Abstract
Aim: This phase III study assessed the efficacy/safety/antiviral activity/pharmacokinetics of bemnifosbuvir, a novel, oral nucleotide analog to treat COVID-19. Patients & methods: Outpatient adults/adolescents with mild-to-moderate COVID-19 were randomized 2:1 to bemnifosbuvir/placebo. Time to symptom alleviation/improvement (primary outcome), risk of hospitalization/death, viral load and safety were evaluated. Results: Although the study was discontinued prematurely and did not meet its primary end point, bemnifosbuvir treatment resulted in fewer hospitalizations (71% relative risk reduction), COVID-19-related medically attended hospital visits, and COVID-19-related complications compared with placebo. No reduction in viral load was observed. The proportion of patients with adverse events was similar; no deaths occurred. Conclusion: Bemnifosbuvir showed hospitalization reduction in patients with variable disease progression risk and was well tolerated.
Clinical Trial Registration: NCT04889040 (ClinicalTrials.gov).
Keywords: AT-527, bemnifosbuvir, COVID-19, oral, outpatient, SARS-CoV-2
Tweetable abstract
#Bemnifosbuvir is a novel, oral, nonmutagenic, nonteratogenic nucleotide analog with low potential for drug–drug interactions or resistance. Bemnifosbuvir showed a 71% reduction in hospitalization for #COVID-19 despite no symptom alleviation/viral load reduction differences. @ateapharma
The emergence of SARS-CoV-2 in 2019 resulted in the catastrophic global COVID-19 pandemic [1]. The overwhelming number of COVID-19 cases, particularly those requiring hospitalization, posed an immense burden to healthcare systems [2], and subsequent variants such as Omicron continue to emerge with varying transmissibility and symptomology [3]. While effective vaccines have been rapidly developed and deployed worldwide [1,4], helping greatly decrease hospitalizations [5], a substantial proportion of the world's population remains at risk and/or unvaccinated for personal or medical reasons, such as being immunocompromised or lack of vaccine supply, especially in low-resource countries [4]. Furthermore, even fully vaccinated individuals may experience breakthrough infections and reinfections [6]. Durability of response after vaccination to different variants appears to be short and is still being investigated [7].
Vaccine limitations necessitate availability of antiviral medications for both prophylactic and acute treatment to mitigate SARS-CoV-2 infection [8]. Global treatment recommendations for COVID-19 are constantly changing [9,10]. Some therapies that were initially deployed (e.g., monoclonal antibodies) are no longer recommended because they have shown decreased efficacy against new SARS-CoV-2 strains [11,12]. The intravenous antiviral remdesivir was approved by the US FDA in 2020 and is indicated for hospitalized and high-risk non-hospitalized COVID-19 patients [13]. However, the variable efficacy and safety reports, combined with its need to be delivered intravenously in a hospital setting, has limited widespread use of remdesivir for treatment of COVID-19; analogs for oral administration are still being investigated [14]. Additionally, single-agent antiviral treatment may be associated with an increased likelihood of treatment-emergent resistance, as described in kidney transplant patients receiving remdesivir [15].
Current oral outpatient treatments include molnupiravir [16] and nirmatrelvir-ritonavir [17]. Increased use of oral antiviral drugs may reduce disease duration, and likelihood of hospitalization for patients with COVID-19, alleviating burdens on healthcare systems [18]. However, global availability of existing therapies is variable, with limited access in various regions [19]. Additionally, some available medications have safety concerns, including potential teratogenesis, viral mutagenesis and drug–drug interactions, thereby limiting their use in certain populations [16,17,20–22]. With the limitations of current anti-COVID-19 therapies, as well as the imminent threat of new variants that may be resistant to current treatments, continued exploration of potential therapeutics is of the utmost importance [23,24], and innovative antiviral agents that harness novel mechanisms of action (MOA) and allow for ease of administration are an imperative [11].
Bemnifosbuvir hemisulfate (AT-527, active metabolite AT-9010; Figure 1) is an oral, non-mutagenic antiviral guanosine analog that inhibits the viral RNA polymerase, an enzyme critical to viral replication [25,26]. With a unique dual MOA, bemnifosbuvir targets the conserved RNA-dependent RNA polymerase (RdRP) active site for RNA chain termination and the nidovirus RdRP-associated nucleotidyltransferase (NiRAN) active site for protein-primed RNA synthesis, which should decrease the likelihood of simultaneous resistance mutations (Figure 1) [26,27]. In vitro, bemnifosbuvir exhibits potent activity against Alpha, Beta, Gamma, Epsilon, Delta and Omicron (BA.1, BA.2, BA.4, BA.5 and XBB) variants [28–30]. Preclinical and phase I results demonstrated that bemnifosbuvir achieved target drug levels in lungs of healthy volunteers and was safe and well tolerated with no teratogenicity [31]. Safety at 550 mg twice daily (BID) was also shown in phase II studies (NCT04396106, NCT04709835) of patients with COVID-19 [32–35]. Results from a phase II study (NCT04396106) suggested that bemnifosbuvir may blunt COVID-19 progression [36]. Furthermore, bemnifosbuvir poses a low risk of interacting with other drugs that may be prescribed to high-risk patients with COVID-19 (e.g., digoxin, rosuvastatin) [29,37–39]. In this phase III study, named MORNINGSKY (NCT04889040) [40], we aimed to assess efficacy, safety, antiviral activity, and pharmacokinetics (PK) of bemnifosbuvir in nonhospitalized adult and adolescent patients with mild-to-moderate COVID-19.
Figure 1. AT-527 mechanism of action and metabolism.
