Abstract
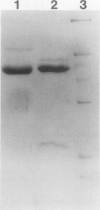
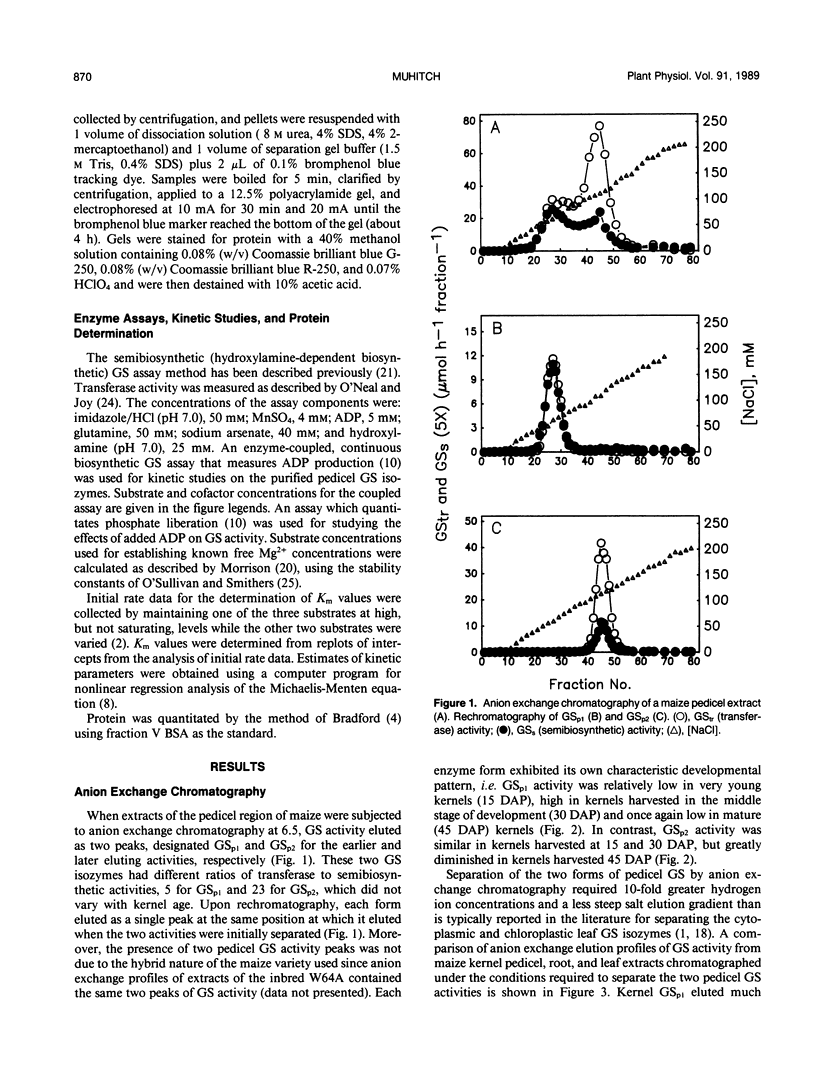
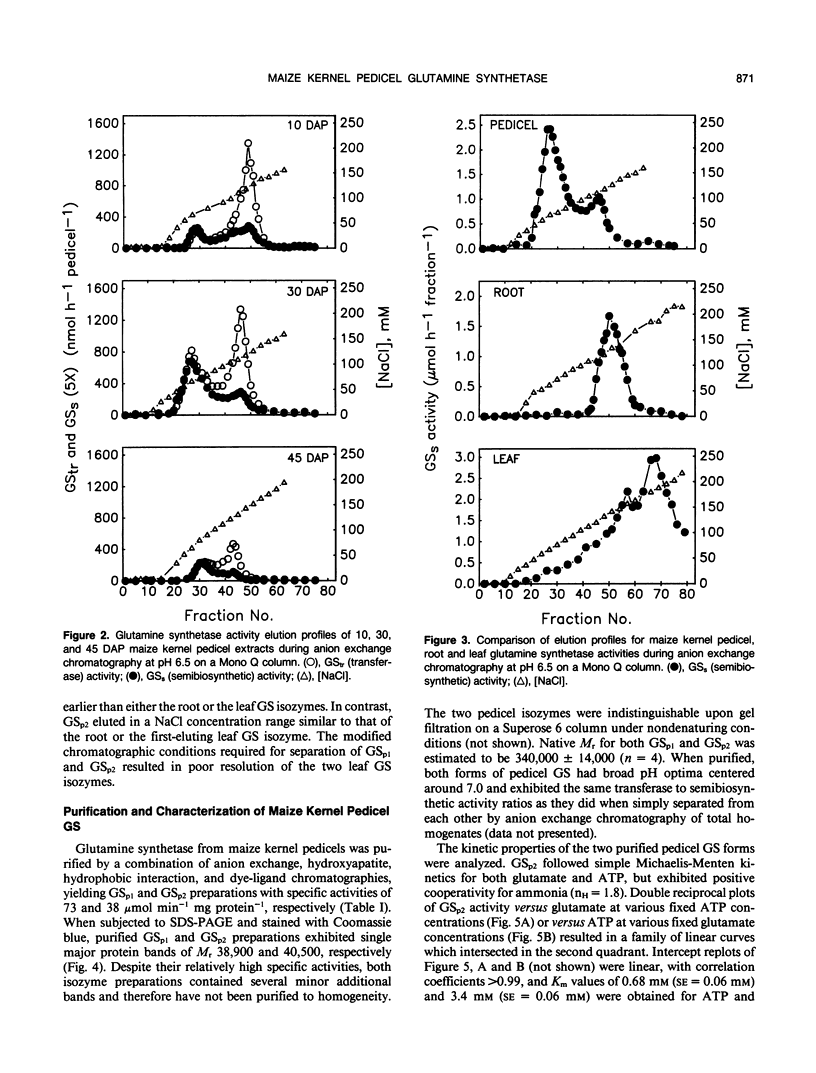
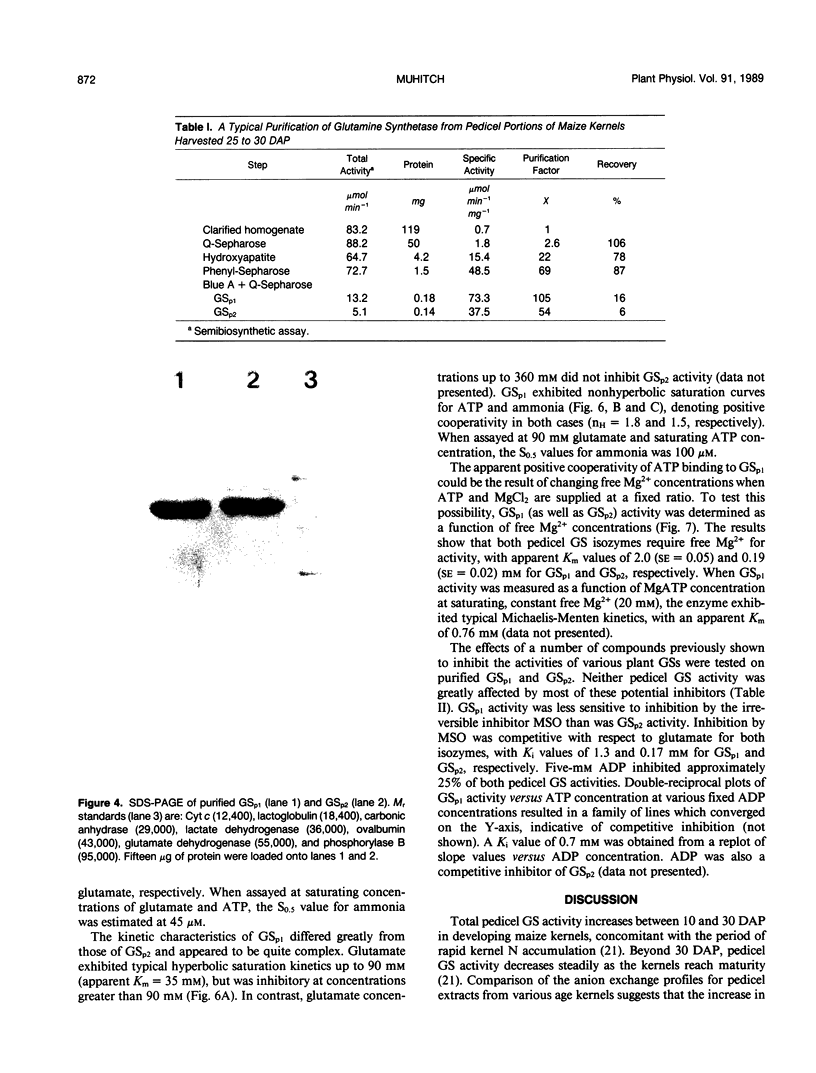
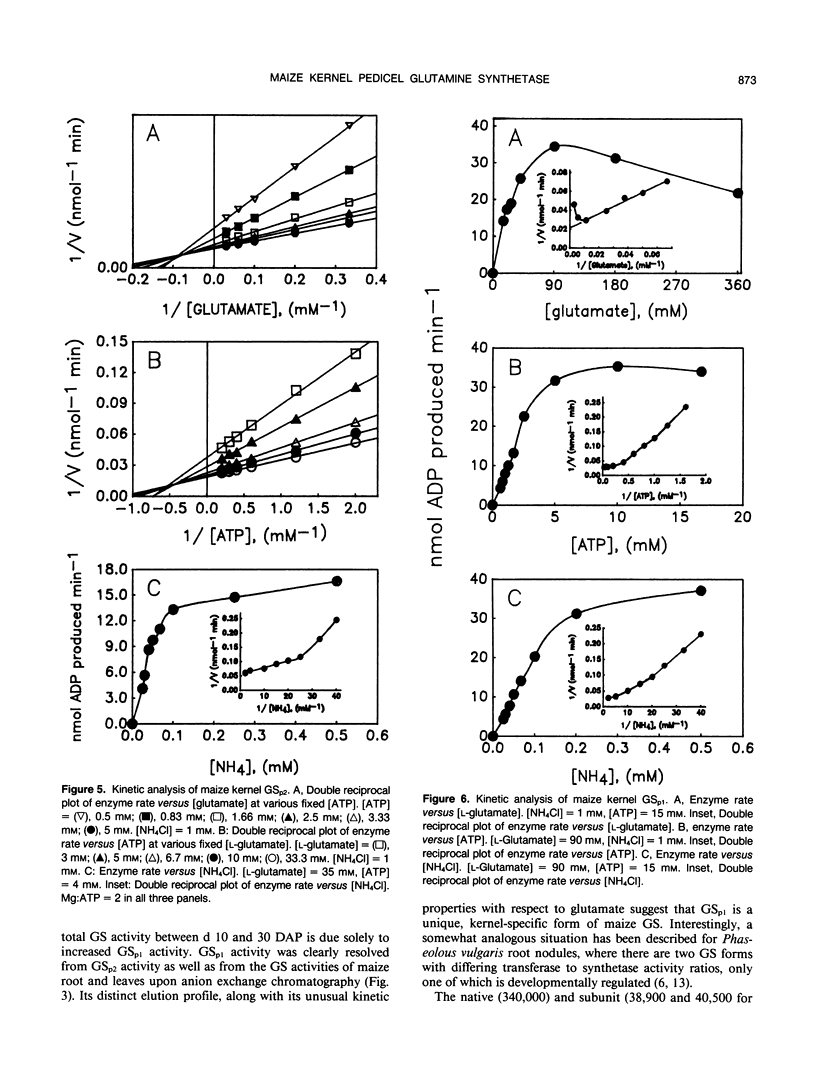
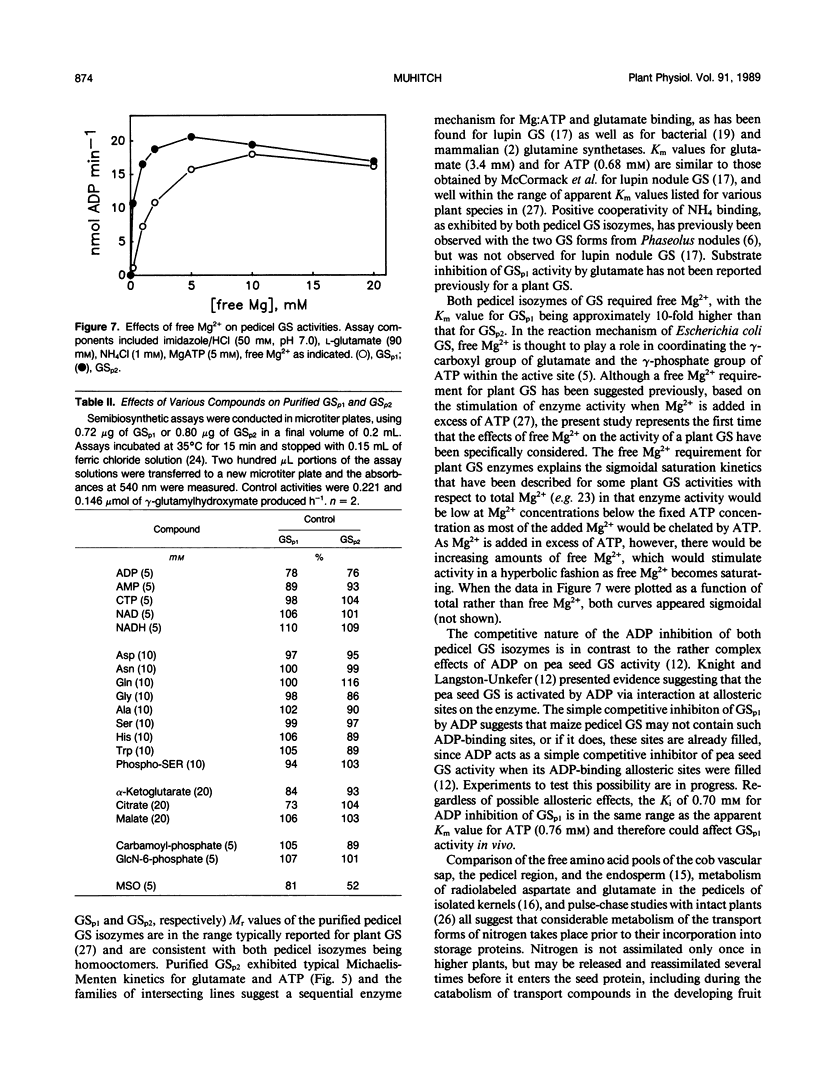
Maize (Zea mays L.) kernel pedicels, including vascular tissues, pedicel parenchyma, placento-chalazal tissue, and the surrounding pericarp, contained two forms of glutamine synthetase (EC 6.3.1.2), separable by anion exchange chromatography under mildly acidic conditions. The earlier-eluting activity (GSp1), but not the later-eluting activity (GSp2), was chromatographically distinct from the maize leaf and root glutamine synthetases. The level of GSp1 activity changed in a developmentally dependent manner while GSp2 activity was constitutive. GSp1 and GSp2 exhibited distinct ratios of transferase to hydroxylamine-dependent synthetase activities (5 and 23, respectively), which did not change with kernel age. Purified pedicel glutamine