Abstract
T cells and natural killer (NK) cells are major effector cells recruited by cancer therapeutic bispecific antibodies; however, differences in the populations of these cells in individual tumors limit the general use of these antibodies. In the present study, trispecific antibodies were created, namely T cell and NK cell engagers (TaKEs), that recruit both T cells and NK cells. Notably, three Fc-fused TaKEs were designed, TaKE1-Fc, TaKE2-Fc and TaKE3-Fc, using variable fragments targeting the epidermal growth factor receptor on tumor cells, CD3 on T cells, and CD16 on NK cells. Among them, TaKE1-Fc was predicted to form a circular tetrabody-like configuration and exhibited the highest production and greatest cancer growth inhibitory effects. TaKE1 was prepared from TaKE1-Fc by digesting the Fc region for further functional evaluation. The resulting TaKE1 exhibited trispecificity via its ability to bind cancer cells, T cells and NK cells, as well as comparable or greater cancer growth inhibitory effects to those of two bispecific antibodies that recruit T cells and NK cells, respectively. A functional trispecific antibody with the potential to exert strong therapeutic effects independent of T cell and NK cell populations was developed.
Keywords: cancer immunotherapy, therapeutic antibody, trispecific antibody, T cell and natural killer cell engager, EGFR, CD3, CD16
Introduction
Conventional monoclonal antibodies, mostly IgG1s, have been widely used as therapies for diseases that are difficult to treat (1,2); however, they have not been effective for cancer treatment. Several strategies for improving the function of therapeutic antibodies have been explored, such as designing non-natural antibody formats, including fusion antibodies, multivalent antibodies and multispecific antibodies, as well as their combinations (3,4). Bispecific antibodies (bsAbs) among multispecific antibodies are a practical non-natural antibody format and have been approved by the United States Food and Drug Administration (5). However, only four cancer therapeutic bsAbs have been approved by the Food and Drug Administration; thus, technologies are needed to accelerate the development of highly functional non-natural antibodies for treating cancer.
Multispecific antibodies were recently developed for treating viral infections and cancer (6). For example, a trispecific antibody targeting different epitopes on the envelope of human immunodeficiency virus-1 showed antigen-binding ability and reduced the effects of viral mutations that typically make therapy challenging (7). Studies on cancer therapeutic multispecific antibodies have recently increased (8). For example, a previous study revealed that trispecific antibodies targeting different targets in cancer cells inhibit cancer growth (9). Multispecific antibodies designed to redirect some types of immune cells as effector cells toward cancer cells have been widely studied especially for developing bsAbs. As effector cells, T cells and NK cells are often used to construct multispecific antibodies (10,11), due to their high abundance in the blood and strong antitumor effects.
In previous studies by the authors, the development and efficacy of bsAbs that recruit T cells or NK cells: Ex3 targeting epidermal growth factor receptor (EGFR) on tumor cells and CD3 on T cells (12,13) and Ex16 targeting EGFR and CD16 on NK cells, were reported (14,15). T cells and NK cells also exist in solid tumors as tumor-infiltrating lymphocytes (TILs); however, a major challenge in cancer therapy is exhaustion of these effector cells (16). To activate such exhausted immune cells, co-stimulatory molecules or agonist antibodies with co-stimulatory signals have been mounted on bsAbs that recruit immune cells to design multispecific antibodies with strong therapeutic effects (17,18). Another concern is the differences in the abundance ratios of T cells and NK cells as TILs, depending on the individual cancer, stage and malignancy (19). Combination therapy with bsAbs that recruit T cells and NK cells may induce therapeutic effects independent of the TIL populations; however, administration of two biologicals is cost-ineffective due to the high production costs of conventional therapeutic antibodies. Therefore, the present study focused on the development of novel trispecific antibodies recruiting both T and NK cells.
In the present study, three Fc-fused trispecific antibodies were designed, namely T-cell and NK-cell engagers (TaKEs), which target EGFR, CD3 and CD16. Among them, TaKE1-Fc was circularly connected with four variable fragments (Fvs) from three different antibody clones. Using highly purified TaKE1 after removing the Fc region, its trispecificity via its ability to bind cancer cells, T cells and NK cells, as well as its comparable or stronger effects than those of the combination of the two bsAbs were confirmed. Functional trispecific antibodies recruiting both T cells and NK cells were created and a novel format for the development of trispecific antibodies was provided.
Materials and methods
Construction of expression vectors and preparation of three types of TaKE-Fc
It has been previously reported that bsAbs use the variable heavy (VH) and variable light (VL) regions of the humanized anti-CD3 antibody OKT3 (12), humanized anti-EGFR antibody 528 (12), mouse anti-CD16 antibody 3G8 (14) and mouse anti-EGFR antibody 225 (20). In the present study, three trispecific antibodies (TaKEs) were designed (Fig. 1). Corresponding gene expression cassettes were designed based on the amino acid sequences of the VH and VL regions and human IgG1 Fc, synthesized, and then inserted into the pCAGGS expression vector (21). All constructed gene sequences for TaKE-Fcs are indicated in Fig. S1, Fig. S2, Fig. S3. All TaKE-Fcs were expressed transiently using Expi293 Expression System (Thermo Fisher Scientific, Inc.) and purified using protein A chromatography (rProtein A Sepharose™ Fast Flow; Cytiva) according to the manufacturer's protocol. Briefly, the column was equilibrated with Tris-HCl buffer containing 200 mM NaCl (pH 8.0). After the culture supernatant was loaded onto the column, the column was washed with Tris-HCl buffer containing 200 mM NaCl (pH 8.0). TaKE-Fcs were eluted using Pierce IgG Elution Buffer (Thermo Fisher Scientific, Inc.).