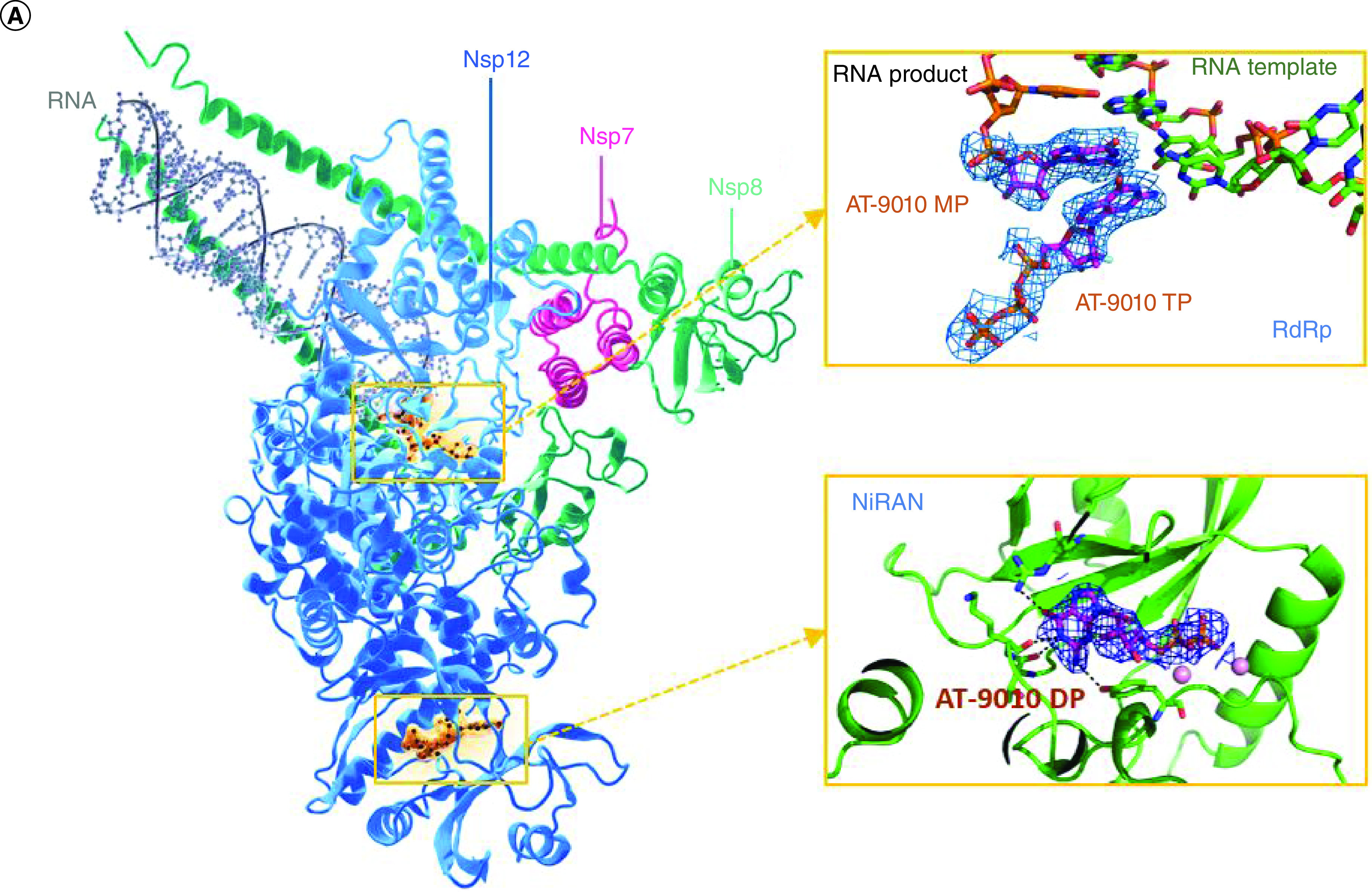
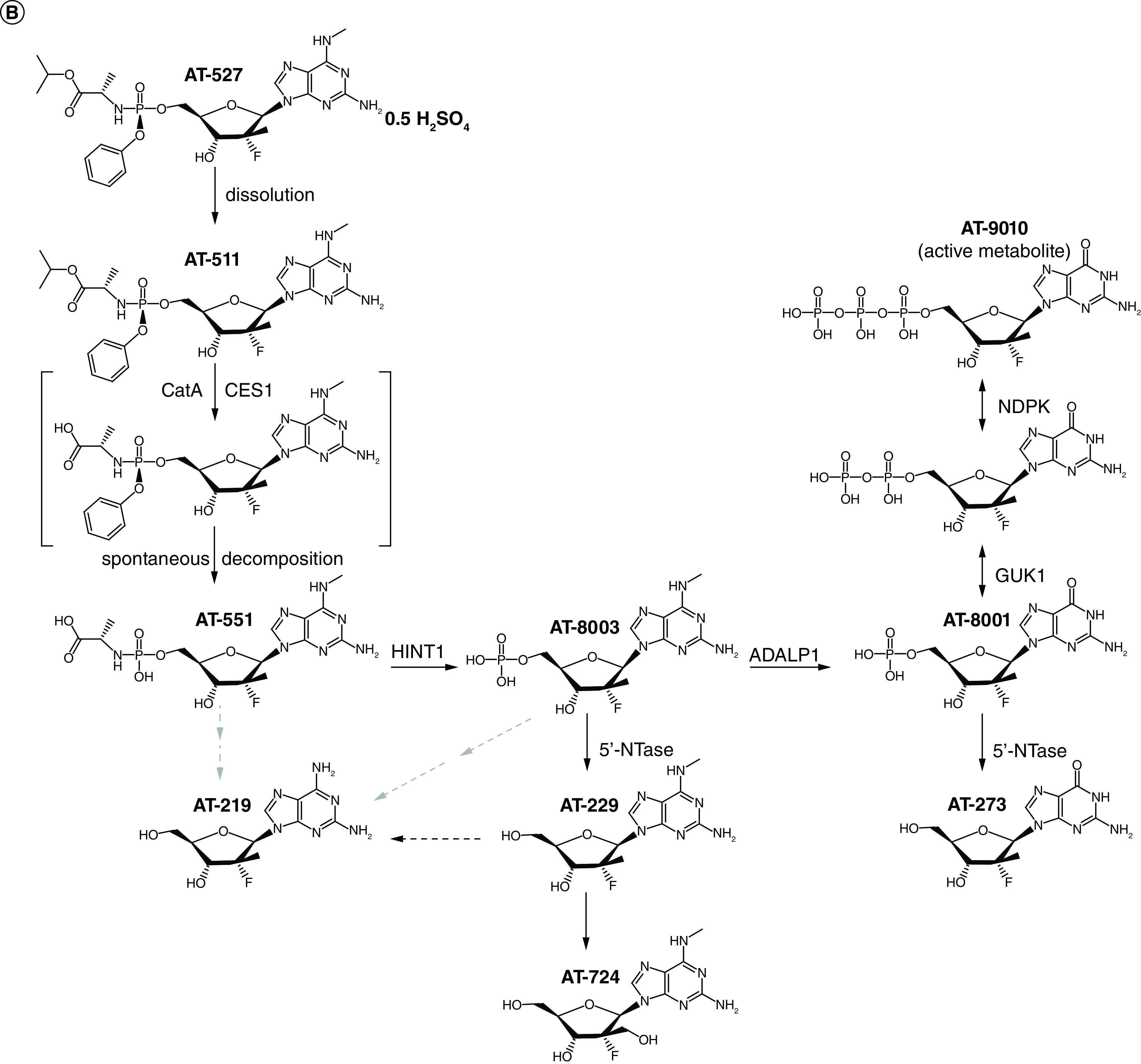
(A) Dual Mechanism of Action of AT-527. Ribbon and stick representation of the cryo-EM structure of nsp7-(nsp8)2–nsp12:AT-9010-terminated-RNA:(AT-9010)2 complex. RNA, AT-9010 and protein shown with the following colors: template RNA, green; RNA product, orange; AT-9010, magenta; nsp7, pink; nsp81 and nsp82, yellow and cyan, respectively; and nsp12in gray and blue for NiRAN and RdRp domains, respectively. Orange boxes are enlarged for RdRp domain and NiRAN domains, respectively, showing experimental cryo-EM map around AT-9010, in the RdRp, one AT-9010 monophosphate (AT-9010-MP) is incorporated at (+1) position, with an incoming AT-9010 occupying the (-1) position. In the NiRAN domain, AT-9010 is bound it its diphosphate form (AT-9010-DP). (B) Metabolism of AT-527. Metabolism of the guanine analog phosphoramidate prodrug AT-527 (top left) and its active triphosphate form AT-9010 (top right).
