. Also, most of the trials consisted of small RTCs limited to specific geographical areas, so the extent of generalizability and trends in different populations are uncertain. Furthermore, the mean age was 38.5 years; therefore, the incidence of MACE, VTE, and all-cause mortality would be low and has limited generalizability in an older patient population. In this study, we chose to focus on 3 of the 5 boxed warnings for JAK inhibitors, and the risks of serious infections and malignant neoplasm warrant further investigation in patients with dermatologic conditions. Finally, the mean follow-up time was 4.9 months, so the association of long-term use of JAK inhibitors is unknown.
Conclusions
In this systematic review and meta-analysis of phase 3 dermatology RCTs, we did not find an elevated risk of adjudicated composite all-cause mortality and MACE or VTE in patients treated with short-term oral or topical JAK inhibitor therapy for IMIDs of the skin. It remains unclear if the cardiovascular risks of JAK inhibitors are primarily due to patient-level cardiovascular risk factors or are drug mediated. Dermatologists should carefully select patients and assess baseline cardiovascular risk factors when considering JAK therapy. Cardiovascular risk assessment should continue for the duration of treatment. Additional RCTs with long-term safety follow-up are needed to better understand the risks of all-cause mortality, MACE, and VTE, and even cancer and infection, with JAK inhibitors used for a dermatologic indication.
eTable. Cochrane risk of assessment bias
eFigure 1. PRISMA flow
eFigure 2. Effect of JAK inhibitors on composite of MACE and all-cause mortality (primary analysis)
eFigure 3. Effect of JAK inhibitors on VTE (primary analysis)
eFigure 4. Funnel plot: effect of JAK inhibitors on composite of MACE and all-cause mortality (secondary analysis)
eFigure 5. Funnel plot: effect of JAK inhibitors on VTE (secondary analysis)
eFigure 6. Secondary analysis
eAppendix. PRISMA checklist
Data Sharing Statement
References
- 1.Damsky W, King BA. JAK inhibitors in dermatology: the promise of a new drug class. J Am Acad Dermatol. 2017;76(4):736-744. doi: 10.1016/j.jaad.2016.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Warren RB, Basey V, Lynam A, Curtis C, Ardern-Jones MR. The risk of venous thromboembolism in atopic dermatitis: a matched cohort analysis in UK primary care. Br J Dermatol. 2023;ljad212. doi: 10.1093/bjd/ljad212 [DOI] [PubMed] [Google Scholar]
- 3.Chen J, Cheng J, Yang H, et al. The efficacy and safety of Janus kinase inhibitors in patients with atopic dermatitis: a systematic review and meta-analysis. J Am Acad Dermatol. 2022;87(2):495-496. doi: 10.1016/j.jaad.2022.03.039 [DOI] [PubMed] [Google Scholar]
- 4.Garshick MS, Ward NL, Krueger JG, Berger JS. Cardiovascular risk in patients with psoriasis: JACC review topic of the week. J Am Coll Cardiol. 2021;77(13):1670-1680. doi: 10.1016/j.jacc.2021.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conrad N, Verbeke G, Molenberghs G, et al. Autoimmune diseases and cardiovascular risk: a population-based study on 19 autoimmune diseases and 12 cardiovascular diseases in 22 million individuals in the UK. Lancet. 2022;400(10354):733-743. doi: 10.1016/S0140-6736(22)01349-6 [DOI] [PubMed] [Google Scholar]
- 6.Silverwood RJ, Forbes HJ, Abuabara K, et al. Severe and predominantly active atopic eczema in adulthood and long term risk of cardiovascular disease: population based cohort study. BMJ. 2018;361:k1786. doi: 10.1136/bmj.k1786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ytterberg SR, Bhatt DL, Mikuls TR, et al. ; ORAL Surveillance Investigators . Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. 2022;386(4):316-326. doi: 10.1056/NEJMoa2109927 [DOI] [PubMed] [Google Scholar]
