Abstract
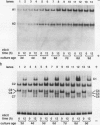
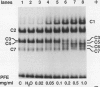
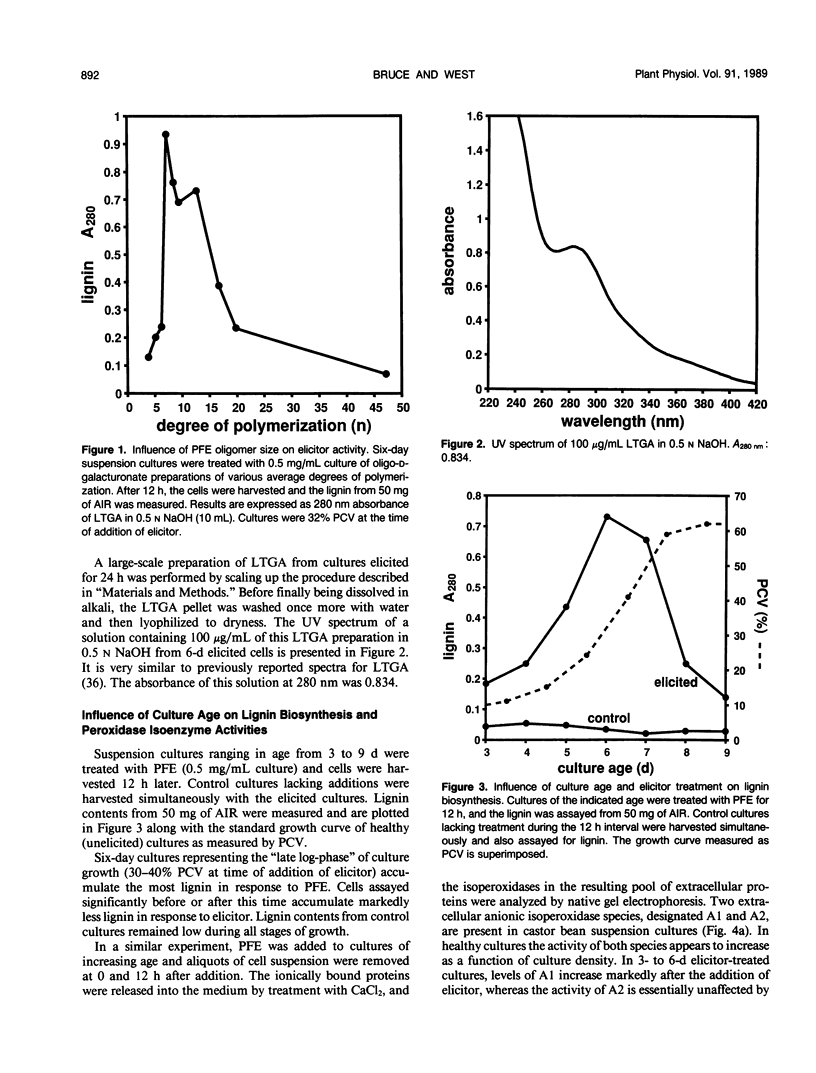
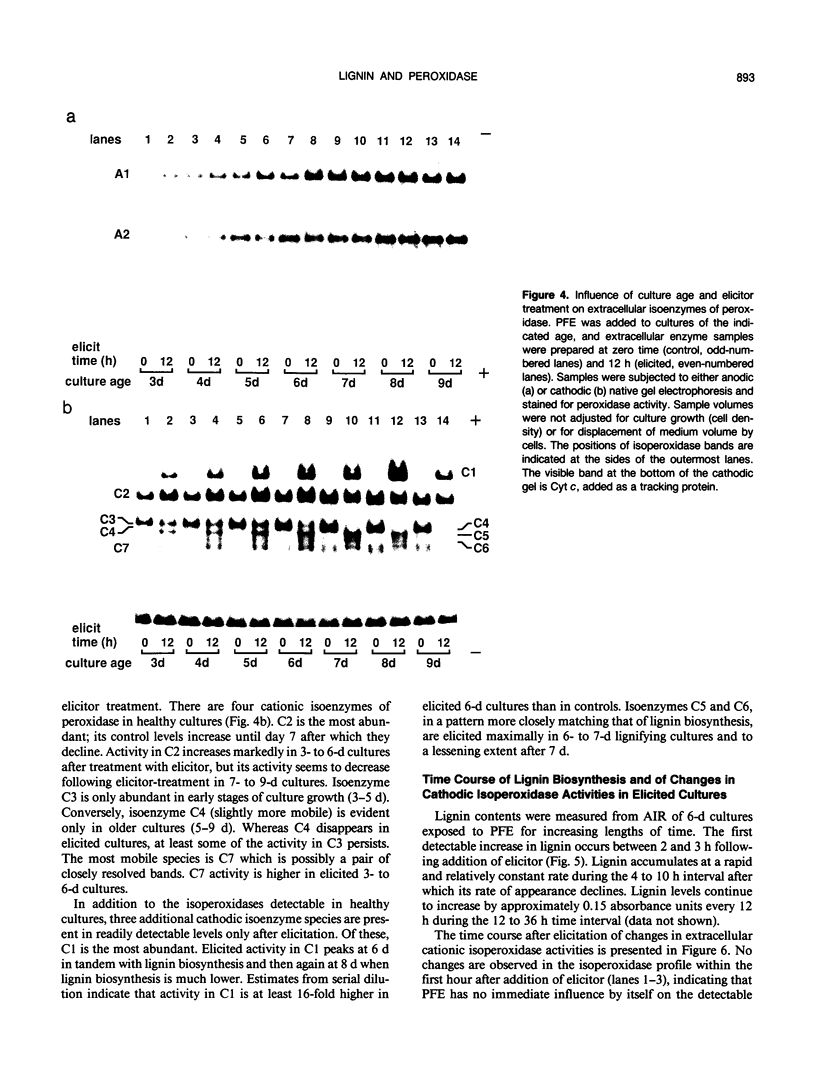
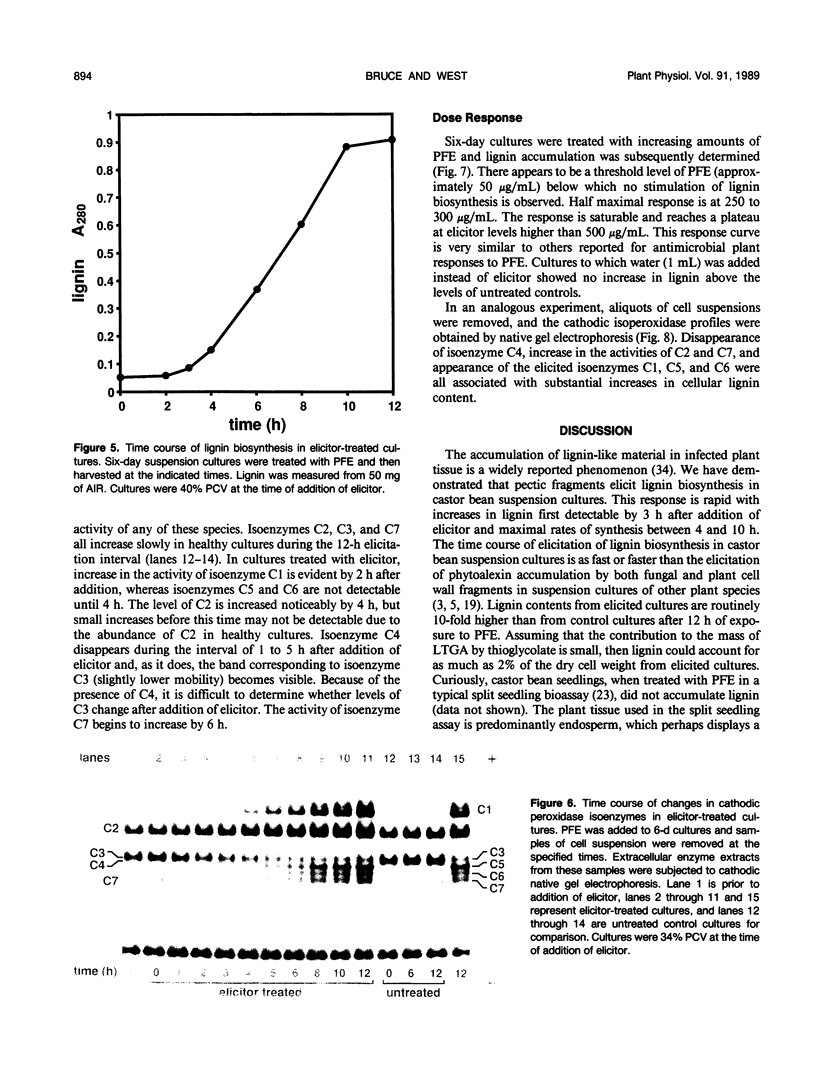
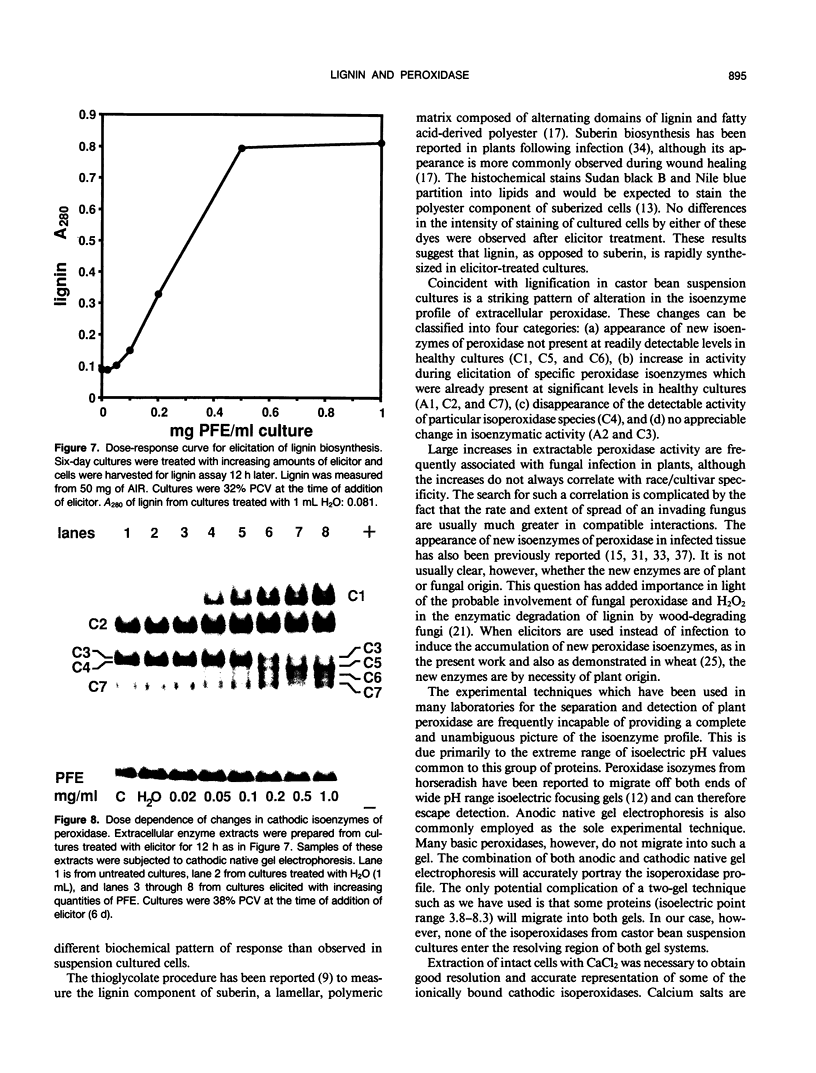
Suspension cultures of castor bean (Ricinus communis L.) which have been treated with pectic fragment elicitor rapidly accumulate lignin as measured by derivatization with thioglycolic acid. The responsiveness of cultured cells to elicitor is dependent on the stage of culture growth. In 6-day (maximally responsive) cultures, increases in lignin are first evident 3 hours after addition of pectic fragment elicitor with maximal rates of lignin synthesis between 4 and 10 hours. The abundance of lignin in cultures after 12 hours of elicitor treatment is 10- to 20-fold higher than in untreated control cultures and can thereby account for as much as 2% of the dry cell weight. Only intermediate sizes of pectic oligomer are active as elicitors of lignin. Half-maximal accumulation of lignin occurs at 250 to 300 micrograms per milliliter of an optimal elicitor preparation with an average degree of polymerization of seven. We consider the synthesis of lignin in elicited cultures to be a mechanism of plant disease resistance which is induced by the elicitor. Plant peroxidases have been proposed to catalyze