Abstract
Background:
Since 2012, WHO has recommended influenza vaccination for health care workers (HCWs), which has different costs than routine infant immunization; however, few cost estimates exist from low- and middle-income countries. Albania, a middle-income country, has self-procured influenza vaccine for some HCWs since 2014, supplemented by vaccine donations since 2016 through the Partnership for Influenza Vaccine Introduction (PIVI). We conducted a cost analysis of HCW influenza vaccination in Albania to inform scale-up and sustainability decisions.
Methods:
We used the WHO’s Seasonal Influenza Immunization Costing Tool (SIICT) micro-costing approach to estimate incremental costs from the government perspective of facility-based vaccination of HCWs in Albania with trivalent inactivated influenza vaccine for the 2018–19 season based on 2016–17 season data from administrative records, key informant consultations, and a convenience sample of site visits. Scenario analyses varied coverage, vaccine presentation, and vaccine prices.
Results:
In the baseline scenario, 13,377 HCWs (70% of eligible HCWs) would be vaccinated at an incremental financial cost of US$61,296 and economic cost of US$161,639. Vaccine and vaccination supplies represented the largest share of financial (89%) and economic costs (44%). Per vaccinated HCW financial cost was US$4.58 and economic cost was US$12.08 including vaccine and vaccination supplies (US$0.49 and US$6.76 respectively without vaccine and vaccination supplies). Scenarios with higher coverage, prefilled syringes, and higher vaccine prices increased total economic and financial costs, although the economic cost per HCW vaccinated decreased with higher coverage as some costs were spread over more HCWs. Across all scenarios, economic costs were <0.07% of Albania’s estimated government health expenditure, and <5.07% of Albania’s estimated immunization program economic costs.
Conclusions:
Cost estimates can help inform decisions about scaling up influenza vaccination for HCWs and other risk groups.
Keywords: Influenza, Economic evaluation, Cost analysis, Health care workers, Low- and middle-income countries
1. Introduction
Every year, an estimated 290,000–650,000 deaths occur due to influenza-associated respiratory diseases [1]. Since 2012, the World Health Organization (WHO) has recommended influenza vaccination for risk groups including health care workers (HCWs), pregnant women, children aged 6–59 months, the elderly, and patients with chronic diseases [2]. Vaccinating these risk groups with a seasonal vaccine for which formulations change each year requires different forecasting, procurement, and service delivery strategies than the year-round routine immunization program targeting infants, with different cost implications for national health systems [3]. Understanding the program costs to deliver influenza vaccine to these at-risk groups is especially important for vaccine introduction and sustainability decisions in low- and middle-income countries (LMICs), in which recent research suggests influenza burden may be higher than previously thought [1]. Resources for health care in LMICs are more constrained, however, and existing vaccination systems are often not oriented towards adult vaccination [4].
Systematic reviews have noted gaps in the availability of economic evidence to inform influenza vaccine decision making in LMICs [5–8]. Recent cost-effectiveness studies in LMICs have used program cost data for other vaccines and/or drawn from settings other than the study country [9,10]. Some recent studies have contributed towards filling this gap, including hypothetical program cost analyses for pregnant women [11], or primary cost data collected alongside a clinical trial for children [12]. However, there remain few studies reporting influenza vaccination program costs based on empirical primary data collection in LMICs for each of the recommended risk groups, in particular for HCWs. WHO recommends that HCWs receive influenza vaccination to protect themselves and their patients as an important part of health facility infection control and pandemic preparedness strategies [2]. Nonetheless, to our knowledge, only one previously published study has reported influenza vaccination program costs for HCWs in a LMIC context [13].
Accordingly, this manuscript presents results from a program cost analysis of seasonal influenza vaccination of HCWs in Albania, a middle-income country in southeastern Europe that transitioned out of Gavi support in 2013 and now largely self-finances its national immunization program (NIP) [14]. The cost analysis was conducted during a pilot of the WHO’s Seasonal Influenza Immunization Costing Tool (SIICT), part of the WHO’s influenza economic “value chain” toolkit and guidance materials to assist governments in generating the economic data needed to inform priority setting and resource allocation for influenza vaccination introduction and expansion [3,11,15]. Albania was selected because it (i) had an established government-funded influenza vaccination program for HCWs that permitted data collection on actual (rather than hypothetical) vaccine delivery costs, and (ii) was in the planning process to expand and sustain government support for influenza vaccination to HCWs and other risk groups (Box 1). Albania currently receives support from the Partnership for Influenza Vaccine Introduction (PIVI), a non-profit public-private partnership that facilitates technical assistance to partner country governments and donations from industry partners to implement influenza vaccination programs [16]. This cost analysis provides insight into the resource requirements, cost drivers, and options for the government of Albania and other middle-income country governments considering scaling up influenza vaccination for HCWs, as well as unit cost estimates that can inform research and policy consideration of the economic value of influenza vaccination.
Box 1. Albania influenza vaccination program overview.
