Abstract
扩张型心肌病(dilated cardiomyopathy, DCM)是心力衰竭重要的原因之一,任何阶段均可出现严重的心血管事件,导致猝死。RNA结合基序蛋白20(RNA-binding motif protein 20, RBM20)基因突变是引起DCM的原因之一,呈家族性聚集,可合并心律失常,具有易猝死、早死亡的特点。该文介绍RBM20基因及其调节TTN基因、CAMK2基因的剪接,总结RBM20基因突变相关DCM的治疗,旨在提高临床工作者对RBM20基因的认识,为精准医学治疗提供新思路。
Keywords: 扩张型心肌病, RBM20基因, 可变剪接
Abstract
Dilated cardiomyopathy (DCM) is a significant contributor to heart failure and can lead to life-threatening cardiovascular events at any stage. RNA-binding motif protein 20 (RBM20) gene mutation is known to be one of the causes of DCM. This mutation exhibits familial aggregation and is associated with arrhythmias, increasing the risk of sudden and early death. This article delves into the characteristics of the RBM20 gene, highlighting its role in regulating alternative splicing of the TTN gene and calcium/calmodulin-dependent protein kinase type II gene. Furthermore, the article provides a summary of treatment options available for DCM caused by RBM20 gene mutations, aiming to enhance clinicians' understanding of the RBM20 gene and provide new ideas for precision medicine treatment.
Keywords: Dilated cardiomyopathy, RBM20 gene, Alternative splicing
扩张型心肌病(dilated cardiomyopathy, DCM)是全球心力衰竭重要的原因之一,表现为心脏结构出现一侧或双侧心室扩大及收缩功能减退,可同时合并心律失常,在疾病的任何阶段均可出现严重心血管事件,导致猝死,最终的治疗手段多为心脏移植。部分DCM原因不明,在特发性DCM中,20%~35%为家族遗传,多为常染色体显性遗传。自1990年发现第1例家族性心肌病致病性基因突变,目前已超过170个基因与心肌病、离子通道病等多种心脏系统疾病相关,超过60个基因可引起DCM[1-2]。2009年,首次发现RNA结合基序蛋白20(RNA binding motif protein 20, RBM20)基因突变可引起DCM[3]。RBM20基因突变所致的心肌病多为家族性DCM,临床表现相似[4],发病率为1%~5%[5]。少数RBM20基因突变所致的心肌病为散发性DCM[6]。RBM20基因又可作为剪接因子,在转录过程中可变剪接TTN基因、TNNT2基因,在心脏发育中起重要作用。RBM20基因突变导致的DCM具有发病年龄小、心力衰竭严重、高病死率的特点[7-9]。目前,RBM20基因已被证实与多种心血管疾病相关,可表现为DCM、心律失常、猝死及心肌广泛纤维化、心肌致密化不全。
1. RBM20基因的结构
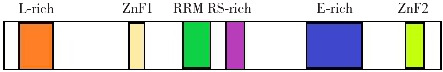
RBM20基因主要在横纹肌中表达,心肌中表达最高,非肌肉组织中几乎不表达[10-11]。RBM20基因位于10号染色体,包含14个外显子,编码含1 227个氨基酸的蛋白质。存在物种间高度保守的功能区域,包括2个锌指(zinc finger, ZnF)结构域,1个RNA识别基序(RNA recognition motif, RRM)结构域,1个富含精氨酸/丝氨酸(arginine-/serine-rich, RS)区域,1个富含亮氨酸(leucine-rich, L-rich)区域,1个富含谷氨酸(glutamate-rich, E-rich)区域[12-13](图1)。其中,与DCM相关的RBM20基因突变多发生在RS区域[2,10,14-15],导致表达的蛋白质功能丧失。E-rich区域和ZnF结构域内突变发生率比RS区域低,但是在这些区域内发生突变后也会降低RBM20基因的剪接活性,影响所调控的基因表达,导致DCM[16-17]。不是所有突变均有意义,如I536T-RBM20基因突变影响基因表达、导致剪接异常,但是不会引起DCM[18]。
图1. RBM20基因结构图 [ZnF]锌指;[RRM] RNA识别基序;[RS-rich]富含精氨酸/丝氨酸;[L-rich]富含亮氨酸;[E-rich]富含谷氨酸。.