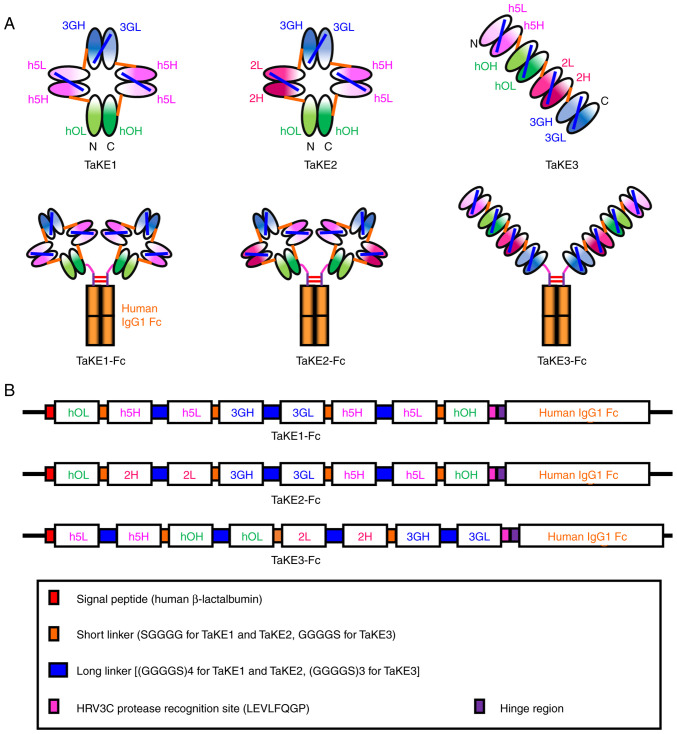
Figure 1.
Construction of TaKEs. (A) Schematic diagrams of three types of TaKEs and their human IgG1 Fc fusion formats. (B) Schematic diagrams of the expression vectors for TaKEs with Fc. TaKE, T cell and natural killer (NK) cell engager; VH, variable heavy; VL, variable light; hOH, the VH regions of the humanized anti-CD3 antibody OKT3; hOL, the VL regions of the humanized anti-CD3 antibody OKT3; h5H, the VH regions of the humanized anti-EGFR antibody 528; h5L, the VL regions of the humanized anti-EGFR antibody 528; 3GH, the VH regions of the mouse anti-CD16 antibody 3G8; 3GL, the VL regions of the mouse anti-CD16 antibody 3G8; 2H, the VH regions of the mouse anti-EGFR antibody 225; 2L, the VL regions of the mouse anti-EGFR antibody 225.
Preparation of TaKE1 and bsAbs
To prepare each TaKE1 without Fc, protein A chromatography-purified TaKE1-Fc was digested with HRV3C protease fused to glutathione S-transferase (PreScission protease; Cytiva), according to the manufacturer's protocol and a previous study by the authors (22). The protease was removed on a Glutathione Sepharose 4 B column (Cytiva), and the flow-through was loaded onto the protein A column again to remove the digested Fc and undigested Fc fusion protein. Gel filtration was performed using a Superdex 200 Increase column (10/300; Cytiva) to fractionate the TaKE1 monomers. Purity and successful preparation were confirmed using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE; SuperSep™ Ace 10–20%, 17 well; FUJIFILM Wako Pure Chemical Corporation) under reducing conditions. Ex3-scDb-LH, a bispecific single-chain diabody targeting EGFR and CD3 (22), was prepared from its Fc fusion format in a similar manner. Ex16-scDb-HL, a bispecific single-chain diabody targeting EGFR and CD16, was prepared using the B. choshinensis expression system (ProteinExpress Co., Ltd.), as previously reported (15). After purification of Ex16-scDb-HL using immobilized metal affinity chromatography directly from the bacterial supernatant, gel filtration was performed using a Superdex 200 Increase column to fractionate the monomer.
Cell lines
Human bile duct carcinoma (TFK-1) was established by the authors (23). CD3-positive lymphokine-activated killer cells with the T-cell phenotype (T-LAK) cells were induced from ethically collected peripheral blood mononuclear cells (CTL-UP1; Cellular Technology Limited) as previously described (24). These cell lines were cultured in a humidified environment (37°C, 5% CO2) in RPMI-1640 medium (Sigma-Aldrich; Merck KGaA) supplemented with 10% fetal bovine serum (Biowest), 100 U/ml penicillin, and 100 µg/ml streptomycin. The NK92/CD16A cell line was kindly provided by Professor Tachibana of Osaka Metropolitan University (Osaka, Japan) and cultured in a humidified environment (37°C, 5% CO2) in MyeloCult H5100 medium (StemCell Technologies, Inc.) supplemented with 100 U/ml of recombinant human interleukin-2 (IMUNACE 35 for Injection; Shionogi & Co., Ltd.) (25).
Flow cytometric analysis
For binding analysis of TaKE1, TFK-1 cells, T-LAK cells and NK92/CD16A cells were used as EGFR-positive, CD3-positive, and CD16-positive cell lines, respectively. Each cell line was incubated with TaKE1 (40 pmol) for 30 min on ice. After being washed with PBS, rabbit anti-Ex3 serum, provided by Immuno-Biological Laboratories Co., Ltd. through polyclonal antibody contract manufacturing service, was added and incubated for 30 min on ice, followed by staining with fluorescein isothiocyanate-labeled anti-rabbit IgG antibody (cat. no. ab6717; Abcam) for 30 min on ice. The fluorescence intensities of TaKE1 for each cell type were measured as binding properties using an Accuri C6 flow cytometer and analyzed using BD Accuri™ C6 Software (version 1.0.264.21; both from BD Biosciences).
In vitro growth inhibition assay
The in vitro growth inhibition of cancer cells was examined using an MTS assay kit (CellTiter 96® AQueous Non-Radioactive Cell Proliferation Assay; Promega Corporation), as previously reported (24). In brief, TFK-1 cells [5,000 cells in 100 µl of RPMI-1640 medium (Sigma-Aldrich: Merck KGaA) supplemented with 10% fetal bovine serum (Biowest), 100 U/ml penicillin, and 100 µg/ml streptomycin] were plated on 96-well, half-area (A/2), flat-bottomed plates (Corning, Inc.). Cells were cultured at 37°C overnight to allow well adhesion. After removal of the culture medium by aspiration, 100 µl of effector cells plus various concentrations of recombinant antibodies were added to each well. After culture of the cells for 16 h at 37°C, each well was washed with PBS three times to remove effector cells and dead target cells, and 90 µl of culture medium plus 10 µl of MTS reagent was added to each well. The plates were incubated for 1 h at 37°C and then read on a microplate reader at a wavelength of 490 nm. Growth inhibition of target cells was calculated as follows: Percentage growth inhibition of target cells=[1-(A490 of experiment-A490 of background)/(A490 of control-A490 of background)] ×100.