Burden: Based on 2014–15 sentinel surveillance data, Albania’s estimated incidence of influenza-associated severe acute respiratory infection (SARI) was 10.3 cases per 100,000 population, with an in-hospital case fatality ratio of 0.6% [23].
Target groups: Albania’s Ministry of Health and Social Protection (MoHSP) has had an influenza vaccine policy for target groups since 2007. Influenza vaccine is currently recommended by Albania’s National Immunization Technical Advisory Group (NITAG) for all people beyond six months of age with government cost subsidies prioritized for: HCWs; children (six months-18 years old); elderly (≥65 years old); individuals with chronic health conditions (respiratory/lung conditions, heart and renal disease, diabetes, immunodeficiencies and other chronic illnesses, obesity); and pregnant women.
Influenza vaccination program history: Influenza vaccination has been offered to HCWs since the 2009 influenza A/H1N1 pandemic, and through a formal program since 2014 when the MoHSP began procuring influenza vaccine in prefilled syringes with funds from the national Health Insurance Fund. Influenza vaccination was offered to HCWs from October to January during the 2014–15, 2015–16 and 2016–17 seasons, but the quantity procured by the government (approximately 10,000 doses each year) covered only 50% of HCWs. Influenza vaccine is offered for all target groups in the private market without any insurance refund or public subsidy policy, and the quantity of vaccine provided in the private market is less than 30% of total doses. Aside from a small-scale World Bank-funded project in 2008–10 and pandemic influenza vaccine procurement in 2009–10, there has been no government or health insurance funding for influenza vaccination of non-HCW populations. In late 2016, through a PIVI-coordinated donation, Albania received 93,500 doses of WHO-prequalified trivalent inactivated influenza vaccine (2016–17 Northern Hemisphere formulation) in single-dose vials without syringes from Hualan Biological Bacterin Co, Ltd, China. Approximately 80% of these doses were administered to vaccinate the remaining HCWs up to the 70% coverage target, as well as other risk groups (pregnant women, elderly persons, and patients with underlying chronic conditions) not covered by government-procured vaccine.
HCW influenza vaccination implementation: The influenza vaccination program for HCWs includes activities and funding by several different government units in Albania. The MoHSP conducts procurement for HCW influenza vaccine based on orders from health facilities with funding from the national Health Insurance Fund. Every health facility funded by the Health Insurance Fund is required to sign a contract with the local distribution company that won the MoHSP tender, and to reimburse a portion of their national Health Insurance Fund allocations to the MoHSP for the number of influenza doses ordered. The Albania Institute of Public Health (the national public health agency under the MoHSP, which is responsible for the NIP and procurement and distribution of all other routine vaccines), provides surveillance, training, social mobilization, and monitoring for the seasonal influenza campaign from their own agency budget allocated to them by MoHSP. At district level, Directorate of Public Health (DPH) staff provide training, coordinate vaccine distribution, and conduct supervision and monitoring with their own budget allocated to them by the MoHSP’s DPH department, (which is separate from the national-level IPH). Health facility staff salaries are paid by the MoHSP through other budget lines unrelated to the national Health Insurance Fund, IPH, or DPH department.
Distribution of PIVI-donated vaccine for HCWs: For PIVI-donated vaccine, IPH used its existing vehicles, cold chain, and staff to distribute the vaccine to DPHs and health facilities in Tirana, and syringes were provided by health facilities from their existing stocks. There are no systematic differences in which HCWs receive the government-procured or the PIVI-donated vaccines by geography or health system level, except that the PIVI-donated vaccines typically arrive later in the season. PIVI-donated vaccines are distributed to all districts in Albania based on quantities requested by health facilities and where health facilities still have HCWs interested in receiving vaccination at the time that PIVI-donated vaccines are available.
2. Methods
2.1. Study design
The cost analysis estimated incremental financial and economic costs of an influenza vaccination program for HCWs from a provider perspective of the Government of Albania, inclusive of the Ministry of Health and Social Protection (MoHSP), national Health Insurance Fund, Institute of Public Health (IPH), Directorates of Public Health (DPHs), and government-funded health care facilities throughout the country. Financial costs were considered to be those incremental monetary expenditures made by the Government of Albania for the HCW influenza vaccination program, while economic costs included all financial costs as well as the value of existing government resources and donations from external partners (e.g., PIVI-donated vaccines) for the HCW influenza vaccination program [17]. The cost analysis time frame for which costs were measured was the 2016–17 flu season, and the analytic horizon for which intervention costs were projected was the 12-month calendar year 2018 for the 2018–19 flu season. Cost categories and program activities included in the analysis were drawn from the SIICT manual and tool (Supplemental Table 1) [17]. Costs related to the pilot project itself and the value of HCW time spent receiving vaccination were excluded from the analysis. The baseline scenario analyzed influenza vaccination to cover 70% of Albania’s 19,110 eligible HCWs using service delivery strategies similar to those deployed during the 2016–17 influenza season at primary, secondary, and tertiary health facilities (Table 1).