the last enzymatic steps in the biosynthesis of both lignin and hydrogen peroxide. Six extracellular isoenzymes of peroxidase (two anionic, designated A1 and A2, and four cationic, designated C2, C3, C4, and C7) are detectable in healthy suspension cultures of castor bean by native gel electrophoresis. Treatment of cultures with elicitor causes substantial changes in the activity of four of these species (A1, C2, C3, and C7). Elicitor treatment also results in the appearance of three new peroxidase isoenzymes that are not readily detectable in healthy cultures (C1, C5, and C6). Increases in the activities of these isoenzymes are concurrent with or slightly precede the accumulation of lignin in elicited 6-day cultures. By 12 hours after addition of elicitor, C1 becomes the most abundant extracellular isoperoxidase. The differential regulation of expression of peroxidase isoenzymes following elicitor treatment suggests that individual isoenzymes of peroxidase may have specific functional roles in the biosynthesis of disease-lignin.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bruce R. J., West C. A. Elicitation of Casbene Synthetase Activity in Castor Bean : THE ROLE OF PECTIC FRAGMENTS OF THE PLANT CELL WALL IN ELICITATION BY A FUNGAL ENDOPOLYGALACTURONASE. Plant Physiol. 1982 May;69(5):1181–1188. doi: 10.1104/pp.69.