Statistical analysis
Data are presented as the mean ± standard deviation are representative of at least two independent experiments. All statistical analyses were performed with EZR (version 1.61; Saitama Medical Center, Jichi Medical University), which is a graphical user interface for R (version 4.2.2; The R Foundation for Statistical Computing). More precisely, it is a modified version of R commander designed to add statistical functions frequently used in biostatistics (26). The significance of the results was analyzed by one-way ANOVA and followed by Tukey's post hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
Design of three types of TaKEs
A total of three types of TaKEs were designed with specificity to cancer cells, T cells and NK cells: i) TaKE1, ii) TaKE2 and iii) TaKE3 (Fig. 1A). All TaKEs contained two Fvs against cancer and one Fv for each effector cell. A previous study revealed that higher affinities against cancer cells than those against effector cells are important for reducing severe adverse effects caused by excessive activation of effector cells (27). A tetrabody-like configuration, formed from a circularly tetramerized single-chain Fv (scFv) (28), was predicted for TaKE1 and TaKE2, as short five amino acid linkers were used as middle linkers of each chimeric single-chain component (Fig. 1B). By contrast, a tandem scFv-based configuration was predicted for TaKE3, as short five amino acid linkers were used to connect each scFv to prevent interactions between different scFvs (Fig. 1B). Two anti-EGFR antibody clones, 528 and 225, competitively target similar epitope regions (29) and show similar binding constants (30,31). To reduce unfavorable interactions between noncognate Fv clones through domain swapping, combinations of 528 and 225 Fv were integrated into TaKE2 and TaKE3. To simplify the purification procedure using protein A chromatography, all TaKEs were prepared in a human IgG1 Fc fusion format. Additionally, the HRV3C protease recognition site (LEVLFQGP) was inserted before the hinge region to produce TaKEs without Fc.
Preparation and evaluation of TaKE-Fcs
To identify functional trispecific antibodies, a cancer growth inhibition assay using protein A-purified TaKE-Fcs was performed. After transient expression using the Expi293 Expression System, TaKE-Fcs were purified from the culture supernatant using protein A chromatography and separated from the products using SDS-PAGE. Bands corresponding to the estimated molecular weights of TaKE1-Fc and TaKE2-Fc (~130 kDa) were observed, whereas these bands were not observed for TaKE3-Fc (Fig. 2A). The yields were 1.7 and 0.23 mg/l for TaKE1-Fc and TaKE2-Fc, respectively. Both TaKE1-Fc and TaKE2-Fc inhibited cancer growth in a dose-dependent manner, although TaKE1-Fc exhibited stronger effects at lower concentrations (Fig. 2B). These results demonstrated that the trispecific antibodies were successfully prepared. TaKE1-Fc exhibited high yield and activity and was selected for further evaluation.
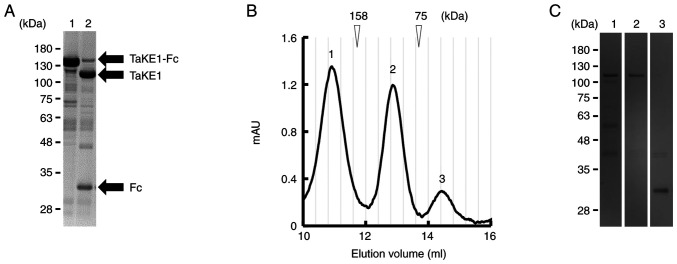
Figure 2.
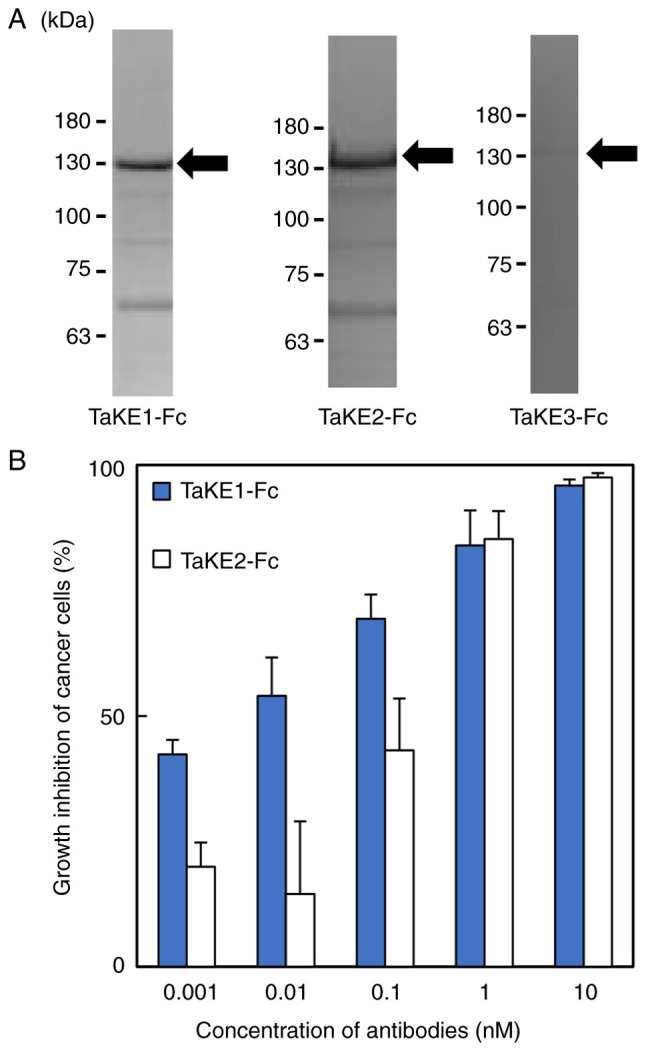
Preparation and evaluation of TaKE-Fcs. (A) Sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis of TaKE-Fcs after protein A purification. (B) Growth inhibition of EGFR-positive TFK-1 cells by each TaKE-Fc. Lymphokine-activated killer cells with T-cell phenotype cells were added to cancer cells at a ratio of 5:1. Data are presented as the mean ± 1 SD. TaKE-Fcs, Fc-fused T cell and natural killer cell engagers; EGFR, epidermal growth factor receptor.
Preparation of TaKE1 with no Fc region
For further functional evaluation using the highly purified samples, TaKE1 with no Fc region was prepared because it can bind and activate NK cells. TaKE1 was prepared from TaKE1-Fc using HRV3C protease digestion (as described in the Materials and methods section; Fig. 3A). After removing the protease and undigested TaKE1-Fc, gel filtration was performed to fractionate the TaKE1 monomer. Three peaks were estimated to correspond to the TaKE1 dimer, TaKE1 monomer and impurities, respectively, from the calibration protein information (Fig. 3B). SDS-PAGE for each fraction supported these results and revealed that TaKE1 monomer was prepared with high purity and with the theoretical molecular weight in the peak 2 fraction (Fig. 3C). Thus, the peak 2 fraction was used for functional evaluation.