synthetases had native relative molecular masses of 340,000, while the subunit relative molecular masses differed slightly at 38,900 and 40,500 for GSp1 and GSp2, respectively. Both GS forms required free Mg2+ with apparent Kms = 2.0 and 0.19 millimolar for GSp1 and GSp2, respectively. GSp1 had an apparent Km for glutamate of 35 millimolar and exhibited substrate inhibition at glutamate concentrations greater than 90 millimolar. In contrast, GSp2 exhibited simple Michaelis-Menten kinetics for glutamate with a Km value of 3.4 millimolar. Both isozymes exhibited positive cooperativity for ammonia, with S0.5 values of 100 and 45 micromolar, respectively. GSp1 appears to be a unique, kernel-specific form of plant glutamine synthetase. Possible functions for the pedicel GS isozymes in kernel nitrogen metabolism are discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison R. D., Todhunter J. A., Purich D. L. Steady state and equilibrium exchange kinetic studies of the sheep brain glutamine synthetase reaction. J Biol Chem. 1977 Sep 10;252(17):6046–6051. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Colanduoni J., Nissan R., Villafranca J. J. Studies of the mechanism of glutamine synthetase utilizing pH-dependent behavior in catalysis and binding. J Biol Chem. 1987 Mar 5;262(7):3037–3043. [PubMed] [Google Scholar]
- Duggleby R. G. A nonlinear regression program for small computers. Anal Biochem. 1981 Jan 1;110(1):9–18. doi: 10.1016/0003-2697(81)90104-4. [DOI] [PubMed] [Google Scholar]
- Ericson M. C. Purification and properties of glutamine synthetase from spinach leaves. Plant Physiol. 1985 Dec;79(4):923–927. doi: 10.1104/pp.79.4.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felker F. C., Shannon J. C. Movement of C-labeled Assimilates into Kernels of Zea mays L: III. AN ANATOMICAL EXAMINATION AND MICROAUTORADIOGRAPHIC STUDY OF ASSIMILATE TRANSFER. Plant Physiol. 1980 May;65(5):864–870. doi: 10.1104/pp.65.5.