2. RBM20基因突变的表达
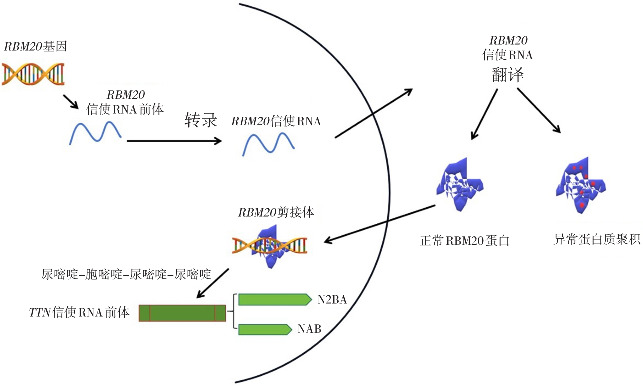
Zhang等[19]表明RS区域发生的突变介导中心核定位信号的中断,可引起严重的DCM。大多数RBM20基因突变定位在RS区域中的精氨酸-丝氨酸-精氨酸-丝氨酸-脯氨酸(arginine-serine-arginine-serine-proline, RSRSP)片段,RSRSP片段的磷酸化干扰了RBM20基因的核定位,在细胞质中形成异常的核糖核蛋白(ribonucleoprotein, RNP)颗粒,考虑这种RNP颗粒是形成DCM心肌改变的重要原因[12,14,20-21]。另有研究表明,RSRSP片段突变使DCM表型更加严重,并发心房颤动及室性心律失常[22]。但是,低水平的磷酸化仍可提高蛋白质表达水平,可能是受反馈机制的作用[23]。RBM20基因是心脏发育过程中核糖核酸处理机制的重要组成成分。RBM20基因突变干扰前体信使核糖核酸(pre-messenger RNA, pre-mRNA)的翻译后修饰过程,生成大量异常蛋白质(图2)。转录过程中,RBM20基因通过RRM结构域抑制上游和下游内含子[17],Upadhyay等[24]发现RRM结构域具有C端螺旋,特异识别含尿嘧啶-胞嘧啶-尿嘧啶-尿嘧啶的RNA基序,成为转录pre-mRNA的基础。
图2. RBM20基因转录翻译及可变剪接TTN基因示意图 图片改自Koelemen等[13]的文章。RBM20基因突变影响转录及翻译过程,导致异常蛋白质聚积。同时,RBM20基因突变通过调整N2BA及NAB亚型比例,可变剪接TTN基因。.
3. RBM20基因的可变剪接
RBM20基因突变导致可变剪接异常,导致靶基因形成致病性的环状RNA,并且在细胞质加工小体内重新生成RNP,导致心肌细胞变异,心脏收缩功能异常[25]。RBM20基因的结构域在定位剪接位点及外显子/内含子的选择中至关重要。通过研究实验大鼠与人类心脏的基因,在内含子结合位点发现一组独特的RNA识别元件,在3'剪接位点下游400碱基对和5'剪接位点上游400碱基对位点与RBM20基因结合,并且,RBM20基因也通过与U1和U2核小核糖核蛋白颗粒(small nuclear ribonucle protein particle, snRNP)结合位点结合,抑制剪接过程[10,26]。
RBM20基因作为一种剪接因子,调节与影响心室舒张功能、肌节组装、离子转运等多个心脏相关基因的剪接[11],包括TTN基因、CAMK2基因、MYH6基因、MYH7基因、RYR2基因、NEXN基因、NPRL2基因、TNNT2基因、PDLIM3基因等[5,11,27]。本文介绍RBM20基因调控TTN基因及CAMK2基因的过程。
3.1. TTN基因
TTN基因突变是引起DCM常见的原因[28],占10%~30%。TTN基因在人类中包含363个外显子,3个亚型,编码的肌联蛋白是人类体积最大的蛋白质,大小超过3 MDa,连接大多数横纹肌中的Z盘和粗丝,调节心肌收缩和弹性,是导致心肌硬化的重要因素[20]。TTN基因主要的2种亚型是N2BA和NAB,心肌顺应性由N2BA和NAB所占比例不同决定。RBM20基因突变显著提高N2BA亚型表达,造成N2BA和NAB比例改变,使肌原纤维被动张力降低,导致心肌病[12,26-27,29](图2)。
3.2. CAMK2基因
CAMK2基因的产物属于丝氨酸/苏氨酸激酶家族的一种多功能蛋白激酶,蛋白激酶中的δ亚型在心脏中占主导地位。RBM20基因突变导致CAMK2基因的产物从常规的-δB和-δC亚型转变为-δA和-δ9亚型,导致L型钙离子电流增加,心肌细胞肌浆网中充满钙离子,细胞内钙离子超负荷,可能是DCM心律失常发生的原因[27,30]。
4. 性别差异
DCM发病率及临床严重程度存在性别差异,男性发病率高于女性,症状更加严重。一项Meta分析表明,相比于女性,男性DCM的全因病死率、心血管病死率及猝死风险更高[31]。RBM20基因突变所致的DCM中,男性患者的临床症状也比女性更严重[6,32]。但是,Lennermann等[33]研究构建的RBM20基因敲除(knockout, KO)小鼠模型,虽然RBM20-KO所致DCM雄性小鼠的基因表达和磷酸化更加显著,但是心脏形态和功能与DCM雌性小鼠相比无性别差异。这种人类及小鼠的发病性别差异的不同可能受行为风险因素如吸烟、肥胖及激素等的影响。
5. 治疗
DCM在临床中大多数仍表现为收缩性心力衰竭。很多患者最后的治疗措施为心脏移植,但供体稀少。目前,已开展多项治疗RBM20基因突变所致心肌病的研究,期望可以治愈心力衰竭。
研究表明,强心苷类药物包括地高辛及洋地黄毒苷可以抑制RBM20基因对TTN基因的可变剪接,降低RBM20基因表达蛋白质的水平,可能改善舒张性心力衰竭[34]。丝氨酸-精氨酸蛋白激酶可以调节RSRSP片段的磷酸化,调控心肌病相关基因的剪接,成为治疗RBM20基因相关心肌病的潜在治疗靶点[21]。应用CRISPR/Cas9基因编辑技术建立体外心肌模型,此模型包含RBM20基因杂合突变的多能干细胞定向分化心肌细胞,通过全反式维甲酸上调RBM20基因表达,部分恢复由RBM20基因缺陷引起的剪接、钙处理异常和收缩功能障碍,有望修复RBM20基因突变所致的DCM[35]。
基因突变的精准校正是一种新的治疗方法。一项研究指出,应用腺嘌呤碱基编辑和先导编辑校正RS区域内的突变,诱导多能干细胞衍生出心肌细胞,使心力衰竭的大鼠恢复心脏功能[14]。目前,关于基因编辑改善致病性RBM20基因变异体修复的方法研究仍在初级阶段。
6. 总结与展望
RBM20基因突变通过多种途径导致DCM,其转录、翻译及调控其他基因可变剪接的过程复杂。目前,对于RBM20基因突变所致DCM的治疗方案多基于基础研究,由于RBM20基因具有物种间高度保守的特征,非同源物种、体内及体外的RBM20基因研究结果有部分不同,对人类DCM的疗效仍需要更多临床研究与基础研究结合进一步明确。
利益冲突声明
所有作者声明无利益冲突。
参 考 文 献
- 1. Brodehl A, Gaertner-Rommel A, Milting H. FLNC (filamin-C): a new(er) player in the field of genetic cardiomyopathies[J]. Circ Cardiovasc Genet, 2017, 10(6): e001959. DOI: 10.1161/CIRCGENETICS.117.001959. [DOI] [PubMed] [Google Scholar]