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Davis K. R., Lyon G. D., Darvill A. G., Albersheim P. Host-Pathogen Interactions : XXV. Endopolygalacturonic Acid Lyase from Erwinia carotovora Elicits Phytoalexin Accumulation by Releasing Plant Cell Wall Fragments. Plant Physiol. 1984 Jan;74(1):52–60. doi: 10.1104/pp.74.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon R. A., Lamb C. J. Stimulation of de novo synthesis of L-phenylalanine ammonia-lyase in relation to phytoalexin accumulation in Colletotrichum lindemuthianum elicitor-treated cell suspension cultures of french bean (Phaseolus vulgaris). Biochim Biophys Acta. 1979 Sep 3;586(3):453–463. doi: 10.1016/0304-4165(79)90035-7. [DOI] [PubMed] [Google Scholar]
- Ebel J., Ayers A. R., Albersheim P. Host-Pathogen Interactions: XII. Response of Suspension-cultured Soybean Cells to the Elicitor Isolated from Phytophthora megasperma var. sojae, a Fungal Pathogen of Soybeans. Plant Physiol. 1976 May;57(5):775–779. doi: 10.1104/pp.57.5.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamborg O. L., Miller R. A., Ojima K. Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res. 1968 Apr;50(1):151–158. doi: 10.1016/0014-4827(68)90403-5. [DOI] [PubMed] [Google Scholar]