Table 1.
Program cost analysis of influenza vaccination of health care workers in Albania: Baseline scenario inputs summary.
| Scenario element | Description | Source |
|---|---|---|
|
| ||
| Target population | 19,110 eligible HCWs | Albania IPH |
| Target coverage rate | 70% (13,377 HCWs); same target coverage rate assumed across all health facilities | Albania IPH |
| Vaccine type | Trivalent inactivated influenza vaccine (Northern Hemisphere formulation) | Albania IPH |
| Vaccine procurement source |
Government-procured: 10,100 doses (including 1% wastage); PIVI-donated: 3546 doses (including 5% wastage); difference in wastage rate due to different vaccine presentations, with lower wastage for prefilled syringes compared to single dose vials. |
Doses: Albania IPH, PIVI Wastage rates: Albania IPH, [29] |
| Vaccine presentation |
Government-procured: pre-filled syringes; PIVI-donated: single-dose vials (syringes procured separately by government) |
Albania IPH, PIVI |
| Vaccine price |
Government-procured: US$5.30 per dose (inclusive of customs) PIVI-donated: US$4.54 per dose (inclusive of customs, plus US$0.04 for government-procured syringe) |
Albania IPH, PIVI, local pharmaceutical distributor |
| Service delivery strategy: Tertiary hospitals (n = 5) and large secondary hospitals (n = 11) with >200 HCWs per facility (18% of HCWs) | Teams of one doctor and one nurse in their own facility dedicated full-time to influenza vaccination performing average 20 vaccinations per working day (incremental cost = days of work throughout flu campaign period) | Assumption based on health facility site visits |
| Service delivery strategy: Mid-size secondary hospitals (n = 11) with 100-199 HCWs per facility (31% of HCWs) | Teams of two nurses in their own facility dedicated full-time to influenza vaccination performing average 20 vaccinations per working day (incremental cost = days of work throughout flu campaign period) | Assumption based on health facility site visits |
| Service delivery strategy: Small secondary hospitals (n = 13) and primary health care facilities (n = 415) with <100 HCWs per facility (51% of HCWs) | Vaccination by facility nurse integrated with regular duties (incremental cost = minutes of nurse’s time per vaccination) | Assumption based on health facility site visits |
HCWs: Health Care Workers.
2.2. Data collection
Data collection and analysis was conducted using the SIICT, an Excel ingredients-based microcosting tool to support planning and cost projections for influenza vaccine introduction [17], an earlier version of which (FLUTool) was piloted in Malawi for the risk group of pregnant women [11]. Data on the type, quantity, and prices of resources used for influenza vaccination activities at all health system levels were collected from government records and a convenience sample of four site visits to health facilities and IPH and DPH facilities during an in-country mission in April 2017, as well as consultations with staff from IPH, MoHSP, the Task Force for Global Health, and the local pharmaceutical supplier for government-procured vaccine (Table 1).
2.3. Baseline scenario analysis
Total financial and economic costs were estimated by multiplying the quantities of resources used (e.g., personnel, per diems, supplies) by their financial and/or economic unit costs and then summing by program activity and overall. Total costs were also disaggregated by recurrent costs (all cost categories except cold storage and capital investments), initial investment costs (nonannualized new capital purchases), and annualized capital costs (assuming a discount rate of 3% for economic costs and straight-line discounting for financial costs); as the cost analysis covers a one-year analytic horizon, no other discounting was performed and all other costs were categorized as recurrent costs. Costs were inflated by 2% based on average annual inflation rates for 2017–18 [18], but otherwise prices from 2016 to 17 were assumed to be indicative of prices in 2018–19. Results were converted into U.S. dollars using an exchange rate of 127 Albanian lek per dollar [19]. Total economic and financial costs were divided by the projected number of HCWs vaccinated under each scenario to obtain cost per HCW vaccinated.
2.4. Scenario analysis and comparison to immunization program budget and government health spending
Alternative scenarios varying coverage (80–85%), vaccine presentation (pre-filled syringes or single-dose vials), and vaccine price (from US$4.54 per dose for PIVI-donated vaccine to US$5.30 per dose for government-procured vaccine, including customs) were explored to compare total and per HCW program costs to the baseline scenario. All alternative scenarios assumed no PIVI donations, i.e., that the Albanian government procures all vaccine and associated supplies regardless of presentation and price, and held wastage rates constant with baseline. Alternative scenarios A, B, and C maintained vaccine prices at current government-procured and PIVI-procured rates; alternative scenarios D and E assumed a single-dose vial vaccine price of US$5.00 per dose for sustainability planning purposes. Syringe costs were held constant with current prices (US$0.04 per syringe) for all single-dose vial scenarios.