- 2. Zahr HC, Jaalouk DE. Exploring the crosstalk between LMNA and splicing machinery gene mutations in dilated cardiomyopathy[J]. Front Genet, 2018, 9: 231. DOI: 10.3389/fgene.2018.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brauch KM, Karst ML, Herron KJ, et al. Mutations in ribonucleic acid binding protein gene cause familial dilated cardiomyopathy[J]. J Am Coll Cardiol, 2009, 54(10): 930-941. DOI: 10.1016/j.jacc.2009.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Robles-Mezcua A, Rodríguez-Miranda L, Morcillo-Hidalgo L, et al. Phenotype and progression among patients with dilated cardiomyopathy and RBM20 mutations[J]. Eur J Med Genet, 2021, 64(9): 104278. DOI: 10.1016/j.ejmg.2021.104278. [DOI] [PubMed] [Google Scholar]
- 5. 李雪银, 李广平. 家族性扩张型心肌病基因突变与精准医学[J]. 中华心力衰竭和心肌病杂志(中英文), 2019, 3(4): 231-234. DOI: 10.3760/cma.j.issn.2096-3076.2019.04.009. [DOI] [Google Scholar]
- 6. Li M, Xia S, Xu L, et al. Genetic analysis using targeted next-generation sequencing of sporadic Chinese patients with idiopathic dilated cardiomyopathy[J]. J Transl Med, 2021, 19(1): 189. DOI: 10.1186/s12967-021-02832-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gaertner A, Klauke B, Brodehl A, et al. RBM20 mutations in left ventricular non-compaction cardiomyopathy[J]. Pediatr Investig, 2020, 4(1): 61-63. DOI: 10.1002/ped4.12184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liatakis I, Prappa E, Gouziouta A, et al. RBM20 mutation and ventricular arrhythmias in a young patient with dilated cardiomyopathy: a case report[J]. Am J Cardiovasc Dis, 2021, 11(3): 398-403. [PMC free article] [PubMed] [Google Scholar]
- 9. 彭昌, 徐合平, 王世远. RBM20基因新发错义突变致家族性儿童扩张性心肌病1例报告[J]. 临床儿科杂志, 2018, 36(12): 912-915. DOI: 10.3969/j.issn.1000-3606.2018.12.007. [DOI] [Google Scholar]
- 10. Maatz H, Jens M, Liss M, et al. RNA-binding protein RBM20 represses splicing to orchestrate cardiac pre-mRNA processing[J]. J Clin Invest, 2014, 124(8): 3419-3430. DOI: 10.1172/JCI74523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guo W, Schafer S, Greaser ML, et al. RBM20, a gene for hereditary cardiomyopathy, regulates titin splicing[J]. Nat Med, 2012, 18(5): 766-773. DOI: 10.1038/nm.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Watanabe T, Kimura A, Kuroyanagi H. Alternative splicing regulator RBM20 and cardiomyopathy[J]. Front Mol Biosci, 2018, 5: 105. DOI: 10.3389/fmolb.2018.