Figure from Shannon A, et al. Nat Commun. 2022. 13(1):621 under the Creative Commons Attribution 4.0 International License: http://creativecommons.org/licenses/by/4.0/
Patients & methods
Study design
MORNINGSKY was a phase III, randomized, double-blind, placebo-controlled study across 50 centers globally [40]. The study protocol and amendments (Supplementary Material) were approved by an Institutional Review Board/Independent Ethics Committee and written informed consent was obtained. The study, cosponsored by F. Hoffmann-La Roche Ltd and Atea Pharmaceuticals, Inc., was performed in accordance with the Declaration of Helsinki, Council for International Organizations of Medical Sciences International Ethical Guidelines and International Conference for Harmonization Good Clinical Practice Guidelines.
Eligible patients were adolescents or adults (aged ≥12 years) with mild-to-moderate COVID-19 with or without high-risk factors for hospitalization. For adults, per industry guidance, high-risk factors included age (≥65 years) and obesity (BMI >30), cardiovascular disease, chronic lung disease, chronic metabolic disease, chronic kidney disease, liver disease or immunocompromised status. Symptom onset ≤5 days before randomization was required, with a positive SARS-CoV-2 diagnostic test ≤72 h before randomization. Inclusion criteria included presence of ≥3 patient-reported COVID-19 symptoms of at least moderate intensity (score ≥2 in a COVID-19 symptom diary) with investigator-determined disease severity. Symptoms included nasal congestion/runny nose, sore throat, cough, shortness of breath, muscle or body aches, fatigue, headache, chills/sweats, feeling hot/feverish, nausea, vomiting, or diarrhea. Patients were excluded if they displayed clinical signs indicative of severe COVID-19, required hospitalization before or at randomization, or were likely to experience deterioration leading to hospitalization [41]. Other key exclusion criteria included treatment with a COVID-19 therapeutic agent (e.g., antivirals, steroids, antibodies), abnormal laboratory results at screening, other active viral or bacterial infection, or COVID-19 vaccination ≤40 days before enrollment.
Randomization & masking
The original sample size of 1386 participants was designed to ensure ≥90% power to detect a 2-day difference in median time to alleviation/improvement of COVID-19 symptoms between the treatment and placebo groups, based on an assumed time of 10 days in the placebo group. Participants were randomly assigned 2:1 to receive oral bemnifosbuvir 550 mg (two 275-mg tablets) or matching placebo tablets BID for 5 days with a 28-day safety and efficacy follow-up period [40]. Patients were stratified at randomization by region and high-risk factor for hospitalization due to COVID-19. Patients were assigned an identification number and treatment through an interactive response system (voice or web based). All study site personnel, patients, cosponsors and their agents and adjudicators were blinded to treatment assignment, except those who required access to patient treatment assignments for their study roles.
Procedures
Following randomization, patients had mandatory visits on days 1, 2, 3, 5,7, and 14 with possible phone calls on days 21 (±2 days), 28 (±2 days), and at end of study, occurring on day 33 (±3 days). Patients reported symptoms as assessed by the patient global impression of severity (PGIS) method (items 1–14) using a Likert scale either twice daily (days 1–14) or daily (days 15–29) in an electronic COVID-19 symptom diary (Supplementary Material) based on an FDA COVID-19 patient-reported outcome instrument [42]. The primary efficacy objective was assessed using items 1–12 of the COVID-19 symptom diary (Supplementary Material). Time to symptom alleviation or improvement was defined as either time from randomization to alleviation (i.e., score of 0 or 1 for new symptoms), or time from randomization to symptom maintenance or improvement (for preexisting symptoms). Vital signs, including SpO2, and routine clinical and laboratory tests were collected at baseline and at prespecified intervals; samples were sent for analysis to the study site's local laboratory. Nasopharyngeal or nasal swabs for SARS-CoV-2 virology tests, genotyping, along with samples for other laboratory tests, were collected at baseline and/or at prespecified intervals and sent to designated central laboratories, the sponsor, or a designee for virology tests and other analyses (Supplementary Material). Adverse events (AEs) were assessed from initiation of study drug until day 33, graded by investigators using National Cancer Institute Common Terminology Criteria for Adverse Events v5.0 and coded using MedDRA v24.1.