- 8.Bieber T, Katoh N, Simpson EL, et al. Safety of baricitinib for the treatment of atopic dermatitis over a median of 1.6 years and up to 3.9 years of treatment: an updated integrated analysis of eight clinical trials. J Dermatolog Treat. 2023;34(1):2161812. doi: 10.1080/09546634.2022.2161812 [DOI] [PubMed] [Google Scholar]
- 9.Bieber T, Thyssen JP, Reich K, et al. Pooled safety analysis of baricitinib in adult patients with atopic dermatitis from 8 randomized clinical trials. J Eur Acad Dermatol Venereol. 2021;35(2):476-485. doi: 10.1111/jdv.16948 [DOI] [PubMed] [Google Scholar]
- 10.King B, Maari C, Lain E, et al. Extended safety analysis of baricitinib 2 mg in adult patients with atopic dermatitis: an integrated analysis from eight randomized clinical trials. Am J Clin Dermatol. 2021;22(3):395-405. doi: 10.1007/s40257-021-00602-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aslam S, Emmanuel P. Formulating a researchable question: a critical step for facilitating good clinical research. Indian J Sex Transm Dis AIDS. 2010;31(1):47-50. doi: 10.4103/0253-7184.69003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higgins JPT, Altman DG, Gøtzsche PC, et al. ; Cochrane Bias Methods Group; Cochrane Statistical Methods Group . The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343(7829):d5928. doi: 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Veroniki AA, Jackson D, Viechtbauer W, et al. Methods to estimate the between-study variance and its uncertainty in meta-analysis. Res Synth Methods. 2016;7(1):55-79. doi: 10.1002/jrsm.1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simpson EL, Sinclair R, Forman S, et al. Efficacy and safety of abrocitinib in adults and adolescents with moderate-to-severe atopic dermatitis (JADE MONO-1): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet. 2020;396(10246):255-266. doi: 10.1016/S0140-6736(20)30732-7 [DOI] [PubMed] [Google Scholar]
- 17.Silverberg JI, Simpson EL, Thyssen JP, et al. Efficacy and safety of abrocitinib in patients with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156(8):863-873. doi: 10.1001/jamadermatol.2020.1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eichenfield LF, Flohr C, Sidbury R, et al. Efficacy and safety of abrocitinib in combination with topical therapy in adolescents with moderate-to-severe atopic dermatitis: the JADE TEEN randomized clinical trial. JAMA Dermatol. 2021;157(10):1165-1173. doi: 10.1001/jamadermatol.2021.2830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reich K, Thyssen JP, Blauvelt A, et al. Efficacy and safety of abrocitinib versus dupilumab in adults with moderate-to-severe atopic dermatitis: a randomised, double-blind, multicentre phase 3 trial. Lancet. 2022;400(10348):273-282. doi: 10.1016/S0140-6736(22)01199-0 [DOI] [PubMed] [Google Scholar]
- 20.Blauvelt A, Silverberg JI, Lynde CW, et al. Abrocitinib induction, randomized withdrawal, and retreatment in patients with moderate-to-severe atopic dermatitis: results from the JAK1 Atopic Dermatitis Efficacy and Safety (JADE) REGIMEN phase 3 trial. J Am Acad Dermatol. 2022;86(1):104-112. doi: 10.1016/j.jaad.2021.05.075 [DOI] [PubMed] [Google Scholar]
- 21.Bieber T, Simpson EL, Silverberg JI, et al. ; JADE COMPARE Investigators . Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med. 2021;384(12):1101-1112. doi: 10.1056/NEJMoa2019380 [DOI] [PubMed] [Google Scholar]