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Koelemen J, Gotthardt M, Steinmetz LM, et al. RBM20-related cardiomyopathy: current understanding and future options[J]. J Clin Med, 2021, 10(18): 4101. DOI: 10.3390/jcm10184101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nishiyama T, Zhang Y, Cui M, et al. Precise genomic editing of pathogenic mutations in RBM20 rescues dilated cardiomyopathy[J]. Sci Transl Med, 2022, 14(672): eade1633. DOI: 10.1126/scitranslmed.ade1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gaertner A, Bloebaum J, Brodehl A, et al. The combined human genotype of truncating TTN and RBM20 mutations is associated with severe and early onset of dilated cardiomyopathy[J]. Genes (Basel), 2021, 12(6): 883. DOI: 10.3390/genes12060883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gaertner A, Klauke B, Felski E, et al. Cardiomyopathy-associated mutations in the RS domain affect nuclear localization of RBM20 [J]. Hum Mutat, 2020, 41(11): 1931-1943. DOI: 10.1002/humu.24096. [DOI] [PubMed] [Google Scholar]
- 17. Dauksaite V, Gotthardt M. Molecular basis of titin exon exclusion by RBM20 and the novel titin splice regulator PTB4 [J]. Nucleic Acids Res, 2018, 46(10): 5227-5238. DOI: 10.1093/nar/gky165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yamamoto T, Sano R, Miura A, et al. I536T variant of RBM20 affects splicing of cardiac structural proteins that are causative for developing dilated cardiomyopathy[J]. J Mol Med (Berl), 2022, 100(12): 1741-1754. DOI: 10.1007/s00109-022-02262-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang Y, Gregorich ZR, Wang Y, et al. Disruption of the nuclear localization signal in RBM20 is causative in dilated cardiomyopathy[J]. JCI insight, 2023, 8(13): e170001. DOI: 10.1172/jci.insight.170001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Murayama R, Kimura-Asami M, Togo-Ohno M, et al. Phosphorylation of the RSRSP stretch is critical for splicing regulation by RNA-binding motif protein 20 (RBM20) through nuclear localization[J]. Sci Rep, 2018, 8(1): 8970. DOI: 10.1038/s41598-018-26624-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sun M, Jin Y, Zhang Y, et al. SR protein kinases regulate the splicing of cardiomyopathy-relevant genes via phosphorylation of the RSRSP stretch in RBM20 [J]. Genes (Basel), 2022, 13(9): 1526. DOI: 10.3390/genes13091526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ihara K, Sasano T, Hiraoka Y, et al. A missense mutation in the RSRSP stretch of Rbm20 causes dilated cardiomyopathy and atrial fibrillation in mice[J]. Sci Rep, 2020, 10(1): 17894. DOI: 10.1038/s41598-020-74800-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang Y, Wang C, Sun M, et al. RBM20 phosphorylation and its role in nucleocytoplasmic transport and cardiac pathogenesis[J]. FASEB J, 2022, 36(5): e22302. DOI: 10.1096/fj.202101811RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Upadhyay SK, Mackereth CD. Structural basis of UCUU RNA motif recognition by splicing factor RBM20 [J]. Nucleic Acids Res, 2020, 48(8): 4538-4550. DOI: 10.1093/nar/gkaa168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fenix AM, Miyaoka Y, Bertero A, et al. Gain-of-function cardiomyopathic mutations in RBM20 rewire splicing regulation and re-distribute ribonucleoprotein granules within processing bodies[J]. Nat Commun, 2021, 12(1): 6324. DOI: 10.1038/s41467-021-26623-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. 