Abstract
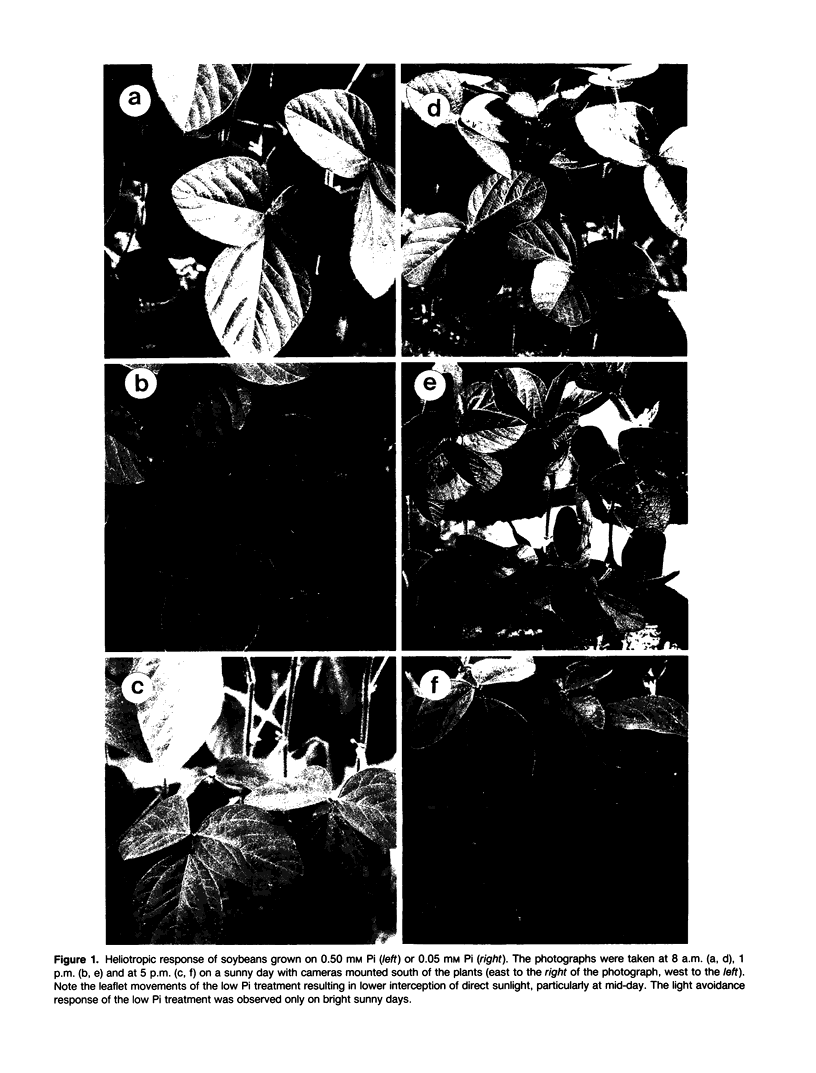
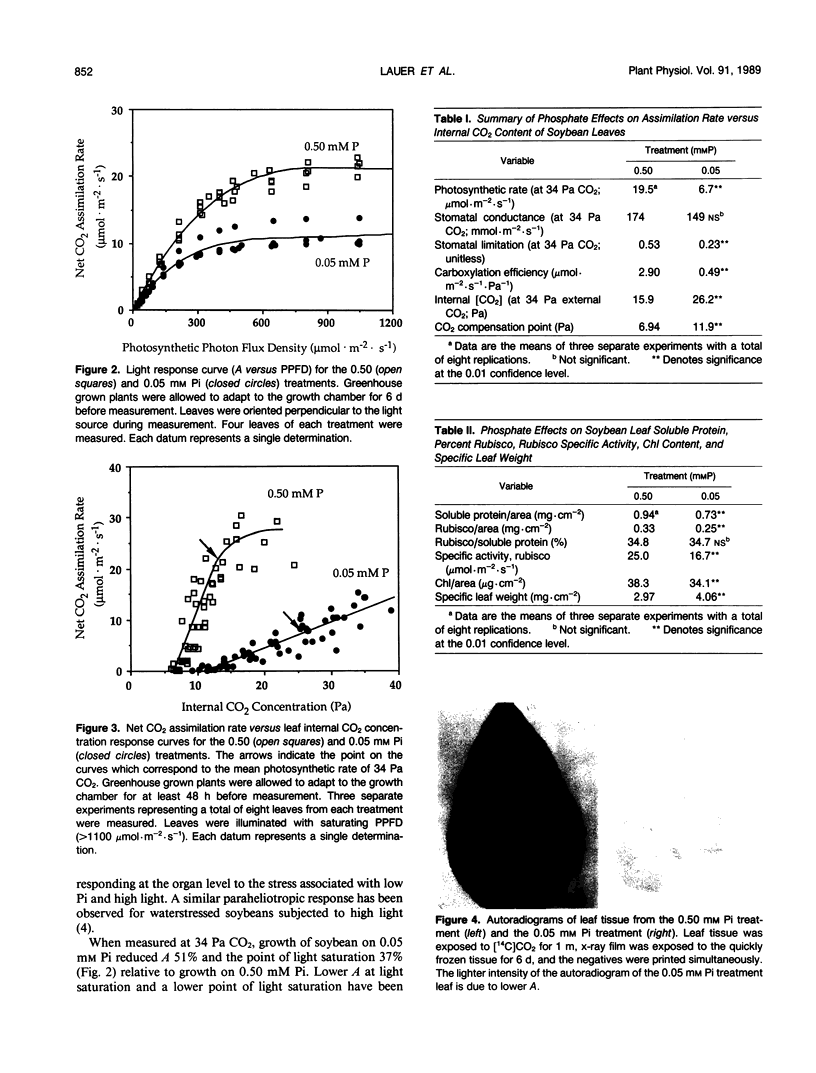
Low phosphate nutrition results in increased chlorophyll fluorescence, reduced photosynthetic rate, accumulation of starch and sucrose in leaves, and low crop yields. This study investigated physiological responses of soybean (Glycine max [L.] Merr.) leaves to low inorganic phosphate (Pi) conditions. Responses of photosynthesis to light and CO2 were examined for leaves of soybean grown at high (0.50 millimolar) or low (0.05 millimolar) Pi. Leaves of low Pi plants exhibited paraheliotropic orientation on bright sunny days rather than the normal diaheliotropic orientation exhibited by leaves of high Pi soybeans. Leaves of plants grown at high Pi had significantly higher light saturation points (1000 versus 630 micromole photons [400-700 nanometers] per square meter per second) and higher apparent quantum efficiency (0.062 versus 0.044 mole CO2 per mole photons) at ambient (34 pascals) CO2 than did low Pi leaves, yet stomatal conductances were similar. High Pi leaves also had significantly higher carboxylation efficiency (2.90 versus 0.49 micromole CO2 per square meter per second per pascal), a lower CO2 compensation point (6.9 versus 11.9 pascals), and a higher photosynthetic rate at 34 pascals CO2 (19.5 versus 6.7 micromoles CO2 per square meter per second) than did low Pi leaves. Soluble protein (0.94 versus 0.73 milligram per square centimeter), ribulose-1,5-bisphosphate carboxylase/oxygenase content (0.33 versus 0.25 milligram per square centimeter), and ribulose-1,5-bisphosphate carboxylase/oxygenase specific activity (25.0 versus 16.7 micromoles per square meter per second) were significantly greater in leaves of plants in the high Pi treatment. The data indicate that Pi stress alters the plant's CO2 reduction characteristics, which may in turn affect the plant's capacity to accommodate normal radiation loads.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackerson R. C. Osmoregulation in Cotton in Response to Water Stress : III. Effects of Phosphorus Fertility. Plant Physiol. 1985 Feb;77(2):309–312. doi: 10.1104/pp.77.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Campbell W. J., Allen L. H., Bowes G. Effects of CO(2) Concentration on Rubisco Activity, Amount, and Photosynthesis in Soybean Leaves. Plant Physiol. 1988 Dec;88(4):1310–1316. doi: 10.1104/pp.88.4.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehleringer J., Forseth I. Solar tracking by plants. Science. 1980 Dec 5;210(4474):1094–1098. doi: 10.1126/science.210.4474.1094. [DOI] [PubMed] [Google Scholar]
