Abstract
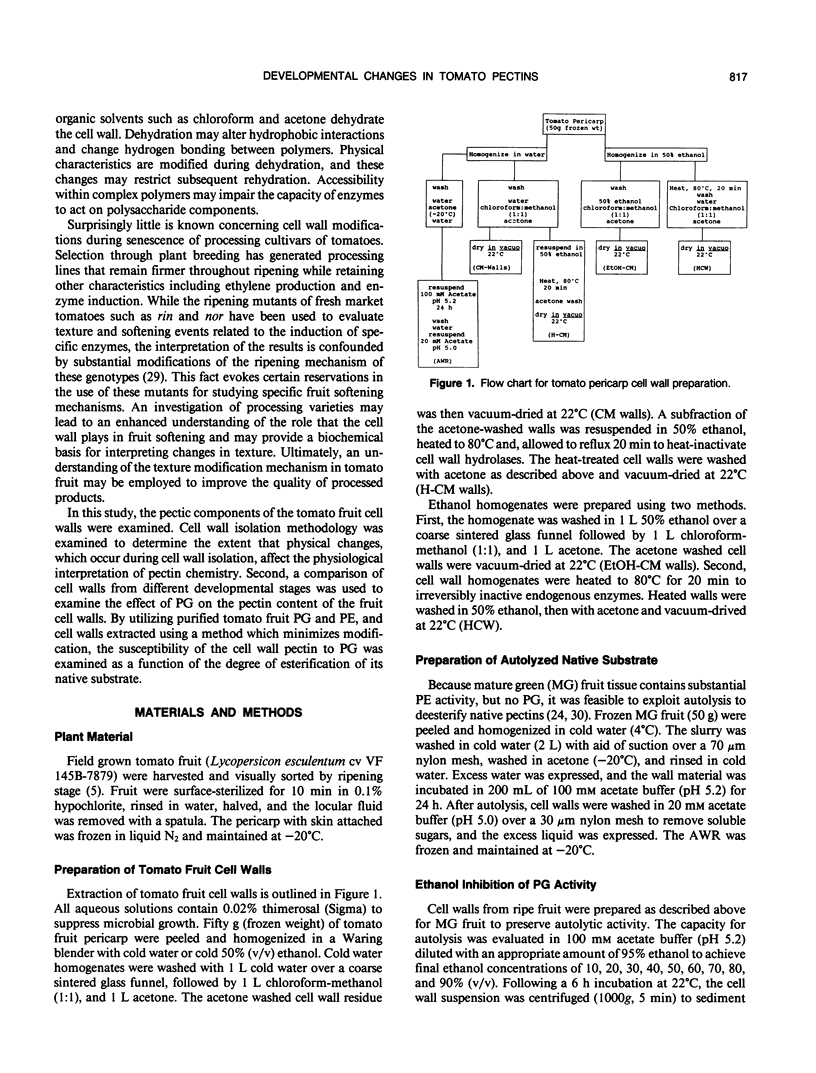
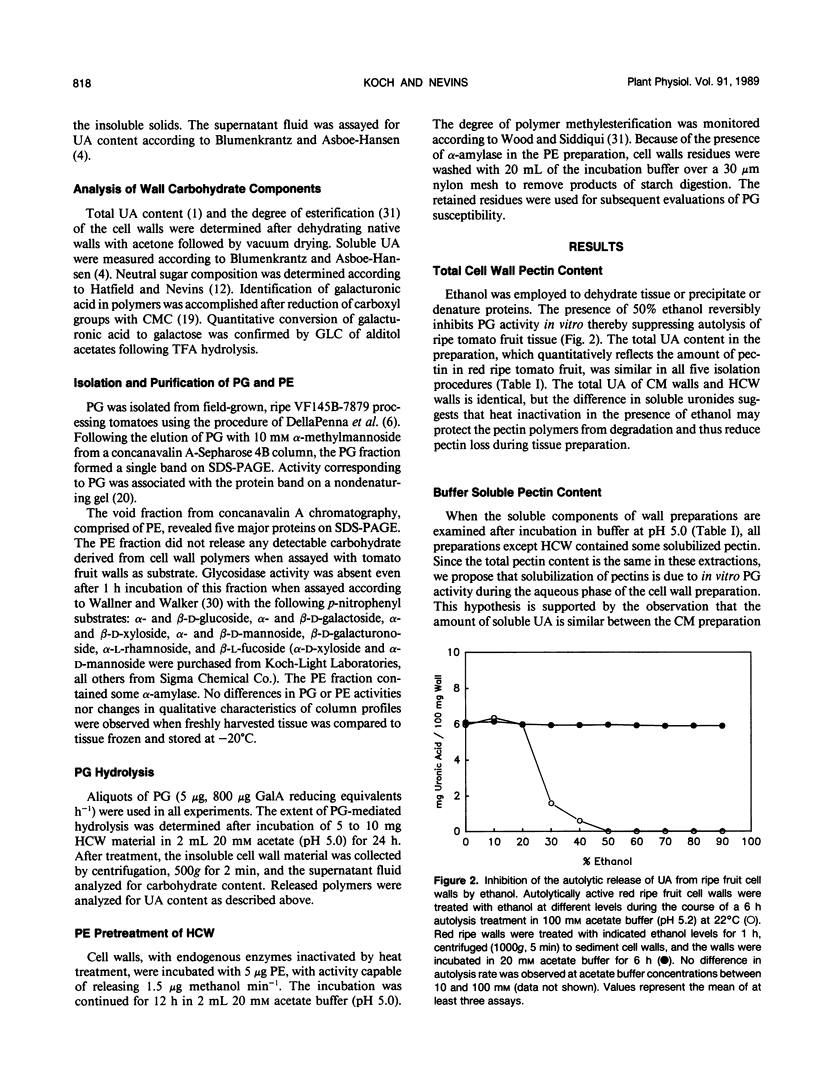
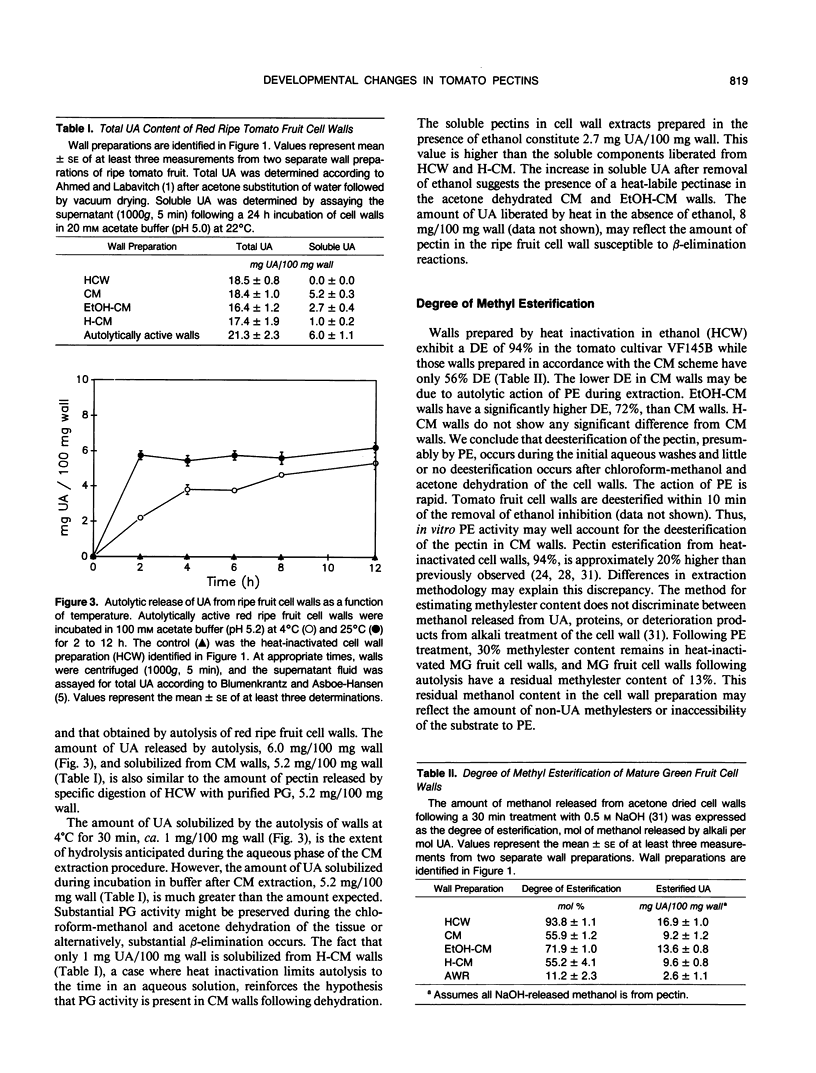
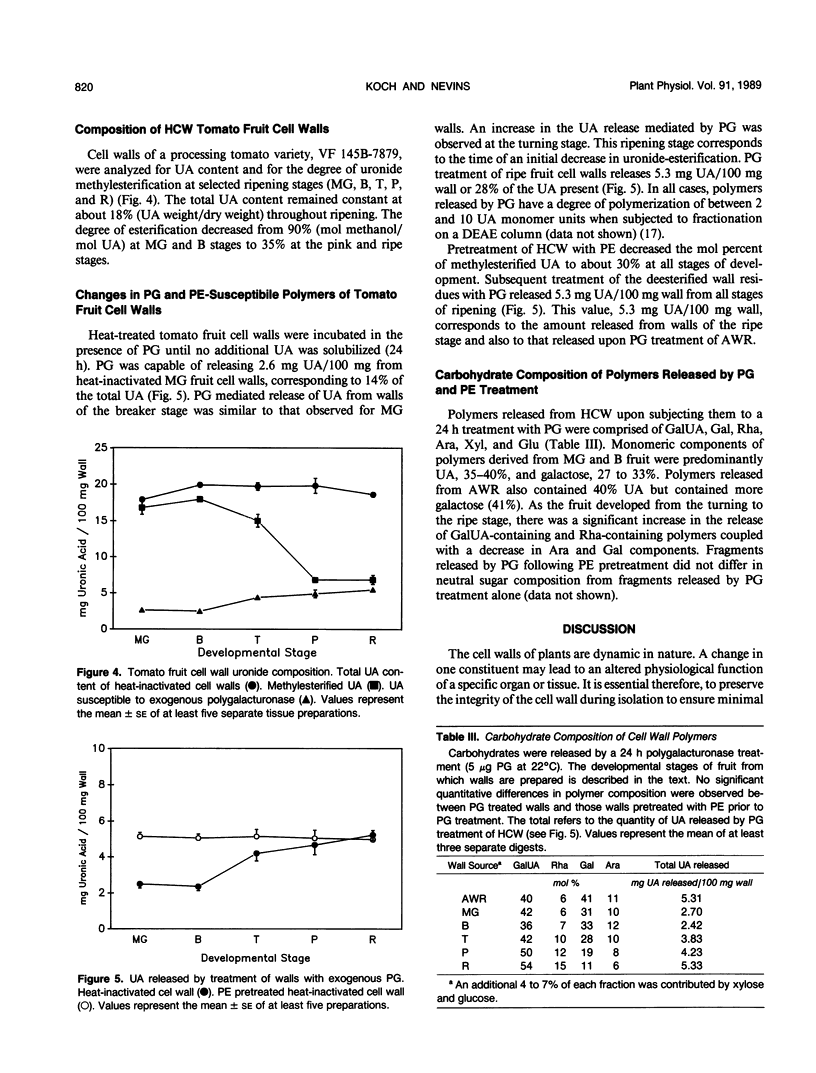
Cell wall isolation procedures were evaluated to determine their effect on the total pectin content and the degree of methylesterification of tomato (Lycopersicon esculentum L.) fruit cell walls. Water homogenates liberate substantial amounts of buffer soluble uronic acid, 5.2 milligrams uronic acid/100 milligrams wall. Solubilization appears to be a consequence of autohydrolysis mediated by polygalacturonase II, isoenzymes A and B, since the uronic acid release from the wall residue can be suppressed by homogenization in the presence of 50% ethanol followed by heating. The extent of methylesterification in heat-inactivated cell walls, 94 mole%, was significantly greater than with water homogenates, 56 mole%. The results suggest that autohydrolysis, mediated by cell wall-associated enzymes, accounts for the solubilization of tomato fruit pectin in vitro. Endogenous enzymes also account for a decrease in the methylesterification during the cell wall preparation. The heat-inactivated cell wall preparation was superior to the other methods studied since it reduces β-elimination during heating and inactivates constitutive enzymes that may modify pectin structure. This heat-inactivated cell wall preparation was used in subsequent enzymatic analysis of the pectin structure. Purified tomato