郑奎, 娄美娜. TTN基因突变致儿童扩张型心肌病的研究进展[J]. 中国当代儿科杂志, 2023, 25(2): 217-222. DOI: 10.7499/j.issn.1008-8830.2208163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lennermann D, Backs J, van den Hoogenhof MMG. New insights in RBM20 cardiomyopathy[J]. Curr Heart Fail Rep, 2020, 17(5): 234-246. DOI: 10.1007/s11897-020-00475-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Domínguez F, Lalaguna L, Martínez-Martín I, et al. Titin missense variants as a cause of familial dilated cardiomyopathy[J]. Circulation, 2023, 147(22): 1711-1713. DOI: 10.1161/CIRCULATIONAHA.122.062833. [DOI] [PubMed] [Google Scholar]
- 29. Li N, Hang W, Shu H, et al. RBM20, a therapeutic target to alleviate myocardial stiffness via titin isoforms switching in HFpEF[J]. Front Cardiovasc Med, 2022, 9: 928244. DOI: 10.3389/fcvm.2022.928244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. van den Hoogenhof MMG, Beqqali A, Amin AS, et al. RBM20 mutations induce an arrhythmogenic dilated cardiomyopathy related to disturbed calcium handling[J]. Circulation, 2018, 138(13): 1330-1342. DOI: 10.1161/CIRCULATIONAHA.117.031947. [DOI] [PubMed] [Google Scholar]
- 31. Long C, Liu X, Xiong Q, et al. Sex differences in dilated cardiomyopathy prognosis[J]. Int Heart J, 2022, 63(1): 36-42. DOI: 10.1536/ihj.20-448. [DOI] [PubMed] [Google Scholar]
- 32. Hey TM, Rasmussen TB, Madsen T, et al. Pathogenic RBM20-variants are associated with a severe disease expression in male patients with dilated cardiomyopathy[J]. Circ Heart Fail, 2019, 12(3): e005700. DOI: 10.1161/CIRCHEARTFAILURE.118.005700. [DOI] [PubMed] [Google Scholar]
- 33. Lennermann DC, Pepin ME, Grosch M, et al. Deep phenotyping of two preclinical mouse models and a cohort of RBM20 mutation carriers reveals no sex-dependent disease severity in RBM20 cardiomyopathy[J]. Am J Physiol Heart Circ Physiol, 2022, 323(6): H1296-H1310. DOI: 10.1152/ajpheart.00328.2022. [DOI] [PubMed] [Google Scholar]
- 34. Liss M, Radke MH, Eckhard J, et al. Drug discovery with an RBM20 dependent titin splice reporter identifies cardenolides as lead structures to improve cardiac filling[J]. PLoS One, 2018, 13(6): e0198492. DOI: 10.1371/journal.pone.0198492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Briganti F, Sun H, Wei W, et al. iPSC modeling of RBM20-deficient DCM identifies upregulation of RBM20 as a therapeutic strategy[J]. Cell Rep, 2020, 32(10): 108117. DOI: 10.1016/j.celrep.2020.108117. [DOI] [PMC free article] [PubMed] [Google Scholar]