Outcomes
The primary efficacy end point was time to alleviation/improvement of COVID-19 symptoms maintained for 21.5 h duration; to allow for flexibility in assessment timing (24 h minus 10%, or 21.5 h, was used). Secondary outcomes included time to alleviation or improvement of COVID-19 symptoms maintained for 43 h duration, proportion of participants with ≥1 COVID-19-related medically attended visit through study end (≤33 days) and proportion of patients requiring hospitalization. Supplementary Table 1 shows a complete listing of secondary end points. Virology secondary end points included change from baseline in amount of SARS-CoV-2 RNA, time to cessation of SARS-CoV-2 viral shedding, proportion of participants positive for SARS-CoV-2 RNA at specified time points and area under the curve in amount of SARS-CoV-2 RNA. Safety and PK assessments were performed. Ad hoc analyses included proportion of patients who were considered standard versus high risk and required hospitalization, had ≥1 COVID-19-related medically attended visit, or COVID-19 complications and proportion of patients aged > or ≤40 years who required hospitalization. An independent adjudication committee evaluated COVID-19-related complications.
Statistical analysis
Data for each end point were analyzed with descriptive statistics by treatment arm and Kaplan–Meier plots, and no formal statistical comparisons were carried out due to the early closure of trial. Efficacy and virology analyses were performed on the efficacy-evaluable set, defined as all randomized patients who received ≥1 dose of treatment and were quantitative reverse transcription polymerase chain reaction-confirmed (RT-qPCR) positive for SARS-CoV-2 during the study. Safety analyses used the safety evaluable set, all randomized participants who received ≥1 dose of treatment. PK analyses were performed on a cohort having ≥1 postdose drug concentration measurement.
Results
Between 28 April 2021 through 2 December 2021, patients were evaluated at 50 study centers located across North America (14.5%), Europe (58.9%) and the rest of the world (26.6%) (Table 1 & Supplementary Table 2). MORNINGSKY was terminated early after careful consideration of the rapid evolution of SARS-CoV-2. Moreover, as this was a placebo-controlled trial, the emergence and increasing availability of standard-of-care treatment options for COVID-19 necessitated early termination. Out of 260 patients screened, 44 patients failed mainly due to not meeting inclusion criteria. The most common reasons for screen failure included lack of a positive SARS-CoV-2 diagnostic test within 72 h prior to randomization, clinical signs indicative of more severe COVID-19 illness requiring hospitalization, and abnormal laboratory test results at screening. Two-hundred and sixteen (15.6%) of the 1386 planned patients were randomized. A total of 207 patients (bemnifosbuvir, 137; placebo, 70) tested positive for SARS-CoV-2 during the study and were included in efficacy analyses (efficacy-evaluable set; Figure 2). One hundred thirty (94.3%) and 61 (85.9%) patients in the bemnifosbuvir and placebo arms received all ten doses of study treatment. One patient in each arm received an extra dose in error.
Table 1. Patient demographic and disease characteristics at baseline (efficacy-evaluable set).
| Characteristic |
Bemnifosbuvir (n = 137) |
Placebo (n = 70) |
Total (n = 207) |
|---|---|---|---|
| Age, years | |||
| Mean (SD) | 41.1 (13.4) | 41.8 (14.9) | 41.3 (13.9) |
| Median (range) | 41.0 (15–81) | 41.0 (13–91) | 41.0 (13–91) |
| Age group, years, n (%) | |||
|---|---|---|---|
| <18 | 1 (0.7) | 2 (2.9) | 3 (1.4) |
| 18–<65 | 129 (94.2) | 63 (90.0) | 192 (92.8) |
| ≥65 | 7 (5.1) | 5 (7.1) | 12 (5.8) |
| ≤40 | 65 (47.4) | 31 (44.3) | 96 (46.4) |
| >40 | 72 (52.6) | 39 (55.7) | 111 (53.6) |
| >50 | 35 (25.5) | 16 (22.9) | 51 (24.6) |
| >65 | 6 (4.4) | 5 (7.1) | 11 (5.3) |
| Sex at birth, n (%) | |||
|---|---|---|---|
| Male | 76 (55.5) | 38 (54.3) | 114 (55.1) |
| Female | 61 (44.5) | 32 (45.7) | 93 (44.9) |
| Race or ethnic group, n (%) | |||
|---|---|---|---|
| White | 102 (74.5) | 47 (67.1) | 149 (72.0) |
| Asian | 24 (17.5) | 14 (20.0) | 38 (18.4) |