Total costs from each scenario were compared to Albania’s estimated NIP recurrent costs and to total government health expenditures (GHE) in 2016. Albania’s estimated annual NIP recurrent costs included vaccine and supplies, IPH human resources costs (salaries and 16.7% benefits) [20], transportation and per diem for vaccine distribution from IPH to DPHs, maintenance and overhead for cold chain equipment and vehicles, and vaccination service delivery time by health facility personnel (assuming 20 minutes per vaccination dose administered, an average health worker salary plus 16.7% benefits of 48,430 Lek per month [21,22], and 635,000 doses administered (birth to age 18) in 2016). IPH and health facility human resource costs were considered economic costs only. Information, education, and communication (IEC)/social mobilization costs for leaflet printing and media training covered by donor funds (WHO) in 2016 were included as an economic cost. All other NIP recurrent costs were considered to be both financial and economic costs for comparability with the perspective used in the SIICT pilot. Albania’s 2016 GHE included budget lines for primary health care, secondary health care, emergency services, public health, and management and administration, all of which were related to aspects of the HCW influenza vaccination program and therefore included in the comparison with scenario results [24].
3. Results
3.1. Baseline scenario results: Total HCWs vaccinated, total costs, and cost per vaccinated HCW
Under the baseline scenario, 13,377 HCWs (70% of 19,110 HCWs eligible for influenza vaccination) would be vaccinated at an estimated total incremental financial cost of US$61,296 and economic cost of US$161,639 in 2018 US dollars (Table 2). As there were no incremental capital costs identified, the total costs are equal to the total incremental recurrent annual costs for the program; all activities were reported to be conducted annually (including training, microplanning, and IEC/social mobilization). Vaccine price per dose, inclusive of customs clearance, was $4.54 for single-dose vials procured by PIVI and $5.30 for pre-filled syringes procured by the Albanian government. Including the cost of vaccine and vaccination supplies (syringes, safety boxes), the financial cost per vaccinated HCW was US$4.58 and the economic cost per vaccinated HCW was US$12.08 (US$0.49 and US$6.76 respectively without vaccine and vaccination supplies).
Table 2.
Total financial and economic costs by recurrent and capital costs and program activity: influenza vaccination of health care workers in Albania (baseline scenario, 2018–19, in 2018 US dollars).
| Cost Type | Program Activity | Financial Cost (US $) |
Economic Cost (US $) |
|---|---|---|---|
|
| |||
| Recurrent Costs | Microplanning | $442 | $3431 |
| Procurement | $54,745 | $71,175 | |
| Distribution | $712 | $12,317 | |
| Training | $292 | $17,840 | |
| Social Mobilization/ | $1802 | $22,162 | |
| IEC Immunization |
$0 | $16,921 | |
| Service Delivery | |||
| Supervision and Monitoring | $3303 | $17,795 | |
| Other Activities | $0 | $0 | |
| Subtotal | $61,296 | $161,639 | |
| Capital Costs | Cold Storage | $0 | $0 |
| (annualized) | Expansion | ||
| Vehicle Acquisition | $0 | $0 | |
| Other Equipment | $0 | $0 | |
| Acquisition | |||
| Other Capital Costs | $0 | $0 | |
| Subtotal | $0 | $0 | |
| TOTAL PROJECTED ANNUAL COSTS | $61,296 | $161,639 | |
3.2. Baseline scenario results: total costs by program activity
Procurement of vaccine represented the program activity with the largest share of both financial costs (89%; US$54,745) and economic costs (44%; US$71,175) (Table 2, Fig. 1, Supplemental Fig. 1). Under the procurement activity, financial costs of procurement included government purchase of vaccine, syringes for the PIVI-donated vaccine in vials, and customs clearance for the PIVI-donated vaccine, while economic costs also included the value of the PIVI-donated vaccine and existing safety boxes. For financial costs, the next largest program activities were supervision and monitoring (5%; US$3,303) and social mobilization/information, education, and communication (IEC) (3%; US$1802); all other program activities represented less than 1% of financial costs. For economic costs, the next largest program activities were social mobilization/IEC (14%; US$22,162), training (11%; US$17,840), and supervision and monitoring (11%; US$17,795). Service delivery immunization activities (i.e., vaccination of HCWs in health facilities) incurred no financial costs and represented only 10% (US $16,921) of economic costs, reflecting mainly the opportunity cost of in-kind government personnel time for those HCWs conducting vaccinations.
Fig. 1.