Figure 3.
Preparation of TaKE from the Fc-fusion format. (A) Reducing SDS-PAGE analysis; lane 1, protein A chromatography-purified TaKE1-Fc; lane 2, after HRV3C protease digestion. (B) Gel filtration of TaKE1 after removal of HRV3C protease by glutathione Sepharose 4B chromatography followed by removal of Fc through protein A. The elution peaks were numbered as 1–3 (peak 1, TaKE1 dimer; peak 2, TaKE1 monomer; peak 3, impurities). (C) Reducing SDS-PAGE for each peak after gel filtration of TaKE1. Lanes 1–3 corresponded to the elution peaks in the chromatograph of B (lane 1, TaKE1 dimer; lane 2, TaKE1 monomer; lane 3, impurities). AU, absorbance unit; TaKE, T cell and natural killer cell engager.
Functional evaluations of TaKE1
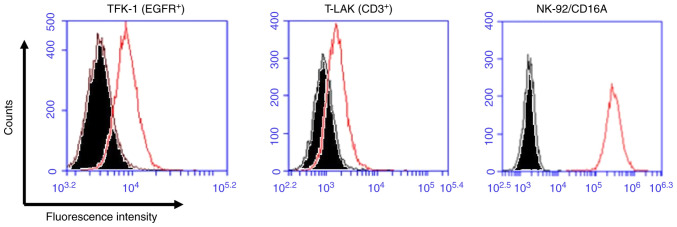
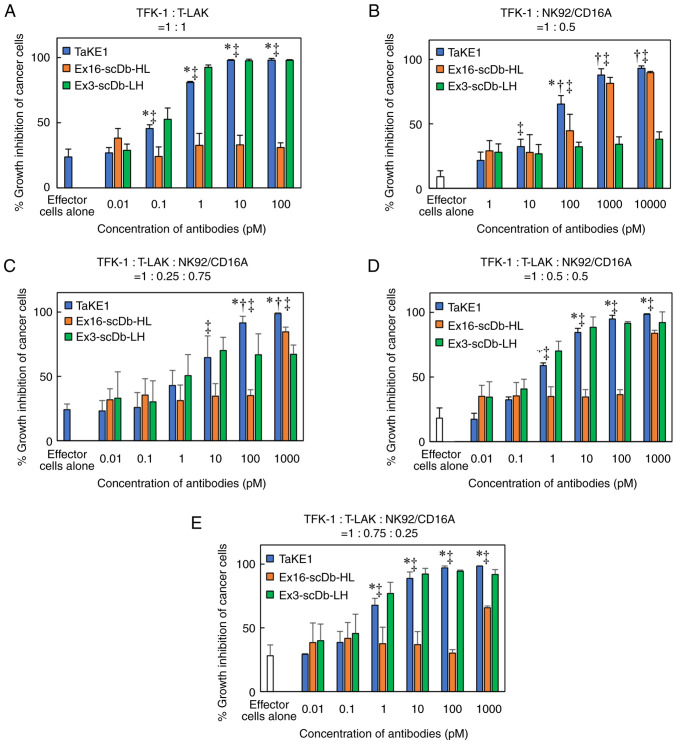
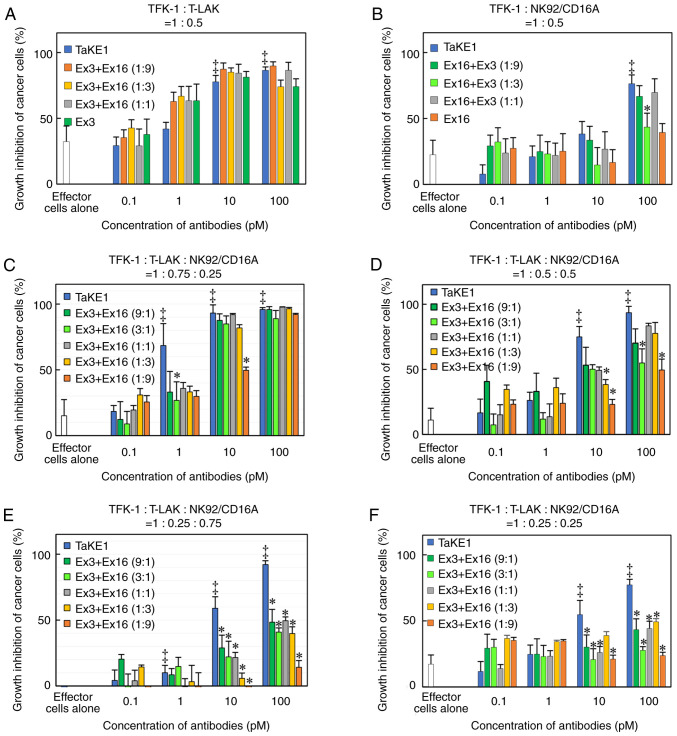
To investigate the trispecific binding abilities of TaKE1, flow cytometric analyses using EGFR-positive TFK-1 cells, CD3-positive T-LAK cells and CD16-positive NK92/CD16A cells were performed. Although the shifts in the fluorescence intensity differed for each target, with relatively low values observed for TFK-1 cells and T-LAK cells and high values observed for NK92/CD16A cells, the trispecificity of TaKE1 was confirmed (Fig. 4). Subsequently, the growth inhibitory effects of TaKE1 versus those of bsAbs were evaluated in an MTS assay using T-LAK cells or NK92/CD16A cells as effector cells (Fig. 5A and B). The bsAbs Ex16-scDb-HL and Ex3-scDb-LH inhibited cancer growth only when the corresponding effector cells, which were NK92/CD16A cells for Ex16-scDb-HL and T-LAK cells for Ex3-scDb-LH, were cocultured. By contrast, TaKE1 revealed comparable effects with Ex16-scDb-HL in NK92/CD16A cells and with Ex3-scDb-LH in T-LAK cells. Subsequently, the effects when both effector cells were present at different ratios were evaluated (Fig. 5C-E). Ex16-scDb-HL was ineffective at concentrations below 100 pM. By contrast, TaKE1 was effective at most concentrations and demonstrated slightly stronger effects than those of Ex3-scDb-LH, particularly at a lower ratio of T-LAK cells. Finally, the effects of TaKE1 were compared with mixtures of Ex16-scDb-HL and Ex3-scDb-LH, under different ratios of effector cells, to investigate the potency of TaKE1 to the combination of two bsAbs (Fig. 6). Under most conditions, TaKE1 exhibited at least comparative effects as a mixture of bsAbs and strong effects in the small population of T-LAK cells. These results indicated the successful preparation of a trispecific antibody that recruits T cells and NK cells, with comparable effects as the combination of two bsAbs.