fruit polygalacturonase and partially purified pectinmethylesterase were used to assess changes in constitutive substrates during tomato fruit ripening. Polygalacturonase treatment of heat-inactivated cell walls from mature green and breaker stages released 14% of the uronic acid. The extent of the release of polyuronides by polygalacturonase was fruit development stage dependent. At the turning stage, 21% of the pectin fraction was released, a value which increased to a maximum of 28% of the uronides at the red ripe stage. Pretreatment of the walls with purified tomato pectinesterase rendered walls from all ripening stages equally susceptible to polygalacturonase. Quantitatively, the release of uronides by polygalacturonase from all pectinesterase treated cell walls was equivalent to polygalacturonase treatment of walls at the ripe stage. Uronide polymers released by polygalacturonase contain galacturonic acid, rhamnose, galactose, arabinose, xylose, and glucose. As a function of development, an increase in the release of galacturonic acid and rhamnose was observed (40 and 6% of these polymers at the mature green stage to 54 and 15% at the red ripe stage, respectively). The amount of galactose and arabinose released by exogenous polygalacturonase decreased during development (41 and 11% from walls of mature green fruit to 11 and 6% at the red ripe stage, respectively). Minor amounts of glucose and xylose released from the wall by exogenous polygalacturonase (4-7%) remained constant throughout fruit development.