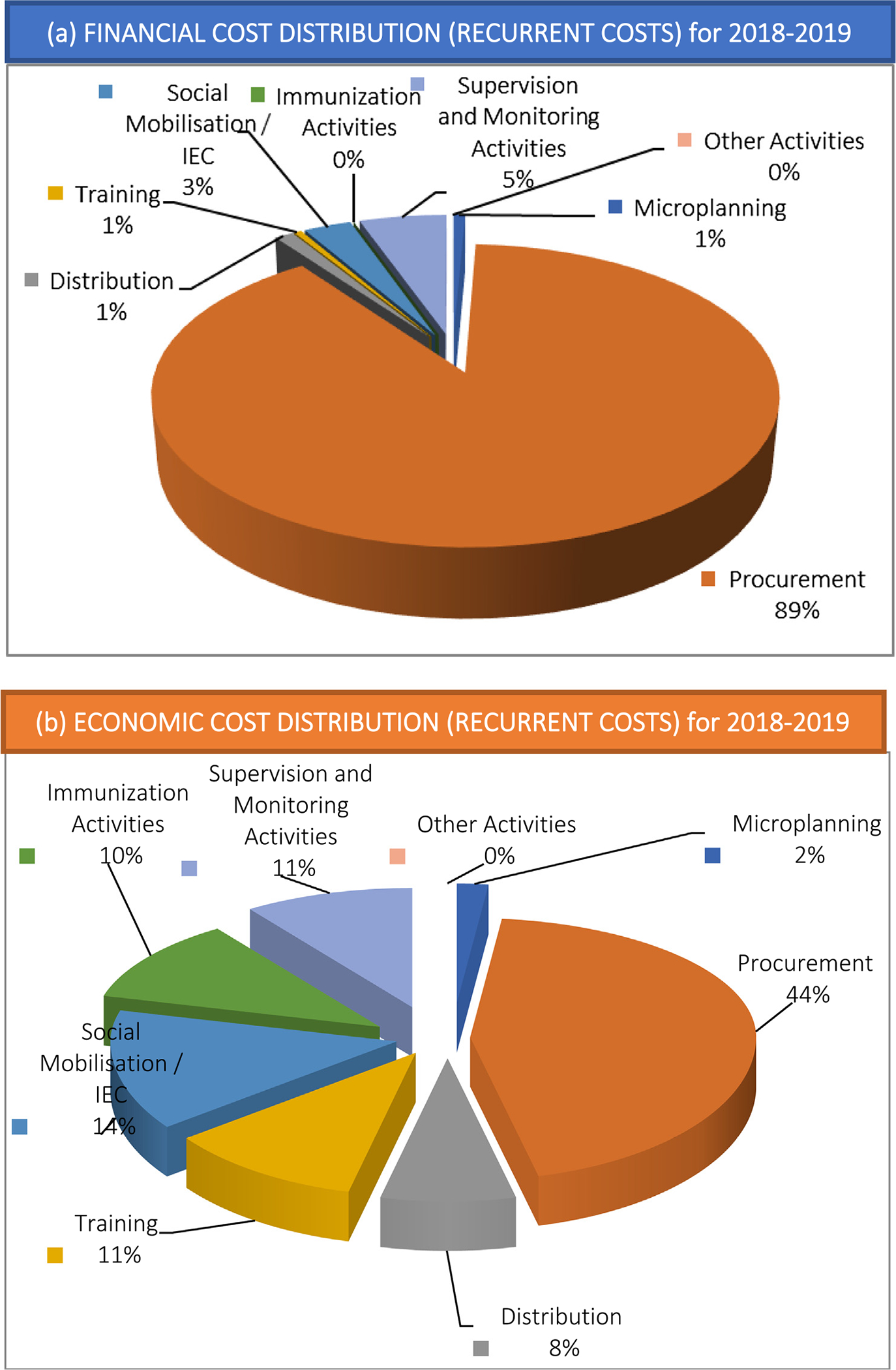
Distribution by program activity of (a) financial costs and (b) economic costs for influenza vaccination of health care workers in Albania (baseline scenario 2018–19)*. (*Note: As there were no incremental capital costs identified, the total costs are equal to the total incremental recurrent annual costs for the program; all activities were reported to be conducted annually (including training, microplanning, and IEC/social mobilization).)
3.3. Alternative scenario analysis costs
Compared to baseline, alternative scenarios with higher coverage targets (scenarios B–E), pre-filled syringes (scenarios A, B), and higher vaccine prices increased total economic and financial costs (Table 3). All alternative scenarios represented an increase in financial costs per HCW vaccinated compared to baseline (due to increased vaccine procurement costs as more expensive prefilled syringe doses were substituted for single-dose vials in scenarios A and B, or as coverage increased in scenarios B–E). The economic costs per HCW vaccinated were lower under alternative scenarios with higher coverage targets (scenarios B–E) due to the effect of some costs (e.g., planning, distribution to DPHs, training) which remain the same in each of the scenarios but which were spread over a larger number of vaccinated HCWs.
Table 3.