| American–Indian or Alaska Native | 8 (5.8) | 6 (8.6) | 14 (6.8) |
| Black or African–American | 3 (2.2) | 1 (1.4) | 4 (1.9) |
| Native Hawaiian or other Pacific Islander | 0 | 1 (1.4) | 1 (0.5) |
| Unknown | 0 | 1 (1.4) | 1 (0.5) |
| Hispanic or Latino | 38 (27.7) | 18 (25.7) | 56 (27.1) |
| Region† | |||
|---|---|---|---|
| North America | 19 (13.9) | 11 (15.7) | 30 (14.5) |
| Europe | 82 (59.9) | 40 (57.1) | 122 (58.9) |
| Rest of world | 36 (26.2) | 19 (27.1) | 55 (26.6) |
| BMI kg/m2 | |||
|---|---|---|---|
| Mean (SD) | 25.69 (4.40) | 27.36 (6.66) | 26.26 (5.32) |
| Median (range) | 25.14 (17.4–40.6) | 26.62 (16.8–50.8) | 25.53 (16.8–50.8) |
| Smoking history, n (%) | |||
|---|---|---|---|
| Nonsmoker | 94 (68.6) | 40 (57.1) | 134 (64.7) |
| Prior smoker | 17 (12.4) | 15 (21.4) | 32 (15.5) |
| Current smoker | 26 (19.0) | 15 (21.4) | 41 (19.8) |
| Presence of high-risk factor, n (%)‡ | |||
|---|---|---|---|
| Yes | 64 (46.7) | 33 (47.1) | 97 (46.9) |
| With presence of high-risk factor, presence of individual adult high-risk factor, n (%)‡ | n = 64 | n = 33 | n = 97 |
|---|---|---|---|
| Age (dependent on protocol version), years§ | 27 (42.2) | 12 (36.4) | 39 (40.2) |
| Obesity (BMI >30 kg/m2) | 23 (35.9) | 18 (54.5) | 41 (42.3) |
| Cardiovascular disease | 29 (45.3) | 18 (54.5) | 47 (48.5) |
| Chronic lung disease | 3 (4.7) | 1 (3.0) | 4 (4.1) |
| Chronic metabolic disease | 14 (21.9) | 6 (18.2) | 20 (20.6) |
| Chronic kidney disease | 1 (1.6) | 2 (6.1) | 3 (3.1) |
| Chronic liver disease | 1 (1.6) | 2 (6.1) | 3 (3.1) |
| Immunocompromised | 6 (9.4) | 1 (3.0) | 7 (7.2) |
| Baseline SARS-CoV-2 RT-qPCR status¶ | n = 137 | n = 69 | n = 206 |
|---|---|---|---|
| Positive | 137 (100.0) | 67 (97.1) | 204 (99.0) |
| Negative | 0 | 2 (2.9) | 2 (1.0) |
| SARS-CoV-2 RT-qPCR viral load (log10 copies/ml)# | n = 137 | n = 69 | n = 206 |
|---|---|---|---|
| Mean (SD) | 6.59 (1.54) | 6.49 (1.61) | 6.56 (1.56) |
| Median (range) | 6.90 (2.5–9.9) | 6.82 (1.8–8.7) | 6.89 (1.8–9.9) |
| SARS-CoV-2 serostatus (spike protein antibody), n (%) | n = 122 | n = 58 | n = 180 |
|---|---|---|---|
| Positive | 69 (56.6) | 32 (55.2) | 101 (56.1) |
| Negative | 53 (43.4) | 26 (44.8) | 79 (43.9) |
| Severe PGIS on day 1, n (%) | n = 137 | n = 70 | n = 207 |
|---|---|---|---|
| Yes | 14 (11.6) | 1 (1.8) | 15 (8.4) |
| COVID-19 vaccination, n (%) | |||
|---|---|---|---|
| Yes (≥1 dose) | 40 (29.2) | 18 (25.7) | 58 (28.0) |
| Vaccine type, n (%) | |||
|---|---|---|---|
| mRNA | 11 (8.0) | 9 (12.9) | 20 (9.7) |
| Viral vector | 21 (15.3) | 5 (7.1) | 27 (13.0) |
| Inactivated | 6 (4.4) | 3 (4.3) | 9 (4.3) |
| Other†† | 2 (1.5) | 1 (1.4) | 3 (1.4) |
| SARS-CoV-2 variant,‡‡ n (%) | n = 123 | n = 64 | n = 187 |
|---|---|---|---|
| Alpha | 12 (9.8) | 8 (12.5) | 20 (10.7) |
| Beta | 1 (0.8) | 0 | 1 (0.5) |
| Gamma | 1 (0.8) | 1 (1.6) | 2 (1.1) |
| Delta | 109 (88.6) | 55 (85.9) | 64 (87.7) |
Countries in North America included Mexico; countries in Europe included Belgium, Denmark, Germany, Portugal, Romania, Switzerland, Turkey and Ukraine; countries in the rest of the world included Argentina, Brazil and Japan.
Patients may have one or multiple high-risk factors.
Presence of a high-risk factor for adult age was defined as >50 years in protocol version 1, and ≥65 years in subsequent protocol versions. Presence of high-risk factor reported as collected in the eCRF.
Patients positive for SARS-CoV-2 RNA is defined as the percentage of patients with a positive virus RNA by RT-qPCR test result above or equal to the LOQ.
Samples reported as BLQ are imputed to the LOQ minus 1 (119 copies/ml). Samples reported as negative are imputed to the LOQ/2 (60 copies/ml).
Includes 2 patients receiving bemnifosbuvir with vaccine type unknown and one patient receiving placebo who received multiple vaccine types.
Patients with a baseline viral load <4 log10 copies/ml were not sequenced for virus variant.
BLQ: Below limit of quantification; eCRF: Electronic case report form; LOQ: Limit of quantification; PGIS: Patient global impression of severity; RT-qPCR: Quantitative reverse transcription polymerase chain reaction; SD: Standard deviation; TCID: Tissue culture infectious dose.
Figure 2. Screening, randomization and analyses.