- Hoyle M. C. High resolution of peroxidase-indoleacetic Acid oxidase isoenzymes from horseradish by isoelectric focusing. Plant Physiol. 1977 Nov;60(5):787–793. doi: 10.1104/pp.60.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin D. F., West C. A. Characteristics of galacturonic Acid oligomers as elicitors of casbene synthetase activity in castor bean seedlings. Plant Physiol. 1984 Apr;74(4):989–992. doi: 10.1104/pp.74.4.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kombrink E., Hahlbrock K. Responses of cultured parsley cells to elicitors from phytopathogenic fungi : timing and dose dependency of elicitor-induced reactions. Plant Physiol. 1986 May;81(1):216–221. doi: 10.1104/pp.81.1.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. C., West C. A. Polygalacturonase from Rhizopus stolonifer, an Elicitor of Casbene Synthetase Activity in Castor Bean (Ricinus communis L.) Seedlings. Plant Physiol. 1981 Apr;67(4):633–639. doi: 10.1104/pp.67.4.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REISFELD R. A., LEWIS U. J., WILLIAMS D. E. Disk electrophoresis of basic proteins and peptides on polyacrylamide gels. Nature. 1962 Jul 21;195:281–283. doi: 10.1038/195281a0. [DOI] [PubMed] [Google Scholar]
- Vance C. P., Anderson J. O., Sherwood R. T. Soluble and cell wall peroxidases in reed canarygrass in relation to disease resistance and localized lignin formation. Plant Physiol. 1976 Jun;57(6):920–922. doi: 10.1104/pp.57.6.920. [DOI] [PMC free article] [PubMed] [Google Scholar]