Alternative scenario analysis results (costs in 2018 US$).
| Scenario | Assumed coverage |
Procurement Volume |
Total vaccine (procurement + syringe) cost* | Total cost of scenario |
Cost per HCW vaccinated (including vaccine) |
Vaccine cost as % of total cost |
Total cost as % of total government health expenditure |
Total cost as % of immunization program cost |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-filled syringe doses | Single dose vials | Financial | Economic | Financial | Economic | Financial | Economic | Financial | Economic | Financial | Economic | |||
|
| ||||||||||||||
| Baseline | 70% | 10,100 | 3,546 | $71,175 | $61,296 | $161,639 | $4.58 | $12.08 | 89% | 44% | 0.02% | 0.06% | 2.29% | 4.75% |
| Alternative A | 70% | 13,511 | 0 | $73,040 | $79,591 | $163,506 | $5.95 | $12.22 | 92% | 45% | 0.03% | 0.06% | 2.98% | 4.80% |
| Alternative B | 80% | 15,441 | 0 | $83,483 | $90,024 | $172,614 | $5.89 | $11.29 | 93% | 48% | 0.04% | 0.07% | 3.37% | 5.07% |
| Alternative C | 80% | 0 | 16,053 | $75,003 | $80,888 | $164,164 | $5.29 | $10.74 | 92% | 46% | 0.03% | 0.06% | 3.02% | 4.82% |
| Alternative D | 80% | 0 | 16,053 | $82,535 | $88,421 | $171,666 | $5.78 | $11.23 | 93% | 48% | 0.03% | 0.07% | 3.31% | 5.04% |
| Alternative E | 85% | 0 | 17,056 | $87,691 | $93,536 | $178,962 | $5.76 | $11.02 | 94% | 49% | 0.04% | 0.07% | 3.50% | 5.25% |
Scenarios A, B: current government-procured price for pre-filled syringe doses (inclusive of customs clearance): US$5.30; Scenario C: current PIVI price for single-dose vials (inclusive of customs clearance): US$4.54. Scenarios D, E: assumed future PIVI sustainability plan price for single-dose vials: US$5.00; Scenarios C, D, E: current price for syringe: US$0.04.
3.4. Comparison to immunization program budget and government health spending
All scenarios, including the baseline scenario, represent a very small share of Albania’s 2016 GHE of US$256.4 million (in 2018 US$, Table 3) [24]. Across all scenarios, financial costs were 0.04% or less of GHE, while economic costs were 0.07% or less. Economic costs were compared to GHE as none of the alternative scenarios included PIVI donations or incremental capital costs, and therefore the scenario economic costs are all funded from annual GHE. Similarly, the financial costs of all scenarios represent a small share of Albania’s estimated 2016 NIP financial cost of US$2.67 million (in 2018 US$), ranging from 2.29% for the baseline scenario to 3.50% for scenario E (85% coverage, procuring only single dose vials) (Table 3). Compared to estimated 2016 NIP economic costs of US $3.41 million (in 2018 US$), the economic costs of all scenarios ranged from 4.75% for the baseline scenario to 5.25% for scenario E.
4. Discussion
Although vaccine procurement costs represented the largest share of both financial and economic costs in Albania’s estimated influenza vaccination program costs for HCWs, the scenario analyses indicated that there might be opportunities to reduce vaccine prices if the government can access alternative presentations and suppliers. Switching from single-dose to multi-dose vials may represent another opportunity to reduce vaccine costs. Such reductions in vaccine price should be weighed against other factors contributing to successful scale-up and sustainability of influenza vaccination in Albania and elsewhere, such as perceptions of the quality of vaccines sourced from different manufacturers and implications for vaccination demand among target groups. Pooled procurement mechanisms in which Albania’s order is grouped with that of other countries to access a lower vaccine price for a larger volume purchased may be another option to reduce vaccine procurement costs; currently, vaccine procurement through UNICEF is the only available mechanism in Albania other than government self-procurement. Information about what other countries are paying for vaccines, such as that available through the WHO’s Market Information for Access to Vaccines (M4IA) platform [25], may also help middle-income countries such as Albania better negotiate with vaccine manufacturers and distributors.
The results of this analysis revealed large net economic costs (i.e., remaining economic costs after deducting financial costs) representing substantial in-kind investment from government, particularly of government personnel time (48% of economic costs), with the value of PIVI-donated vaccine doses accounting for only 10% of economic costs. Even though PIVI vaccine was donated, the government of Albania used existing personnel, cold chain equipment, and vehicles to distribute it, totaling 8% of economic costs. Quantifying these opportunity costs of existing health system resource use is important in all new vaccine introduction decisions but especially so for seasonal influenza vaccination given the intensive effort required during the seasonal campaigns; as previous literature suggests potential negative impacts on other health services during vaccination campaigns [26–28], additional human and equipment resources may need to be allocated to ensure successful scale-up of seasonal influenza vaccination efforts.
The government of Albania increased its vaccine purchase in the 2018–19 season in accordance with the multi-year sustainability plan developed with PIVI. PIVI support and vaccine donations were reduced in the 2018–19 influenza season and are scheduled to phase out completely after the 2020–21 flu season, requiring increased financial commitments to vaccine procurement from the government of Albania to maintain influenza vaccination coverage for HCWs and other risk groups that have benefited from PIVI-donated vaccines. While this scheduled reduction in PIVI financial support and donation quantity year-over-year motivated the cost analysis, the phase out was not explicitly modeled in the baseline or scenario analysis. Even for the highest cost scenarios explored, the analysis suggests that influenza vaccination for HCWs is likely to represent only a small share of the national immunization program budget and of government health expenditures overall. Moreover, the process of conducting the SIICT pilot enabled immunization program staff to identify opportunities to increase efficiency and reduce costs in vaccine distribution, social mobilization, and service delivery. Using the results of this cost analysis in cost-effectiveness and budget impact analyses can help inform resource allocation decisions between sustained or increased influenza vaccination versus other health investments.