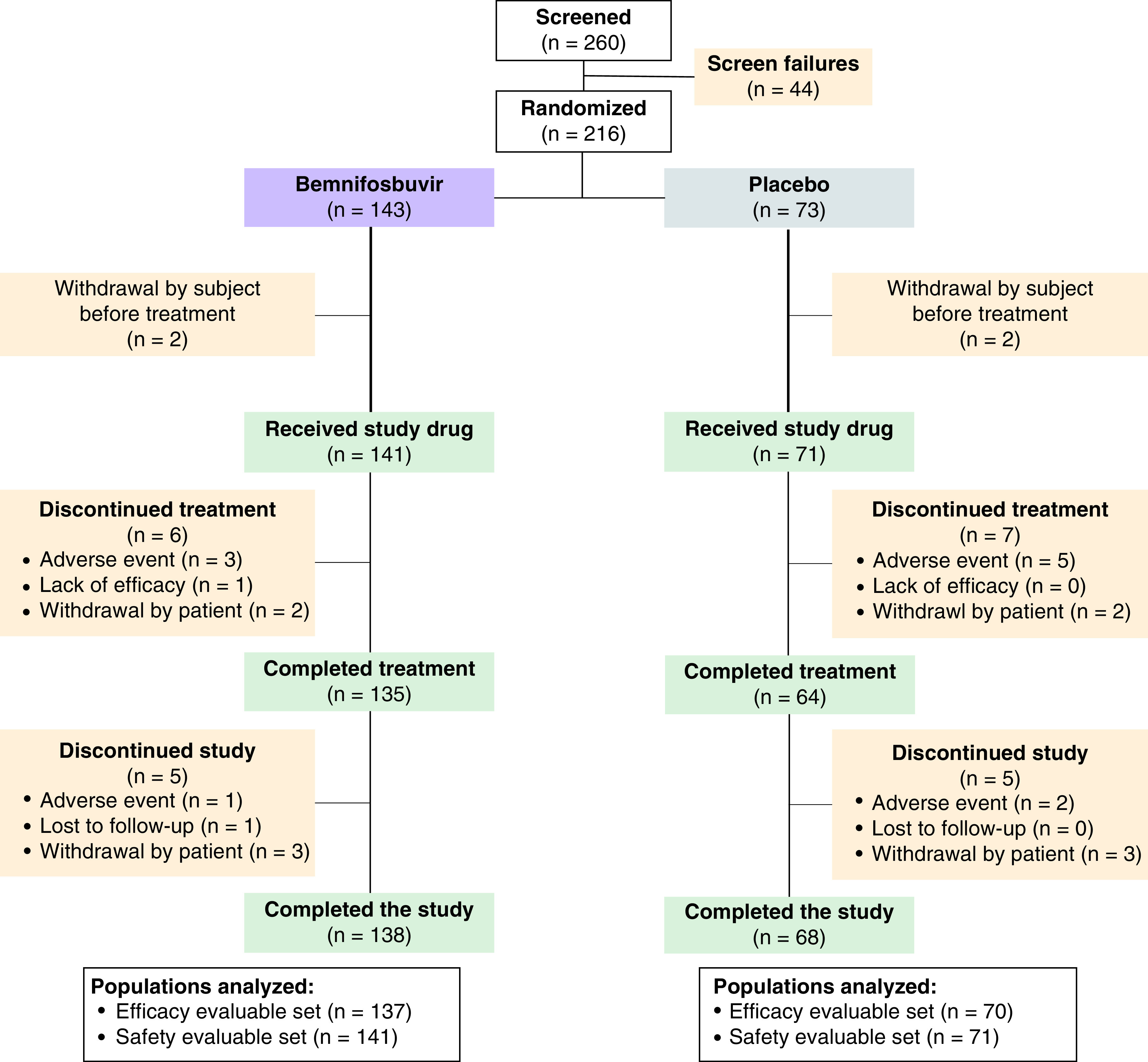
Patient baseline characteristics and demographics were generally well balanced between groups, with slight imbalances in patients who were immunocompromised, with a BMI >30 kg/m2, and with more severe day 1 PGIS (Table 1). Notably, the cohort included both vaccinated and unvaccinated patients, with 40 (29.2%) patients in the bemnifosbuvir group and 18 (25.7%) in the placebo group who received ≥1 doses of a vaccine against COVID-19. Both treatment groups had patients with high-risk factors, 64 (46.7%) in the bemnifosbuvir group and 33 (47.1%) in the placebo group. Across treatment groups, 164 patients (87.7%) were infected with the Delta strain.
Table 2 provides a summary of primary and secondary efficacy end points. Bemnifosbuvir did not meet its primary end point, with longer median time for symptom alleviation or improvement time than placebo, 3.9 days (94.5 h) versus 3.1 days (73.7 h), respectively (Supplementary Figure 1). However, in regard to secondary end points, patients receiving bemnifosbuvir experienced lower rates of hospitalizations for COVID-19, COVID-19-related complications, COVID-19-related medically attended visits, and posttreatment infections compared with placebo (Figure 3A-C; Table 2). The relative risk of requiring hospitalization for COVID-19 was 71% lower for those receiving bemnifosbuvir versus placebo (4 [2.9%; 97.5% CI, 0.00–6.51] vs 7 [10%; 97.5% CI, 1.25–18.75] patients, respectively; Figure 3A). In an ad hoc exploratory analysis for patients aged >40 years (MORNINGSKY patients median age, 40 years), bemnifosbuvir reduced relative risk of hospitalization by 82%, compared with a 5% relative risk reduction for patients aged ≤40 years (Table 2). All hospitalizations were in-patient admissions where patients were hospitalized for no less than 3 days. All were reported as serious AEs and were for events related to COVID-19 (e.g., pneumonia, hypoxia, respiratory failure) as judged by the investigator and further adjudicated by a blinded adjudication committee.
Table 2. Summary of the primary and secondary efficacy end point results.
| Key end points |
Bemnifosbuvir (n = 137) |
Placebo (n = 70) |
|---|---|---|
| Primary end point: time to symptom alleviation or improvement maintained for ≥21.5 h† | ||
| Patients with event, n (%) | 128 (93.4) | 64 (91.4) |
| Median (97.5% CI) time to event,‡ h | 94.5 (68.1–131.7) | 73.7 (47.1–105.8) |
| Range, h | 0–687 | 0–687 |
| Secondary efficacy end points | ||
|---|---|---|
| Proportion of patients requiring hospitalization for COVID-19 | ||
| Patients requiring hospitalization, n (%) | 4 (2.9) | 7 (10.0) |
| 97.5% CI | 0.00–6.51 | 1.25–18.75 |
| High risk,§ n (%) | 3/64 (4.7%) | 5/33 (15.2%) |
| 97.5% CI | 0.00–11.39 | 0.00–30.66 |
| Standard risk,§ n (%) | 1/73 (1.4%) | 2/37 (5.4%) |
| 97.5% CI | 0.00–5.10 | 0.00–15.09 |
| Proportion of patients with ≥1 COVID-19-related medically attended visit throughout study | ||
| Patients with ≥1 visit, n (%) | 14 (10.2) | 10 (14.3) |
| 97.5% CI | 4.05–16.38 | 4.20–24.37 |
| Frequency of COVID-19-related complications | ||
| Patients with complications, n (%) | 6 (4.4) | 7 (10.0) |
| 97.5% CI | 0.10–8.66 | 1.25–18.75 |
| Proportion of patients with any posttreatment infection | ||
| Patients with posttreatment infection, n (%) | 13 (9.5) | 10 (14.3) |
| 97.5% CI | 3.51–15.47 | 4.20–24.37 |
| Proportion of patients with all-cause mortality | ||
| Patients with all-cause mortality, n (%) | 0 | 0 |
| Proportion of patients requiring hospitalization for COVID-19 (ad hoc) | ||
| Patients aged ≤40 y, n (%) | 2/65 (3.1) | 1/31 (3.2) |
| 97.5% CI | 0–8.65 | 0–11.95 |
| Patients aged >40 y, n (%) | 2/72 (2.8) | 6/39 (15.4) |
| 97.5% CI | 0–7.81 | 1.15–29.62 |
Defined as time from randomization to the first time at which all COVID-19 symptoms from items 1–12 of the COVID-19 symptom diary were either alleviated, maintained, or improved for a minimum duration of 21.5 h.
Median time to event with 97.5% CIs estimated from the Kaplan–Meier curves. 97.5% CI for rates constructed using the Wald with continuity correction method.
Ad hoc analyses.
CI: Confidence interval.
Figure 3. Secondary Endpoints.