- Fredeen A. L., Rao I. M., Terry N. Influence of Phosphorus Nutrition on Growth and Carbon Partitioning in Glycine max. Plant Physiol. 1989 Jan;89(1):225–230. doi: 10.1104/pp.89.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt H. W., Chon C. J., Maronde D. Role of orthophosphate and other factors in the regulation of starch formation in leaves and isolated chloroplasts. Plant Physiol. 1977 Jun;59(6):1146–1155. doi: 10.1104/pp.59.6.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph M. C., Randall D. D. Photosynthesis in Polyploid Tall Fescue : II. PHOTOSYNTHESIS AND RIBULOSE-1, 5-BISPHOSPHATE CARBOXYLASE OF POLYPLOID TALL FESCUE. Plant Physiol. 1981 Oct;68(4):894–898. doi: 10.1104/pp.68.4.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauer M. J., Blevins D. G., Sierzputowska-Gracz H. P-Nuclear Magnetic Resonance Determination of Phosphate Compartmentation in Leaves of Reproductive Soybeans (Glycine max L.) as Affected by Phosphate Nutrition. Plant Physiol. 1989 Apr;89(4):1331–1336. doi: 10.1104/pp.89.4.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radin J. W., Hartung W., Kimball B. A., Mauney J. R. Correlation of Stomatal Conductance with Photosynthetic Capacity of Cotton Only in a CO(2)-Enriched Atmosphere: Mediation by Abscisic Acid? Plant Physiol. 1988 Dec;88(4):1058–1062. doi: 10.1104/pp.88.4.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesay A., Stewart C. R., Shibles R. M. Effects of KCN and Salicylhydroxamic Acid on Respiration of Soybean Leaves at Different Ages. Plant Physiol. 1986 Oct;82(2):443–447. doi: 10.1104/pp.82.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintermans J. F., de Mots A. Spectrophotometric characteristics of chlorophylls a and b and their pheophytins in ethanol. Biochim Biophys Acta. 1965 Nov 29;109(2):448–453. doi: 10.1016/0926-6585(65)90170-6. [DOI] [PubMed] [Google Scholar]
- Wong S. C., Cowan I. R., Farquhar G. D. Leaf Conductance in Relation to Rate of CO(2) Assimilation: I. Influence of Nitrogen Nutrition, Phosphorus Nutrition, Photon Flux Density, and Ambient Partial Pressure of CO(2) during Ontogeny. Plant Physiol. 1985 Aug;78(4):821–825. doi: 10.1104/pp.78.4.821. [DOI] [PMC free article] [PubMed] [Google Scholar]