Figure 4.
Binding activity of TaKE for EGFR-positive TFK-1 cells, CD3-positive T-LAK cells and NK-92/CD16A cells. Each cell was incubated with PBS as a negative control (shaded area) and with TaKE1 (open area), after which rabbit anti-Ex3 serum was added, followed by staining with FITC-labeled anti-rabbit IgG antibody. TaKE, T cell and natural killer cell engager; T-LAK, lymphokine-activated killer cells with the T-cell phenotype.
Figure 5.
Comparison of growth inhibitory effects of TaKE with each bispecific antibody for EGFR-positive TFK-1 cells. The purified antibodies were added to TFK-1 cells with CD3-positive T-LAK cells, NK-92/CD16A cells, or both cells, and growth inhibition was evaluated in an MTS assay. The ratio of each cell was indicated in each panel (A-E). Significant differences between TaKE1 and Ex16-scDb-HL (*P<0.05) or Ex3-scDb-LH (†P<0.05) or effector cells alone (‡P<0.05). Statistical analysis was conducted using the one-way ANOVA test. TaKE, T cell and natural killer cell engager; T-LAK, lymphokine-activated killer cells with the T-cell phenotype.
Figure 6.
Comparison of growth inhibitory effects of TaKE with the combination of two types of bispecific antibodies for EGFR-positive TFK-1 cells. The purified antibodies were added to TFK-1 cells with CD3-positive T-LAK cells, NK-92/CD16A cells, or both cells, and growth inhibition was evaluated in an MTS assay. The ratio of each cell and bispecific antibody is indicated in each panel (A-F). Concentrations for mixture of bsAbs are indicated as total concentration of bsAbs. Significant differences between TaKE1 and mixture of bsAbs (*P<0.05) or effector cells alone (‡P<0.05). Statistical analysis was carried out using the one-way ANOVA test. TaKE, T cell and natural killer cell engager; T-LAK, lymphokine-activated killer cells with the T-cell phenotype; Ex3, Ex3-scDb-LH; Ex16, Ex16-scDb-HL.
Discussion
To induce therapeutic effects independent of the populations of T cells and NK cells among TILs, three trispecific antibodies were designed, namely TaKEs. All TaKEs contained two Fvs against cancer and one Fv for each effector cell for effective tumor targeting and reducing overactivation of effector cells (27), as well as a human Fc region to simplify protein A purification. TaKE1-Fc and TaKE2-Fc, which were predicted to form circular tetrabody-like configurations, were expressed transiently in Expi293 cells (Fig. 2A), whereas TaKE3-Fc with four scFvs connected tandemly was not expressed. In scFvs, the length and composition of the polypeptide linker between the VH and VL domains strongly influences formation of the multimeric structure; such scFv dimers, trimers and tetramers are known as diabodies, triabodies and tetrabodies, respectively (32–36). Multimerization depends on the antibody clones. It has been previously reported that anti-EGFR scFv and Ex3 diabodies both include anti-EGFR Fv clone 528 and partially form stable tetrabody (37) and tetrabody-like structures (38), respectively. This characteristic may have contributed to the successful preparation of TaKE1-Fc and TaKE2-Fc, as well as the higher expression yields and activities of TaKE1-Fc with two 528 Fvs than those of TaKE2-Fc.
For further functional evaluation, the Fc region of TaKE1-Fc was removed through HRV3C protease digestion because the Fc region can bind and activate NK cells. Although complete digestion of Fc was confirmed using SDS-PAGE (Fig. 3A), the peak corresponding to the TaKE1 dimer revealed a similar intensity as the monomer peak (Fig. 3B and C). Formation of multimers by domain swapping often occurs during the development of multispecific and/or multivalent antibodies with multiple Fv domains, as it has been previously reported (22,39). The length of the linkers and domain order of Fvs, which can markedly affect the function (22,40), are currently being optimized by the authors, to reduce the formation of such multimers and increase the number of monomers for further functional evaluation.
Although TaKE1 showed trispecificity with the ability to bind TFK-1 cells, T-LAK cells, and NK92/CD16A cells (Fig. 4), strong interactions were clearly observed only in NK92/CD16A cells. Anti-Ex3 serum was used as the detection antibody because TaKE1 possesses no potential detection tags; the epitopes in TaKE1 for anti-Ex3 serum may be hidden by binding of TaKE1 to EGFR on TFK-1 cells and CD3 on T-LAK cells. TaKE1 was effective in the presence of either NK92/CD16A cells or T-LAK cells, unlike bsAbs (Fig. 5A and B), supporting the trispecificity of TaKE1. In the presence of both effector cells, TaKE1 was more effective than Ex16-scDb-HL at most concentrations and than Ex3-scDb-LH only at a lower ratio of T-LAK cells (Fig. 5 C-E). Although further detailed evaluation is needed, these results indicate that TaKE1 binds predominantly to T-LAK cells. TaKE1 also demonstrated comparable or higher cancer growth inhibitory effects than the combination of the two types of bsAbs (Fig. 6). Although other in vitro studies such as cell cycle analysis and apoptosis analysis, and in vivo studies to evaluate antitumor effects should be performed, a functional trispecific antibody that may recruit both T and NK cells was developed.