There are few other studies in LMIC settings against which to compare the estimated costs of influenza vaccination for HCWs in Albania. In a cost-effectiveness analysis of influenza vaccination of HCWs in Colombia as a strategy to prevent influenza infection among cancer patients, Chicaiza-Becerra included only vaccine costs ($2.32 per dose in 2007 US$) [13], assuming that other logistical costs were negligible, which the current analysis demonstrates they are not. Further empirical program cost analyses of this type are needed in other LMICs to build the evidence base about influenza vaccination strategies for different risk groups.
4.1. Limitations
The analysis results are subject to several limitations. First, the pilot team visited only a few sites in Tirana and Durres, and did not visit any secondary-level (district) hospital or sites in more rural areas. The service delivery models used at the sites visited may not be representative of all sites in Albania; however, these assumed models were validated with the IPH pilot team members who had visited and provided supervision and technical assistance to DPH and health facilities throughout the country. Second, past prices from the 2016–17 influenza vaccination campaign may not be a good indication of future prices, although the IPH influenza program managers did not anticipate any particular price changes for the resources analyzed. Third, some activities included in the pilot scenario were new activities for 2018–19 season (e.g., printing of IEC posters) and estimated resource quantities and prices for these activities were based on budget projections and price quotations; actual costs may differ once implemented. Fourth, some costs (e.g., IPH personnel time) were difficult to extract and itemize as influenza-specific costs from shared costs, which may have led to over- or under-estimation of total costs. Fifth, economic costs may be underestimated as the SIICT test version used for the pilot did not include functionality to estimate the economic cost of existing capital equipment used (e.g., vehicles, cold storage), only the value of new capital investments. Finally, as the tool adopts a one-year analytic horizon, costs of non-capital activities that may not need to be repeated every year (e.g., development of IEC materials) are not amortized over multiple years, potentially overestimating these costs in the first year and underestimating them in subsequent years; however, as influenza strains and vaccines vary annually, the pilot team felt that most activities for influenza vaccination campaign preparation would need to be repeated each year to provide updated information.
5. Conclusions
This cost analysis provides insight into resource requirements and cost drivers for Albania and potentially other middle-income countries considering scaling up influenza vaccination for HCWs, as well as unit cost estimates that can inform cost-effectiveness and budget impact analyses to characterize the economic value of influenza vaccination. In addition to providing cost estimates, program cost analysis exercises, such as that conducted in Albania using the SIICT, can aid program planning and budget advocacy by engaging relevant program and financing stakeholders in developing scenarios, articulating assumptions, and reviewing results of vaccination strategies and resources required. The SIICT can be used to estimate program costs for multiple risk groups and strategies as one component in the WHO economic value chain toolkit and guidance materials to characterize the economic and social costs and benefits of influenza vaccination, which together can better inform influenza vaccine introduction decisions and broader health resource allocation.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the input received during pilot planning, execution, and/or manuscript preparation from the following (affiliations at the time of the study): Marc Perut (WHO), Raymond Hutubessy (WHO), Philipp Lambach (WHO), Justin Ortiz (WHO), Pernille Jorgenson (WHO), Eduardo AzzizBaumgartner (CDC), Joseph Bresee (CDC), Susan Chu (CDC), Anna Hidle (CDC), Ledia Agolli (SECID), Artan Simaku (IPH), and officials at the Albanian Ministry of Health and the sites visited during the pilot mission.
Funding
This work was supported by funding from the U.S. Centers for Disease Control and Prevention.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Disclaimer
The views expressed in this report are those of the authors and do not reflect the official positions of the U.S. Centers for Disease Control and Prevention or Albania Institute of Public Health.
Ethical clearance
The project was determined to not be human subjects research by the U.S. Centers for Disease Control and Prevention.
Appendix A. Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2019.10.027.