- 22.Blauvelt A, Teixeira HD, Simpson EL, et al. Efficacy and safety of upadacitinib vs dupilumab in adults with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2021;157(9):1047-1055. doi: 10.1001/jamadermatol.2021.3023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guttman-Yassky E, Teixeira HD, Simpson EL, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021;397(10290):2151-2168. doi: 10.1016/S0140-6736(21)00588-2 [DOI] [PubMed] [Google Scholar]
- 24.Reich K, Teixeira HD, de Bruin-Weller M, et al. Safety and efficacy of upadacitinib in combination with topical corticosteroids in adolescents and adults with moderate-to-severe atopic dermatitis (AD Up): results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2021;397(10290):2169-2181. doi: 10.1016/S0140-6736(21)00589-4 [DOI] [PubMed] [Google Scholar]
- 25.Katoh N, Ohya Y, Murota H, et al. Safety and efficacy of upadacitinib for atopic dermatitis in Japan: 2-year interim results from the phase 3 Rising Up study. Dermatol Ther (Heidelb). 2023;13(1):221-234. doi: 10.1007/s13555-022-00842-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simpson EL, Lacour JP, Spelman L, et al. Baricitinib in patients with moderate-to-severe atopic dermatitis and inadequate response to topical corticosteroids: results from two randomized monotherapy phase III trials. Br J Dermatol. 2020;183(2):242-255. doi: 10.1111/bjd.18898 [DOI] [PubMed] [Google Scholar]
- 27.Bieber T, Reich K, Paul C, et al. ; BREEZE-AD4 study group . Efficacy and safety of baricitinib in combination with topical corticosteroids in patients with moderate-to-severe atopic dermatitis with inadequate response, intolerance or contraindication to ciclosporin: results from a randomized, placebo-controlled, phase III clinical trial (BREEZE-AD4). Br J Dermatol. 2022;187(3):338-352. doi: 10.1111/bjd.21630 [DOI] [PubMed] [Google Scholar]
- 28.Simpson EL, Forman S, Silverberg JI, et al. Baricitinib in patients with moderate-to-severe atopic dermatitis: results from a randomized monotherapy phase 3 trial in the United States and Canada (BREEZE-AD5). J Am Acad Dermatol. 2021;85(1):62-70. doi: 10.1016/j.jaad.2021.02.028 [DOI] [PubMed] [Google Scholar]
- 29.Torrelo A, Rewerska B, Galimberti M, et al. Efficacy and safety of baricitinib in combination with topical corticosteroids in paediatric patients with moderate-to-severe atopic dermatitis with an inadequate response to topical corticosteroids: results from a phase III, randomized, double-blind, placebo-controlled study (BREEZE-AD PEDS). Br J Dermatol. 2023;189(1):23-32. doi: 10.1093/bjd/ljad096 [DOI] [PubMed] [Google Scholar]
- 30.Papp K, Szepietowski JC, Kircik L, et al. Efficacy and safety of ruxolitinib cream for the treatment of atopic dermatitis: results from 2 phase 3, randomized, double-blind studies. J Am Acad Dermatol. 2021;85(4):863-872. doi: 10.1016/j.jaad.2021.04.085 [DOI] [PubMed] [Google Scholar]
- 31.King B, Ohyama M, Kwon O, et al. ; BRAVE-AA Investigators . Two phase 3 trials of baricitinib for alopecia areata. N Engl J Med. 2022;386(18):1687-1699. doi: 10.1056/NEJMoa2110343 [DOI] [PubMed] [Google Scholar]
- 32.Papp KA, Menter MA, Abe M, et al. ; OPT Pivotal 1 and OPT Pivotal 2 investigators . Tofacitinib, an oral Janus kinase inhibitor, for the treatment of chronic plaque psoriasis: results from two randomized, placebo-controlled, phase III trials. Br J Dermatol. 2015;173(4):949-961. doi: 10.1111/bjd.14018 [DOI] [PubMed] [Google Scholar]
- 33.Zhang J, Tsai TF, Lee MG, et al. The efficacy and safety of tofacitinib in Asian patients with moderate to severe chronic plaque psoriasis: a phase 3, randomized, double-blind, placebo-controlled study. J Dermatol Sci. 2017;88(1):36-45. doi: 10.1016/j.jdermsci.2017.05.004 [DOI] [PubMed] [Google Scholar]