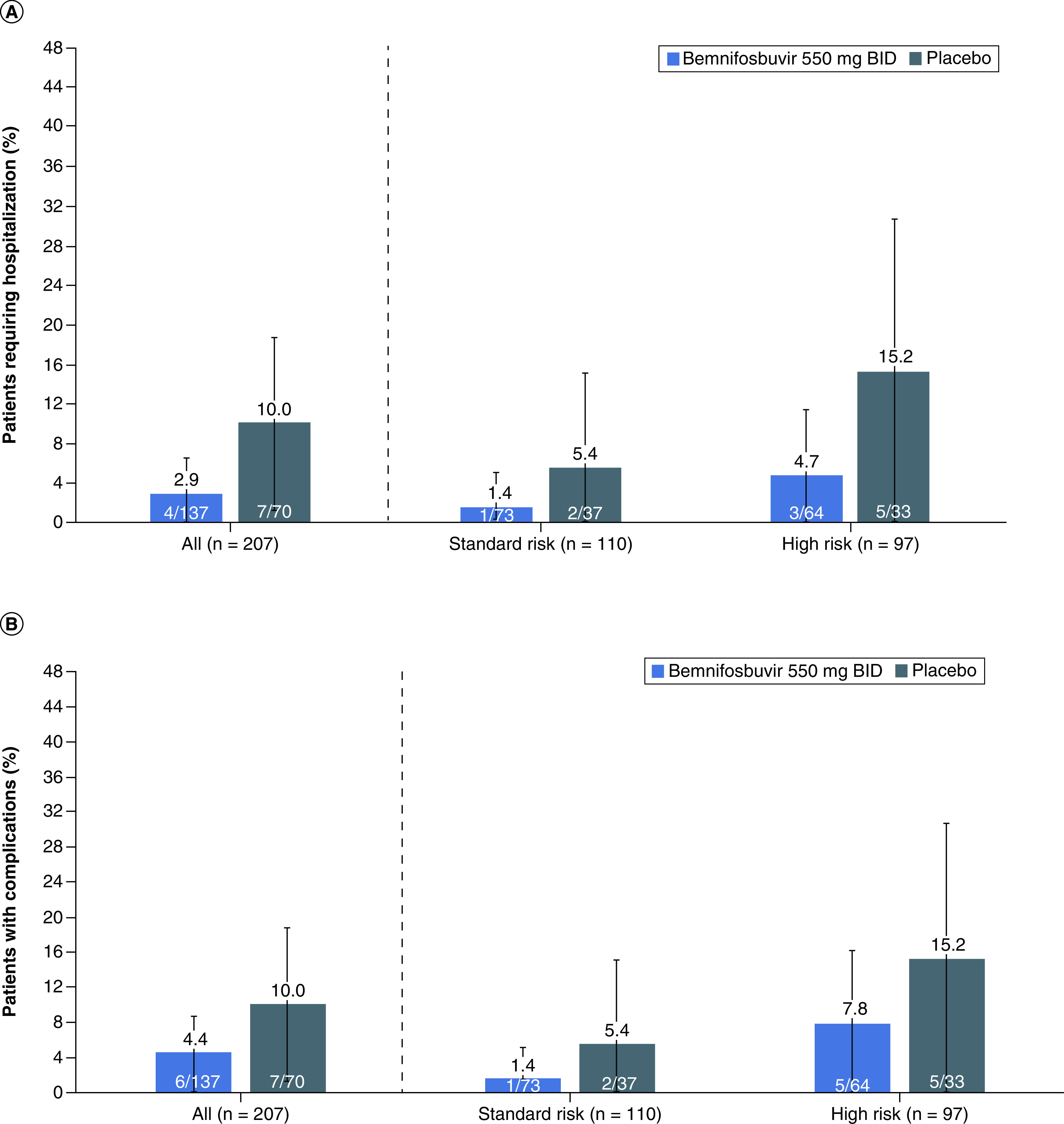
(A) Rate of hospitalization. BID, twice daily. Error bars denote the 97.5% confidence interval (CI). (B) Rate of COVID-19 Complications. BID, twice daily. Error bars denote the 97.5% CI. (C) Rate of medically attended visits related to COVID-19. BID, twice daily. Error bars denote the 97.5% CI.
Secondary symptom-based end point results are summarized in Supplementary Table 3. No clear differences in any of the virology end points between the bemnifosbuvir and placebo arms were observed (Supplementary Table 4). PK results are presented in Supplementary Table 5.
Overall, bemnifosbuvir was well tolerated, with a safety profile comparable with placebo and no new safety signals observed. Most AEs were grade 1 or 2 (Table 3), and the most common treatment-related AEs were gastrointestinal (Supplementary Table 6). AEs leading to treatment discontinuation occurred in 4 (2.8%) patients receiving bemnifosbuvir compared with 5 (7.0%) patients receiving placebo. No deaths were observed during the study.
Table 3. Summary of adverse events in the safety-evaluable set.
| Category, n (%) | Bemnifosbuvir (n = 141) | Placebo (n = 71) | Total (n = 212) |
|---|---|---|---|
| AEs† | 87 | 51 | 138 |
| Deaths | 0 | 0 | 0 |
| Patients who discontinued owing to an AE | 1 (0.7) | 2 (2.8) | 3 (1.4) |
| Patients with ≥1: | |||
| AE | 55 (39.0) | 26 (36.69) | 81 (38.2) |
| AE with fatal outcome | 0 | 0 | 0 |
| Serious AE | 7 (9.9) | 5 (3.5) | 12 (5.7) |
| Serious AE leading to treatment discontinuation | 4 (5.6) | 2 (1.4) | 6 (2.8) |
| Treatment-related serious AE | 0 | 0 | 0 |
| AE leading to treatment discontinuation | 4 (2.8) | 5 (7.0) | 9 (4.2) |
| Treatment-related AE | 21 (14.9) | 3 (4.2) | 24 (11.3) |
| Treatment-related AE leading to treatment discontinuation | 2 (1.4) | 1 (1.4) | 3 (1.4) |
| AE severity (by worst grade) | |||
| Grade 1 | 23 (16.3) | 14 (19.7) | 37 (17.5) |
| Grade 2 | 27 (19.1) | 6 (8.5) | 33 (15.6) |
| Grade 3 | 4 (2.8) | 6 (8.5) | 10 (4.7) |
| Grade 4 | 1 (0.7) | 0 | 1 (0.7) |
Multiple occurrences of the same AE in 1 individual are counted only once except for “Total number of AEs” row in which multiple occurrences of the same AE are counted separately.