Various multispecific and/or multivalent antibody formats have been designed using Fvs, as well as single-domain antibodies, such as VHH (17,41,42). However, these formats may not be functional and practical because their effects are highly dependent on the therapeutic strategy, targets and antibody clones. Especially, in the series of the comprehensive development of bsAbs, an improvement of ~1,000-times was identified in the cytotoxicity by screening effective combinations of antibody clones even for the same targets (43). Antibody combinations and structural formats may produce steric hindrance in the cross-linking of target cells (22,44), and these may also affect the efficacy of trispecific antibodies. In actuality, the TaKE format with a circular tetrabody-like configuration also has antibody clone dependencies; TaKE1 was superior to TaKE2 and further investigation of antibody clones may result in the enhancement of cytotoxicity. However, the highly potent cytotoxicity would raise concerning side effects. In this case, a design of prodrug antibodies is effective (45), and the universal design of prodrug antibodies was recently reported by the authors (46). One advantage of the TaKE format without the Fc region is the possibility of producing these antibodies using a cost-effective microbial expression system. The authors of the present study have previously prepared anti-EGFR tetrabody (37) and Ex3 tetrabody (38), both of which are similar in size to TaKE, using Escherichia coli and reported the preparation of functional bsAbs using the gram-positive Brevibacillus choshinensis expression system (13). The authors are working to prepare TaKEs using microbial expression systems.
While the development of complex multispecific antibodies has been progressing, one limitation is methods to evaluate and compare the efficacy of them precisely. General methods for in vitro growth inhibition assay can not always reflect the behavior of cancer therapeutic multispecific antibodies in solid tumors with TILs. The use of a xenografted mouse model with a surgical resection containing TILs from cancer patients is a possibility (47); however, this involves extensive challenges especially in the regulations. The generation of spheroids formed from cancer cell lines containing some effector cells is an ideal alternative method (48). A cancer spheroid model with T cells and NK cells would be helpful to evaluate and compare trispecific antibodies such as TaKEs in the future.
In the development of multispecific antibodies, the designs to redirect some types of immune cells, especially T cells and NK cells, toward cancer cells have been largely studied (10,11). However, one limitation is the differences in the abundance ratios of T cells and NK cells in tumor cells (19) and administration of two bsAbs recruiting T cells and NK cells is not a realistic therapeutic strategy due to the high production costs. Although the preparation of homogeneous molecules with high yields is required for additional in vitro investigations and in vivo experiments to strengthen the utility of the TaKE, a functional trispecific antibody with potentially strong therapeutic effects that are independent of the T cell and NK cell populations was generated.
Supplementary Material
Acknowledgements
The authors would like to thank Ms Hiromi Ogata (Department of Biomolecular Engineering, Graduate School of Engineering, Tohoku University, Sendai, Japan), Ms Mika Ohta, Ms Sayuri Murasaki and Ms Maiko Ueki (all from Department of Biotechnology and Life Science, Graduate School of Engineering, Tokyo University of Agriculture and Technology, Tokyo, Japan) for their excellent technical assistance.
Glossary
Abbreviations
- NK
natural killer
- TaKE
T cell and NK cell engager
- bsAbs
bispecific antibodies
- EGFR
epidermal growth factor receptor
- TILs
tumor-infiltrating lymphocytes
- Fv
variable fragment
- scFv
single-chain Fv
- SDS-PAGE
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- T-LAK
lymphokine-activated killer cells with T-cell phenotype
Funding Statement
The present research was funded by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (JSPS) (grant nos. 21K18321 and 22H02915).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
RA and IK conceived the study. RA, TN and IK designed the study. KK, AK and SS conducted the experiments. RA and KK analyzed the data and confirm the authenticity of all the raw data. KK and RA wrote the manuscript. TN and IK provided conceptual advice for the study. RA and KK edited the manuscript. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
The present study was approved (approval no. R2-60) by the Biosafety Subcommittee for Safe Handling of Living Modified Organisms at Tokyo University of Agriculture and Technology (Tokyo, Japan) and carried out according to the guidelines of the committee.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Kaplon H, Reichert JM. Antibodies to watch in 2018. MAbs. 2018;10:183–203. doi: 10.1080/19420862.2018.1415671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ecker DM, Jones SD, Levine HL. The therapeutic monoclonal antibody market. MAbs. 2015;7:9–14. doi: 10.4161/19420862.2015.989042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lou H, Cao X. Antibody variable region engineering for improving cancer immunotherapy. Cancer Commun (Lond) 2022;42:804–827. doi: 10.1002/cac2.12330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arlotta KJ, Owen SC. Antibody and antibody derivatives as cancer therapeutics. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2019;11:e1556. doi: 10.1002/wnan.1556. [DOI] [PubMed] [Google Scholar]
- 5.Nunez-Prado N, Compte M, Harwood S, Álvarez-Méndez A, Lykkemark S, Sanz L, Álvarez-Vallina L. The coming of age of engineered multivalent antibodies. Drug Discov Today. 2015;20:588–594. doi: 10.1016/j.drudis.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 6.Jaiswal D, Verma S, Nair DT, Salunke DM. Antibody multispecificity: A necessary evil? Mol Immunol. 2022;152:153–161. doi: 10.1016/j.molimm.2022.10.012. [DOI] [PubMed] [Google Scholar]
