Abstract
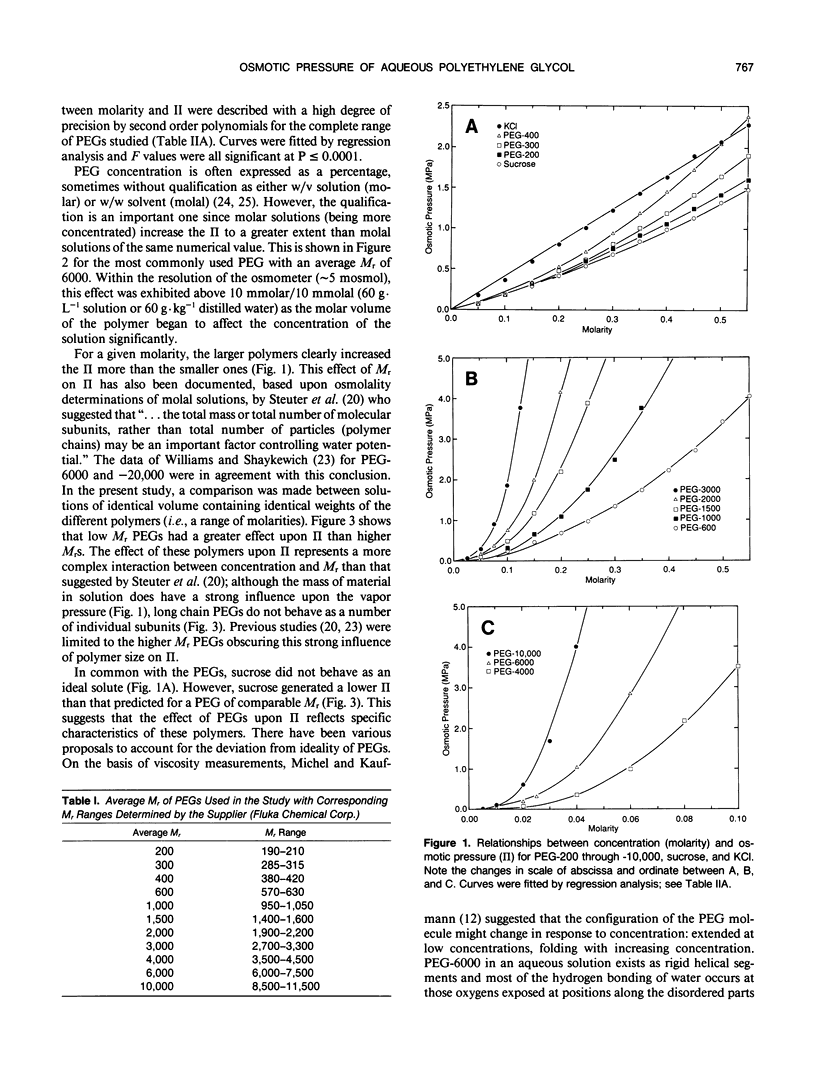
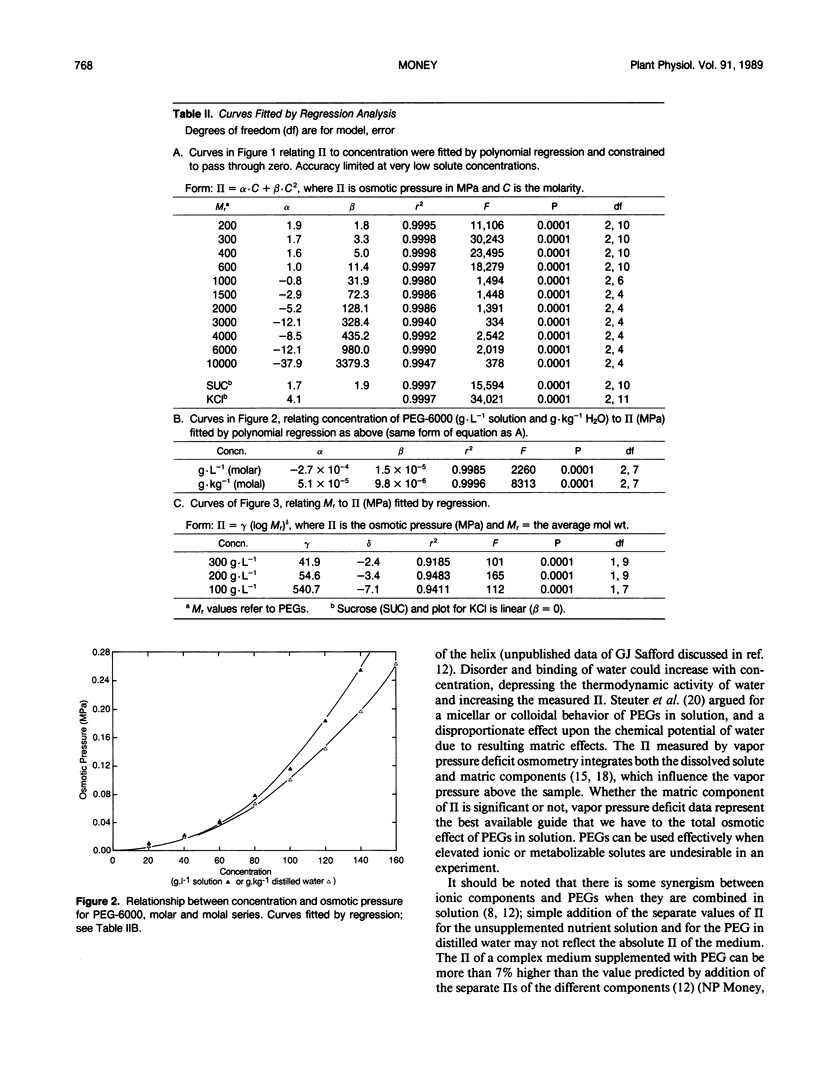
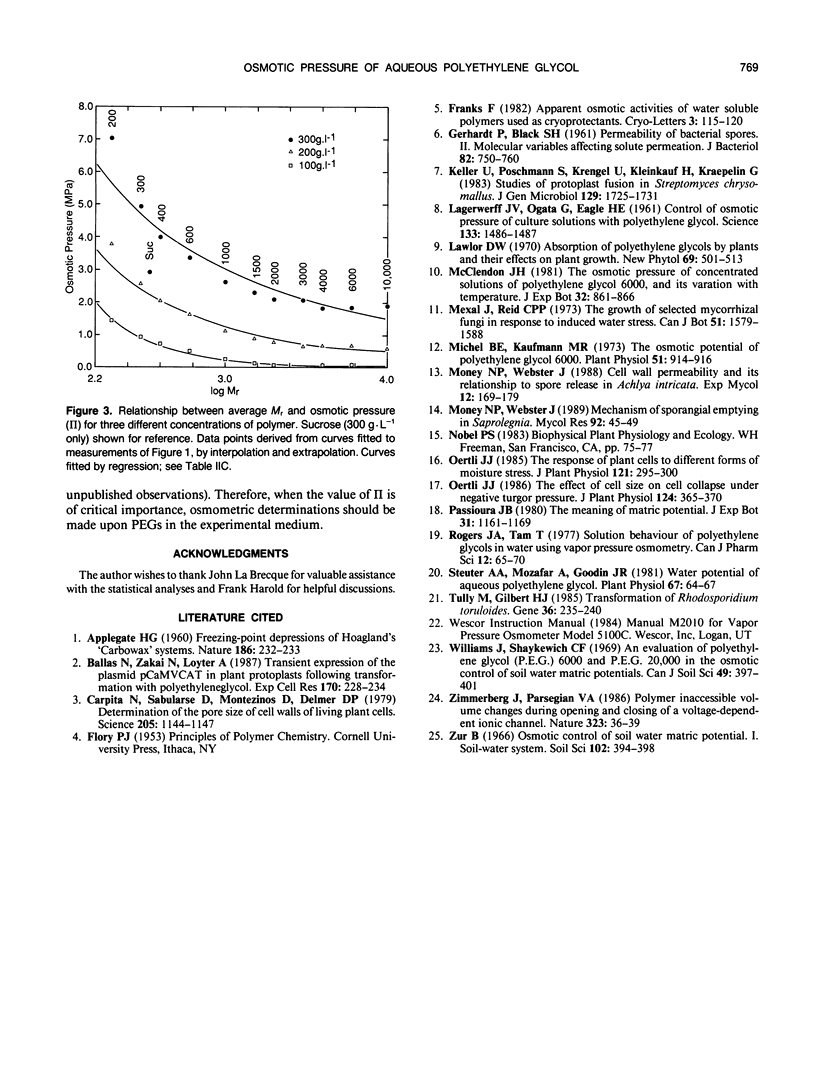
Osmotic pressures (II) of aqueous solutions of polyethylene glycols (PEGs) of average relative molecular weight (Mr) between 200 and 10,000 were measured using vapor pressure deficit osmometry. The relationships between molarity and II were described with high precision by second order polynomials for each of the PEGs studied. In contrast to previous reports, equivalent weights of different polymers in solution did not generate the same II; low Mr PEGs generated a higher II than the higher Mr PEGs. The effect of PEGs upon II represents an interaction between concentration and Mr.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballas N., Zakai N., Loyter A. Transient expression of the plasmid pCaMVCAT in plant protoplasts following transformation with polyethyleneglycol. Exp Cell Res. 1987 May;170(1):228–234. doi: 10.1016/0014-4827(87)90132-7. [DOI] [PubMed] [Google Scholar]