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALBERSHEIM P., NEUKOM H. DEUEL H: Splitting of pectin chain molecules in neutral solutions. Arch Biochem Biophys. 1960 Sep;90:46–51. doi: 10.1016/0003-9861(60)90609-3. [DOI] [PubMed] [Google Scholar]
- Blumenkrantz N., Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal Biochem. 1973 Aug;54(2):484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- Dellapenna D., Alexander D. C., Bennett A. B. Molecular cloning of tomato fruit polygalacturonase: Analysis of polygalacturonase mRNA levels during ripening. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6420–6424. doi: 10.1073/pnas.83.17.6420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross K. C., Wallner S. J. Degradation of Cell Wall Polysaccharides during Tomato Fruit Ripening. Plant Physiol. 1979 Jan;63(1):117–120. doi: 10.1104/pp.63.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber S. C., Israel D. W. Biochemical Basis for Partitioning of Photosynthetically Fixed Carbon between Starch and Sucrose in Soybean (Glycine max Merr.) Leaves. Plant Physiol. 1982 Mar;69(3):691–696. doi: 10.1104/pp.69.3.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackey G. D., Gross K. C., Wallner S. J. Loss of tomato cell wall galactan may involve reduced rate of synthesis. Plant Physiol. 1980 Sep;66(3):532–533. doi: 10.1104/pp.66.3.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisker N., Retig N. Detection of polygalacturonase and pectin lyase isoenzymes in polyacrylamide gels. J Chromatogr. 1974 Sep 11;96(2):245–249. doi: 10.1016/s0021-9673(00)98570-4. [DOI] [PubMed] [Google Scholar]