- 7.Steinhardt JJ, Guenaga J, Turner HL, McKee K, Louder MK, O'Dell S, Chiang CI, Lei L, Galkin A, Andrianov AK, et al. Rational design of a trispecific antibody targeting the HIV-1 Env with elevated anti-viral activity. Nat Commun. 2018;9:877. doi: 10.1038/s41467-018-03335-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhong X, D'Antona AM. Recent advances in the molecular design and applications of multispecific biotherapeutics. Antibodies (Basel) 2021;10:13. doi: 10.3390/antib10020013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castoldi R, Jucknischke U, Pradel LP, Arnold E, Klein C, Scheiblich S, Niederfellner G, Sustmann C. Molecular characterization of novel trispecific ErbB-cMet-IGF1R antibodies and their antigen-binding properties. Protein Eng Des Sel. 2012;25:551–559. doi: 10.1093/protein/gzs048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamakura D, Asano R, Yasunaga M. T cell bispecific antibodies: An antibody-based delivery system for inducing antitumor immunity. Pharmaceuticals (Basel) 2021;14:1172. doi: 10.3390/ph14111172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrini S, Cambiaggi A, Cantoni C, Canevari S, Mezzanzanica D, Colnaghi MI, Moretta L. Targeting of T or NK lymphocytes against tumor cells by bispecific monoclonal antibodies: Role of different triggering molecules. Int J Cancer Suppl. 1992;7:15–18. [PubMed] [Google Scholar]
- 12.Asano R, Sone Y, Makabe K, Tsumoto K, Hayashi H, Katayose Y, Unno M, Kudo T, Kumagai I. Humanization of the bispecific epidermal growth factor receptor × CD3 diabody and its efficacy as a potential clinical reagent. Clin Cancer Res. 2006;12:4036–4042. doi: 10.1158/1078-0432.CCR-06-0059. [DOI] [PubMed] [Google Scholar]
- 13.Asano R, Kuroki Y, Honma S, Akabane M, Watanabe S, Mayuzumi S, Hiyamuta S, Kumagai I, Sode K. Comprehensive study of domain rearrangements of single-chain bispecific antibodies to determine the best combination of configurations and microbial host cells. MAbs. 2018;10:854–863. doi: 10.1080/19420862.2018.1476815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Asano R, Nakayama M, Kawaguchi H, Kubota T, Nakanishi T, Umetsu M, Hayashi H, Katayose Y, Unno M, Kudo T, Kumagai I. Construction and humanization of a functional bispecific EGFR CD16 diabody using a refolding system. FEBS J. 2012;279:223–233. doi: 10.1111/j.1742-4658.2011.08417.x. [DOI] [PubMed] [Google Scholar]
- 15.Kuwahara A, Nagai K, Nakanishi T, Kumagai I, Asano R. Functional domain order of an anti-EGFR × Anti-CD16 bispecific diabody involving NK cell activation. Int J Mol Sci. 2020;21:8914. doi: 10.3390/ijms21238914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meermeier EW, Welsh SJ, Sharik ME, Du MT, Garbitt VM, Riggs DL, Shi CX, Stein CK, Bergsagel M, Chau B, et al. Tumor burden limits bispecific antibody efficacy through T cell exhaustion averted by concurrent cytotoxic therapy. Blood Cancer Discov. 2021;2:354–369. doi: 10.1158/2643-3230.BCD-21-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beha N, Harder M, Ring S, Kontermann RE, Müller D. IL15-based trifunctional antibody-fusion proteins with costimulatory TNF-superfamily ligands in the single-chain format for cancer immunotherapy. Mol Cancer Ther. 2019;18:1278–1288. doi: 10.1158/1535-7163.MCT-18-1204. [DOI] [PubMed] [Google Scholar]
- 18.Wu L, Seung E, Xu L, Rao E, Lord DM, Wei RR, Cortez-Retamozo V, Ospina B, Posternak V, Ulinski G, et al. Trispecific antibodies enhance the therapeutic efficacy of tumor-directed T cells through T cell receptor co-stimulation. Nat Cancer. 2020;1:86–98. doi: 10.1038/s43018-019-0004-z. [DOI] [PubMed] [Google Scholar]
- 19.Geissler K, Fornara P, Lautenschlager C, Holzhausen HJ, Seliger B, Riemann D. Immune signature of tumor infiltrating immune cells in renal cancer. Oncoimmunology. 2015;4:e985082. doi: 10.4161/2162402X.2014.985082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Asano R, Nagai K, Makabe K, Takahashi K, Kumagai T, Kawaguchi H, Ogata H, Arai K, Umetsu M, Kumagai I. Structural considerations for functional anti-EGFR × anti-CD3 bispecific diabodies in light of domain order and binding affinity. Oncotarget. 2018;9:13884–13893. doi: 10.18632/oncotarget.24490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miyazaki J, Takaki S, Araki K, Tashiro F, Tominaga A, Takatsu K, Yamamura K. Expression vector system based on the chicken beta-actin promoter directs efficient production of interleukin-5. Gene. 1989;79:269–277. doi: 10.1016/0378-1119(89)90209-6. [DOI] [PubMed] [Google Scholar]
- 22.Asano R, Shimomura I, Konno S, Ito A, Masakari Y, Orimo R, Taki S, Arai K, Ogata H, Okada M, et al. Rearranging the domain order of a diabody-based IgG-like bispecific antibody enhances its antitumor activity and improves its degradation resistance and pharmacokinetics. MAbs. 2014;6:1243–1254. doi: 10.4161/mabs.29445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saijyo S, Kudo T, Suzuki M, Katayose Y, Shinoda M, Muto T, Fukuhara K, Suzuki T, Matsuno S. Establishment of a new extrahepatic bile duct carcinoma cell line, TFK-1. Tohoku J Exp Med. 1995;177:61–71. doi: 10.1620/tjem.177.61. [DOI] [PubMed] [Google Scholar]
- 24.Asano R, Watanabe Y, Kawaguchi H, Fukazawa H, Nakanishi T, Umetsu M, Hayashi H, Katayose Y, Unno M, Kudo T, Kumagai I. Highly effective recombinant format of a humanized IgG-like bispecific antibody for cancer immunotherapy with retargeting of lymphocytes to tumor cells. J Biol Chem. 2007;282:27659–27665. doi: 10.1074/jbc.M704719200. [DOI] [PubMed] [Google Scholar]
- 25.Nakadate Y, Kodera Y, Kitamura Y, Shirasawa S, Tachibana T, Tamura T, Koizumi F. KRAS mutation confers resistance to antibody-dependent cellular cytotoxicity of cetuximab against human colorectal cancer cells. Int J Cancer. 2014;134:2146–2155. doi: 10.1002/ijc.28550. [DOI] [PubMed] [Google Scholar]
- 26.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xie Z, Shi M, Feng J, Yu M, Sun Y, Shen B, Guo N. A trivalent anti-erbB2/anti-CD16 bispecific antibody retargeting NK cells against human breast cancer cells. Biochem Biophys Res Commun. 2003;311:307–312. doi: 10.1016/j.bbrc.2003.09.211. [DOI] [PubMed] [Google Scholar]