- 34.McInnes IB, Anderson JK, Magrey M, et al. Trial of upadacitinib and adalimumab for psoriatic arthritis. N Engl J Med. 2021;384(13):1227-1239. doi: 10.1056/NEJMoa2022516 [DOI] [PubMed] [Google Scholar]
- 35.Mease PJ, Lertratanakul A, Anderson JK, et al. Upadacitinib for psoriatic arthritis refractory to biologics: SELECT-PsA 2. Ann Rheum Dis. 2021;80(3):312-320. doi: 10.1136/annrheumdis-2020-218870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mease P, Hall S, FitzGerald O, et al. Tofacitinib or adalimumab versus placebo for psoriatic arthritis. N Engl J Med. 2017;377(16):1537-1550. doi: 10.1056/NEJMoa1615975 [DOI] [PubMed] [Google Scholar]
- 37.Gladman D, Rigby W, Azevedo VF, et al. Tofacitinib for psoriatic arthritis in patients with an inadequate response to TNF inhibitors. N Engl J Med. 2017;377(16):1525-1536. doi: 10.1056/NEJMoa1615977 [DOI] [PubMed] [Google Scholar]
- 38.Leng X, Lin W, Liu S, et al. Efficacy and safety of tofacitinib in Chinese patients with active psoriatic arthritis: a phase 3, randomised, double-blind, placebo-controlled study. RMD Open. 2023;9(1):2559. doi: 10.1136/rmdopen-2022-002559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosmarin D, Passeron T, Pandya AG, et al. ; TRuE-V Study Group . Two phase 3, randomized, controlled trials of ruxolitinib cream for vitiligo. N Engl J Med. 2022;387(16):1445-1455. doi: 10.1056/NEJMoa2118828 [DOI] [PubMed] [Google Scholar]
- 40.Nakagawa H, Nemoto O, Igarashi A, et al. Delgocitinib ointment in pediatric patients with atopic dermatitis: a phase 3, randomized, double-blind, vehicle-controlled study and a subsequent open-label, long-term study. J Am Acad Dermatol. 2021;85(4):854-862. doi: 10.1016/j.jaad.2021.06.014 [DOI] [PubMed] [Google Scholar]
- 41.Nakagawa H, Nemoto O, Igarashi A, Saeki H, Kaino H, Nagata T. Delgocitinib ointment, a topical Janus kinase inhibitor, in adult patients with moderate to severe atopic dermatitis: a phase 3, randomized, double-blind, vehicle-controlled study and an open-label, long-term extension study. J Am Acad Dermatol. 2020;82(4):823-831. doi: 10.1016/j.jaad.2019.12.015 [DOI] [PubMed] [Google Scholar]
- 42.King B, Zhang X, Harcha WG, et al. Efficacy and safety of ritlecitinib in adults and adolescents with alopecia areata: a randomised, double-blind, multicentre, phase 2b-3 trial. Lancet. 2023;401(10387):1518-1529. doi: 10.1016/S0140-6736(23)00222-2 [DOI] [PubMed] [Google Scholar]
- 43.Reich K, Kabashima K, Peris K, et al. Efficacy and safety of baricitinib combined with topical corticosteroids for treatment of moderate to severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156(12):1333-1343. doi: 10.1001/jamadermatol.2020.3260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bachelez H, van de Kerkhof PC, Strohal R, et al. ; OPT Compare Investigators . Tofacitinib versus etanercept or placebo in moderate-to-severe chronic plaque psoriasis: a phase 3 randomised non-inferiority trial. Lancet. 2015;386(9993):552-561. doi: 10.1016/S0140-6736(14)62113-9 [DOI] [PubMed] [Google Scholar]
- 45.Chen TL, Lee LL, Huang HK, Chen LY, Loh CH, Chi CC. Association of risk of incident venous thromboembolism with atopic dermatitis and treatment with Janus kinase inhibitors: a systematic review and meta-analysis. JAMA Dermatol. 2022;158(11):1254-1261. doi: 10.1001/jamadermatol.2022.3516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johnson BB, Franco AI, Beck LA, Prezzano JC. Treatment-resistant atopic dermatitis: challenges and solutions. Clin Cosmet Investig Dermatol. 2019;12:181-192. doi: 10.2147/CCID.S163814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kincaid CM, Arnold JD, Mesinkovska NA. Baricitinib as the first systemic treatment for severe alopecia areata. Expert Rev Clin Immunol. 2023;19(6):565-573. doi: 10.1080/1744666X.2023.2200166 [DOI] [PubMed] [Google Scholar]