- Rushing J. W., Huber D. J. In vitro characterization of tomato fruit softening : the use of enzymically active cell walls. Plant Physiol. 1984 Aug;75(4):891–894. doi: 10.1104/pp.75.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmadge K. W., Keegstra K., Bauer W. D., Albersheim P. The Structure of Plant Cell Walls: I. The Macromolecular Components of the Walls of Suspension-cultured Sycamore Cells with a Detailed Analysis of the Pectic Polysaccharides. Plant Physiol. 1973 Jan;51(1):158–173. doi: 10.1104/pp.51.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Themmen A. P., Tucker G. A., Grierson D. Degradation of isolated tomato cell walls by purified polygalacturonase in vitro. Plant Physiol. 1982 Jan;69(1):122–124. doi: 10.1104/pp.69.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallner S. J., Walker J. E. Glycosidases in Cell Wall-degrading Extracts of Ripening Tomato Fruits. Plant Physiol. 1975 Jan;55(1):94–98. doi: 10.1104/pp.55.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood P. J., Siddiqui I. R. Determination of methanol and its application to measurement of pectin ester content and pectin methyl esterase activity. Anal Biochem. 1971 Feb;39(2):418–428. doi: 10.1016/0003-2697(71)90432-5. [DOI] [PubMed] [Google Scholar]