- Carpita N., Sabularse D., Montezinos D., Delmer D. P. Determination of the pore size of cell walls of living plant cells. Science. 1979 Sep 14;205(4411):1144–1147. doi: 10.1126/science.205.4411.1144. [DOI] [PubMed] [Google Scholar]
- GERHARDT P., BLACK S. H. Permeability of bacterial spores. II. Molecular variables affecting solute permeation. J Bacteriol. 1961 Nov;82:750–760. doi: 10.1128/jb.82.5.750-760.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller U., Pöschmann S., Krengel U., Kleinkauf H., Kraepelin G. Studies of protoplast fusion in Streptomyces chrysomallus. J Gen Microbiol. 1983 Jun;129(6):1725–1731. doi: 10.1099/00221287-129-6-1725. [DOI] [PubMed] [Google Scholar]
- LAGERWERFF J. V., OGATA G., EAGLE H. E. Control of osmotic pressure of culture solutions with polyethylene glycol. Science. 1961 May 12;133(3463):1486–1487. doi: 10.1126/science.133.3463.1486. [DOI] [PubMed] [Google Scholar]
- Michel B. E., Kaufmann M. R. The osmotic potential of polyethylene glycol 6000. Plant Physiol. 1973 May;51(5):914–916. doi: 10.1104/pp.51.5.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steuter A. A. Water potential of aqueous polyethylene glycol. Plant Physiol. 1981 Jan;67(1):64–67. doi: 10.1104/pp.67.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tully M., Gilbert H. J. Transformation of Rhodosporidium toruloides. Gene. 1985;36(3):235–240. doi: 10.1016/0378-1119(85)90178-7. [DOI] [PubMed] [Google Scholar]
- Zimmerberg J., Parsegian V. A. Polymer inaccessible volume changes during opening and closing of a voltage-dependent ionic channel. Nature. 1986 Sep 4;323(6083):36–39. doi: 10.1038/323036a0. [DOI] [PubMed] [Google Scholar]