- 48.Hendricks AJ, Lio PA, Shi VY. Management recommendations for dupilumab partial and non-durable responders in atopic dermatitis. Am J Clin Dermatol. 2019;20(4):565-569. doi: 10.1007/s40257-019-00436-8 [DOI] [PubMed] [Google Scholar]
- 49.Chung WS, Peng CL, Lin CL, et al. Rheumatoid arthritis increases the risk of deep vein thrombosis and pulmonary thromboembolism: a nationwide cohort study. Ann Rheum Dis. 2014;73(10):1774-1780. doi: 10.1136/annrheumdis-2013-203380 [DOI] [PubMed] [Google Scholar]
- 50.Durante A, Bronzato S. The increased cardiovascular risk in patients affected by autoimmune diseases: review of the various manifestations. J Clin Med Res. 2015;7(6):379-384. doi: 10.14740/jocmr2122w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ogdie A, Yu Y, Haynes K, et al. Risk of major cardiovascular events in patients with psoriatic arthritis, psoriasis and rheumatoid arthritis: a population-based cohort study. Ann Rheum Dis. 2015;74(2):326-332. doi: 10.1136/annrheumdis-2014-205675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maqsood MH, Weber BN, Haberman RH, Lo Sicco KI, Bangalore S, Garshick MS. Cardiovascular and venous thromboembolic risk with Janus kinase inhibitors in immune-mediated inflammatory diseases: a systematic review and meta-analysis of randomized trials. ACR Open Rheumatol. 2022;4(10):912-922. doi: 10.1002/acr2.11479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Charles-Schoeman C, Buch MH, Dougados M, et al. Risk of major adverse cardiovascular events with tofacitinib versus tumour necrosis factor inhibitors in patients with rheumatoid arthritis with or without a history of atherosclerotic cardiovascular disease: a post hoc analysis from ORAL Surveillance. Ann Rheum Dis. 2023;82(1):119-129. doi: 10.1136/ard-2022-222259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Karpouzas G, Szekanecz Z, Baecklund E, et al. POS0519: relationship between disease activity and major adverse events in patients with rheumatoid arthritis on tofacitinib or TNF inhibitors: a post hoc analysis of ORAL Surveillance. Ann Rheum Dis. 2022;81(suppl 1):517-518. doi: 10.1136/annrheumdis-2022-eular.1238 [DOI] [Google Scholar]
- 55.Taylor PC, Bieber T, Alten R, et al. Baricitinib safety for events of special interest in populations at risk: analysis from randomised trial data across rheumatologic and dermatologic indications. Adv Ther. 2023;40(4):1867-1883. doi: 10.1007/s12325-023-02445-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shah JT, Shah KT, Femia AN, et al. Cardiovascular risk management in patients treated with JAK inhibitors. J Cardiovasc Pharmacol. Published online August 10, 2023. doi: 10.1097/FJC.0000000000001470 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Cochrane risk of assessment bias
eFigure 1. PRISMA flow
eFigure 2. Effect of JAK inhibitors on composite of MACE and all-cause mortality (primary analysis)
eFigure 3. Effect of JAK inhibitors on VTE (primary analysis)
eFigure 4. Funnel plot: effect of JAK inhibitors on composite of MACE and all-cause mortality (secondary analysis)
eFigure 5. Funnel plot: effect of JAK inhibitors on VTE (secondary analysis)
eFigure 6. Secondary analysis
eAppendix. PRISMA checklist
Data Sharing Statement