- 28.Gall FL, Kipriyanov SM, Moldenhauer G, Little M. Di-, tri- and tetrameric single chain Fv antibody fragments against human CD19: Effect of valency on cell binding. FEBS Lett. 1999;453:164–168. doi: 10.1016/S0014-5793(99)00713-9. [DOI] [PubMed] [Google Scholar]
- 29.Sato JD, Kawamoto T, Le AD, Mendelsohn J, Polikoff J, Sato GH. Biological effects in vitro of monoclonal antibodies to human epidermal growth factor receptors. Mol Biol Med. 1983;1:511–529. [PubMed] [Google Scholar]
- 30.Makabe K, Nakanishi T, Tsumoto K, Tanaka Y, Kondo H, Umetsu M, Sone Y, Asano R, Kumagai I. Thermodynamic consequences of mutations in vernier zone residues of a humanized anti-human epidermal growth factor receptor murine antibody, 528. J Biol Chem. 2008;283:1156–1166. doi: 10.1074/jbc.M706190200. [DOI] [PubMed] [Google Scholar]
- 31.Li SQ, Schmitz KR, Jeffrey PD, Wiltzius JJW, Kussie P, Ferguson KM. Structural basis for inhibition of the epidermal growth factor receptor by cetuximab. Cancer Cell. 2005;7:301–311. doi: 10.1016/j.ccr.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 32.Pei XY, Holliger P, Murzin AG, Williams RL. The 2.0-A resolution crystal structure of a trimeric antibody fragment with noncognate VH-VL domain pairs shows a rearrangement of VH CDR3. Proc Natl Acad Sci USA. 1997;94:9637–9642. doi: 10.1073/pnas.94.18.9637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dolezal O, Pearce LA, Lawrence LJ, McCoy AJ, Hudson PJ, Kortt AA. ScFv multimers of the anti-neuraminidase antibody NC10: Shortening of the linker in single-chain Fv fragment assembled in V(L) to V(H) orientation drives the formation of dimers, trimers, tetramers and higher molecular mass multimers. Protein Eng. 2000;13:565–574. doi: 10.1093/protein/13.8.565. [DOI] [PubMed] [Google Scholar]
- 34.Power BE, Doughty L, Shapira DR, Burns JE, Bayly AM, Caine JM, Liu Z, Scott AM, Hudson PJ, Kortt AA. Noncovalent scFv multimers of tumor-targeting anti-Lewis(y) hu3S193 humanized antibody. Protein Sci. 2003;12:734–747. doi: 10.1110/ps.0228503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Le Gall F, Reusch U, Moldenhauer G, Little M, Kipriyanov SM. Immunosuppressive properties of anti-CD3 single-chain Fv and diabody. J Immunol Methods. 2004;285:111–127. doi: 10.1016/j.jim.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 36.Hudson PJ, Kortt AA. High avidity scFv multimers; diabodies and triabodies. J Immunol Methods. 1999;231:177–189. doi: 10.1016/S0022-1759(99)00157-X. [DOI] [PubMed] [Google Scholar]
- 37.Asano R, Koyama N, Hagiwara Y, Masakari Y, Orimo R, Arai K, Ogata H, Furumoto S, Umetsu M, Kumagai I. Anti-EGFR scFv tetramer (tetrabody) with a stable monodisperse structure, strong anticancer effect, and a long in viFvo half-life. FEBS Open Bio. 2016;6:594–602. doi: 10.1002/2211-5463.12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Asano R, Ikoma K, Sone Y, Kawaguchi H, Taki S, Hayashi H, Nakanishi T, Umetsu M, Katayose Y, Unno M, et al. Highly enhanced cytotoxicity of a dimeric bispecific diabody, the hEx3 tetrabody. J Biol Chem. 2010;285:20844–20849. doi: 10.1074/jbc.M110.120444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Asano R, Ikoma K, Shimomura I, Taki S, Nakanishi T, Umetsu M, Kumagai I. Cytotoxic enhancement of a bispecific diabody by format conversion to tandem single-chain variable fragment (taFv): The case of the hEx3 diabody. J Biol Chem. 2011;286:1812–1818. doi: 10.1074/jbc.M110.172957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Asano R, Kumagai T, Nagai K, Taki S, Shimomura I, Arai K, Ogata H, Okada M, Hayasaka F, Sanada H, et al. Domain order of a bispecific diabody dramatically enhances its antitumor activity beyond structural format conversion: The case of the hEx3 diabody. Protein Eng Des Sel. 2013;26:359–367. doi: 10.1093/protein/gzt009. [DOI] [PubMed] [Google Scholar]
- 41.Wang J, Kang G, Yuan H, Cao X, Huang H, de Marco A. Research progress and applications of multivalent, multispecific and modified nanobodies for disease treatment. Front Immunol. 2021;12:838082. doi: 10.3389/fimmu.2021.838082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu X, Demarest SJ. Building blocks for bispecific and trispecific antibodies. Methods. 2019;154:3–9. doi: 10.1016/j.ymeth.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 43.Sugiyama A, Umetsu M, Nakazawa H, Niide T, Onodera T, Hosokawa K, Hattori S, Asano R, Kumagai I. A semi high-throughput method for screening small bispecific antibodies with high cytotoxicity. Sci Rep. 2017;7:2862. doi: 10.1038/s41598-017-03101-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maejima A, Ishibashi K, Kim H, Kumagai I, Asano R. Evaluation of intercellular cross-linking abilities correlated with cytotoxicities of bispecific antibodies with domain rearrangements using AFM force-sensing. Biosens Bioelectron. 2021;178:113037. doi: 10.1016/j.bios.2021.113037. [DOI] [PubMed] [Google Scholar]
- 45.Lucchi R, Bentanachs J, Oller-Salvia B. The masking game: Design of activatable antibodies and mimetics for selective therapeutics and cell control. ACS Cent Sci. 2021;7:724–738. doi: 10.1021/acscentsci.0c01448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maejima A, Suzuki S, Makabe K, Kumagai I, Asano R. Incorporation of a repeated polypeptide sequence in therapeutic antibodies as a universal masking procedure: A case study of T cell-engaging bispecific antibodies. N Biotechnol. 2023;77:80–89. doi: 10.1016/j.nbt.2023.07.004. [DOI] [PubMed] [Google Scholar]
- 47.Schlereth B, Fichtner I, Lorenczewski G, Kleindienst P, Brischwein K, da Silva A, Kufer P, Lutterbuese R, Junghahn I, Kasimir-Bauer S, et al. Eradication of tumors from a human colon cancer cell line and from ovarian cancer metastases in immunodeficient mice by a single-chain Ep-CAM-/CD3-bispecific antibody construct. Cancer Res. 2005;65:2882–2889. doi: 10.1158/0008-5472.CAN-04-2637. [DOI] [PubMed] [Google Scholar]
- 48.Fujii H, Tanaka Y, Nakazawa H, Sugiyama A, Manabe N, Shinoda A, Shimizu N, Hattori T, Hosokawa K, Sujino T, et al. Compact seahorse-shaped T cell-activating antibody for cancer therapy. Adv Ther. 2018;1:1700031. doi: 10.1002/adtp.201700031. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article.