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight T. J., Langston-Unkefer P. J. Adenine nucleotides as allosteric effectors of pea seed glutamine synthetase. J Biol Chem. 1988 Aug 15;263(23):11084–11089. [PubMed] [Google Scholar]
- Mc Cormack D. K., Farnden K. J., Boland M. J. Purification and properties of glutamine synthetase from the plant cytosol fraction of lupin nodules. Arch Biochem Biophys. 1982 Oct 15;218(2):561–571. doi: 10.1016/0003-9861(82)90380-0. [DOI] [PubMed] [Google Scholar]
- McNally S. F., Hirel B., Gadal P., Mann A. F., Stewart G. R. Glutamine Synthetases of Higher Plants : Evidence for a Specific Isoform Content Related to Their Possible Physiological Role and Their Compartmentation within the Leaf. Plant Physiol. 1983 May;72(1):22–25. doi: 10.1104/pp.72.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek T. D., Villafranca J. J. Kinetic mechanism of Escherichia coli glutamine synthetase. Biochemistry. 1980 Nov 25;19(24):5513–5519. doi: 10.1021/bi00565a008. [DOI] [PubMed] [Google Scholar]
- Morrison J. F. Approaches to kinetic studies on metal-activated enzymes. Methods Enzymol. 1979;63:257–294. doi: 10.1016/0076-6879(79)63013-6. [DOI] [PubMed] [Google Scholar]
- O'Sullivan W. J., Smithers G. W. Stability constants for biologically important metal-ligand complexes. Methods Enzymol. 1979;63:294–336. doi: 10.1016/0076-6879(79)63014-8. [DOI] [PubMed] [Google Scholar]
- O'neal D., Joy K. W. Glutamine synthetase of pea leaves: divalent cation effects, substrate specificity, and other properties. Plant Physiol. 1974 Nov;54(5):773–779. doi: 10.1104/pp.54.5.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernollet J. C., Huet J. C., Moutot F., Morot-Gaudry J. F. Relationship between Photosynthesis and Protein Synthesis in Maize: II. Interconversions of the Photoassimilated Carbon in the Ear Leaf and in the Intermediary Organs to Synthesize the Seed Storage Proteins and Starch. Plant Physiol. 1986 Jan;80(1):216–222. doi: 10.1104/pp.80.1.216. [DOI] [PMC free article] [PubMed] [Google Scholar]