References
- [1].Iuliano AD et al. Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. Lancet 2018;391(10127):1285–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].World Health Organization. Meeting of the Strategic Advisory Group of Experts on immunization, April 2012 – conclusions and recommendations. Wkly Epidemiol Rec 2012;87(21):201–16. [PubMed] [Google Scholar]
- [3].Newall AT et al. WHO guide on the economic evaluation of influenza vaccination. Influenza Other Respi Viruses 2017;12:211–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Neuzil KM et al. Data and product needs for influenza immunization programs in low- and middle-income countries: Rationale and main conclusions of the WHO preferred product characteristics for next-generation influenza vaccines. Vaccine 2017;35(43):5734–7. [DOI] [PubMed] [Google Scholar]
- [5].Peasah SK et al. Influenza cost and cost-effectiveness studies globally – A review. Vaccine 2013;31:5339–48. [DOI] [PubMed] [Google Scholar]
- [6].Imai C et al. A systematic review and meta-analysis of the direct epidemiological and economic effects of seasonal influenza vaccination on healthcare workers. PLoS One 2018;13(6):e0198685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ott JJ et al. Influenza vaccines in low and middle income countries. Human Vacc Immunother 2013;9(7):1500–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Francisco de, Shapovalova N et al. A systematic review of the social and economic burden of influenza in low- and middle-income countries. Vaccine 2015;33:6537–44. [DOI] [PubMed] [Google Scholar]
- [9].de Boer PT et al. The cost-effectiveness of trivalent and quadrivalent influenza vaccination in communities in South Africa, Vietnam and Australia. Vaccine 2018;36(7):997–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Orenstein E et al. Cost-effectiveness of maternal influenza immunization in Bamako, Mali: a decision analysis. PLoS One 2017;12(2):e0171499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Pecenka C et al. Maternal influenza immunization in Malawi: Piloting a maternal influenza immunization program costing tool by examining a prospective program. PLoS One 2017;12(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kittikraisak W et al. Cost-effectiveness of inactivated seasonal influenza vaccination in a cohort of Thai children <60 months of age. PLoS One 2017;12 (8):e0183391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Chicaíza-Becerra LA et al. Evaluación Económica de la Vacuna contra la Influenza aplicada al Personal de Salud que Atiende Pacientes Oncológicos Hospitalizados. Rev. Salud Pública 2008;10(5):756–66. [DOI] [PubMed] [Google Scholar]
- [14].Zoidze A, Goguadze K, Chikovani I. Final evaluation of Gavi support to Albania: final report. Tbilisi: Curatio Foundation; 2015. [Google Scholar]
- [15].World Health Organization. Maternal influenza immunization: Guidance to inform introduction of influenza vaccine in low and middle-income countries: Economic guidance. 2018. [cited 2018 September 25]; Available from: http://www.who.int/immunization/research/development/influenza_maternal_immunization/en/index2.html.
- [16].Partnership for Influenza Vaccine Introduction (PIVI). PIVI’s work with Albania. 2017. [cited 2019 April 10]; Available from: https://pivipartners.org/albania/.
- [17].World Health Organization. WHO maternal seasonal influenza vaccine programme: planning and costing user’s guide (pilot version 1.0). Geneva: World Health Organization; 2016. [Google Scholar]
- [18].Albania Institute of Statistics. Consumer Price Index; 2018. [cited 2019 May 29]; Available from: http://www.instat.gov.al/en/themes/prices/consumer-price-index/#tab2.
- [19].Bank of Albania. Exchange rate archive; 2017. [cited 2019 April 30]; Available from:https://www.bankofalbania.org/Markets/Official_exchange_rate/Exchage_rate_archive/.
- [20].Decision Counsel of Minister, Decision Counsel of Minister (DCM) no. 555 on 11 August 2011. Tirana: Government of Albania; 2013. [Google Scholar]
- [21].Administrative Board Commission (ABC) of Health Insurance Institute, Order no. 9 on 31 July 2008. Tirana: Government of Albania; 2008. [Google Scholar]
- [22].Administrative Board Commission (ABC) of Health Insurance Institute, Order no. 29 on 29 August 2013. Tirana: Government of Albania; 2013. [Google Scholar]
- [23].Bino S, Simaku A, Olsen SJ. Annual Influenza Epidemics and Burden in Albania, 2009–2018. Unpublished internal data. Tirana: Institute for Public Health; 2017. [Google Scholar]
- [24].Ministria E Shendetesise dhe Mbrojtjes Sociale. Tabelat e raportimit për intervalet kohore: 3 muaj, 9 muaj dhe vjetore si dhe relacionet përkatëse. 2016. [cited 2018 May 31]; Available from: http://www.shendetesia.gov.al/al/publikime/raportet/shendetesia1516991290/tabelat-e-raportimit-per-intervalet-kohore-3-muaj-9-muaj-dhe-vjetore-si-dhe-relacionet-perkatese.
- [25].World Health Organization. MI4A: market information for access to vaccines; 2018. [cited 2019 April 19]; Available from: https://www.who.int/immunization/programmes_systems/procurement/v3p/platform/en/.
- [26].Coulibaly Y et al. Programme activities: a major burden for district health systems?. Trop Med Int Health 2008;13:1430–2. [DOI] [PubMed] [Google Scholar]
- [27].Hanvoravongchai P et al. Impact of measles elimination activities on immunization services and health systems: findings from six countries. J Infect Dis 2011;204(Suppl 1):S82–9. [DOI] [PubMed] [Google Scholar]
- [28].Mounier-Jack S et al. One year of campaigns in Cameroon: effects on routine health services. Health Policy Plan 2016;31(9):1225–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