AE: Adverse event.
Discussion
Although definitive conclusions cannot be drawn owing to early study termination and the consequent small sample size, the outcomes of this study contribute important results that can further guide investigation of treatments for patients with mild-to-moderate COVID-19 in outpatient settings. Results of the symptom-based end points tended to favor the placebo group, but trends were observed with a reduced rate of hospitalizations, COVID-19 complications, and COVID-19-related medically attended visits in patients receiving bemnifosbuvir. While the observed 10% hospitalization rate for patients receiving placebo is comparable to some studies [43], it is higher than that reported for other previous clinical studies [44,45]. Despite this, patients receiving bemnifosbuvir had decreased hospitalization rates that were more apparent when comparing patients aged >40 years in an ad hoc analysis. However, it must be contextualized that a limitation of this study is that the population was not all vaccinated; those who had not received vaccinations would likely benefit more from treatment with bemnifosbuvir than those who were vaccinated. In addition, the population was located in different regions worldwide, and most patients were infected with the Delta strain, which may have influenced hospitalization risk. This study also established the tolerable safety profile of bemnifosbuvir, comparable with placebo.
MORNINGSKY did not meet the primary efficacy end point, which could be attributed to multiple factors, including challenges related to measuring COVID-19 symptoms with the current patient-reported outcome tools. This is reflected in the FDA guidance for outpatient studies at the beginning of the pandemic, which highlighted that the time course of COVID-19 symptoms was continually evolving, and certain symptoms may take longer than others to resolve [42]. Additionally, variability of SARS-CoV-2 strains infecting a heterogeneous population further complicated symptom readouts (Table 1) [46]. Other clinical studies for COVID-19 treatments have shown variable success with symptom-based end points, although not all results were statistically significant. In the PINETREE clinical study, which was terminated early, 34.8% of patients receiving remdesivir reported symptom alleviation by day 14, compared with 25% of patients receiving placebo [47]. The MOVE-OUT study for molnupiravir showed similar results: resolution of COVID-19 symptoms was more likely, and progression less likely in the molnupiravir group compared with placebo [43]. In an analysis of patients treated with nirmatrelvir-ritonavir or placebo, difference in symptoms between treated and placebo groups was statistically significant, and patients continued having fewer symptoms at 30 days after diagnosis [48]. However, the EPIC-SR study in patients treated with nirmatrelvir-ritonavir did not meet the primary end point of sustained alleviation of symptoms [49]. COVID-19 rebound is a concern with nirmatrelvir-ritonavir and may impact time to symptom alleviation [50]. Designing trials based on patient-reported symptom alleviation is difficult as symptoms continue to change with each new variant.
Although viral load reduction, measured by RNA copy number in the nasopharynx, is commonly used to determine antiviral activity with COVID-19, correlations between reduction and clinical outcomes have been inconsistent. In our study, bemnifosbuvir had no effect on viral load. While some antivirals with varying MOAs have shown reductions in nasopharyngeal viral load, associated with clinical study outcomes, remdesivir, which has a similar MOA to bemnifosbuvir, did not cause substantial decrease in nasopharyngeal viral load in human studies, despite showing clinical benefit [47]. Further, there is no firm correlation between viral load reduction and symptom alleviation. Interestingly, in a phase II study evaluating bemnifosbuvir in high-risk hospitalized patients with COVID-19, bemnifosbuvir treatment resulted in greater reduction of viral load from baseline compared with placebo [33,51]. However, in a phase II study on outpatients with mild-to-moderate COVID-19, no viral load differences were observed with bemnifosbuvir [32].
Although the MORNINGSKY primary end point was not met, relative reduction in risk of hospitalization with bemnifosbuvir was 71%, despite including vaccinated and unvaccinated patients with high and low risk for progression. Interpretation of this result is limited, as the study was limited by sample size. The reduction observed in patients treated with nirmatrelvir-ritonavir who were vaccinated and with ≥1 risk factor for progressing to severe illness was 57% (n = 361) and 88.9% (n = 389) in patients who were unvaccinated, nonhospitalized, and at high risk for progression to severe COVID-19 [44,49]. Remdesivir also demonstrated relative reduction in risk of hospitalization, 87%, (n = 279) for patients who had ≥1 risk factor for progression to severe COVID-19 [47]. Monoclonal and combination antibody treatments have shown similar relative risk reduction compared with placebo in patients with mild-to-moderate COVID-19 who received treatment within 3 days of symptom onset [52–54].
Risk of hospitalization is an end point that may influence real-world outcomes including economic and health resource impact of COVID-19. It is a commonly used end point for COVID-19 clinical studies, with 43% of phase III randomized-controlled treatment trials in the beginning of 2020 using an ordinal scale often including hospitalization as an end point [55], including early studies for remdesivir [56], and is the basis by which multiple treatments have received approval/emergency use authorization [13,16,17].
In April 2023, bemnifosbuvir was granted ‘Fast Track Designation’ by the FDA, which may facilitate its development and evaluation for the treatment of COVID-19 [57]. Bemnifosbuvir is now being evaluated for the treatment of COVID-19 in outpatients, regardless of vaccination status, who are at high risk for COVID-19 disease progression in the international phase III clinical trial, SUNRISE-3 (NCT05629962) [25,58].
Conclusion
With continued evolution of the COVID-19 pandemic since the MORNINGSKY study, the pandemic landscape has changed owing to the emergence of Omicron variants and increased presence of hybrid immunity derived from vaccination and prior infection or reinfection [6]. Additionally, recommendations for treatments that were initially effective have been revised due to decreased susceptibility of more recent variants [12]. With the continual emergence of new SARS-CoV-2 strains, modalities with novel MOAs are needed. Bemnifosbuvir with its dual MOA may show that the chance of mutations conferring resistance to this drug is lower than seen for other drugs with single MOAs [26]. The conserved sequence of the SARS-CoV-2 RdRP active sites [26,27] suggests that bemnifosbuvir should be active against all variants reported to date, including Omicron subvariants BA.4 and BA.5 confirmed by in vitro data [28]. In this phase III study, patients receiving bemnifosbuvir experienced reduced rates of hospitalizations and fewer COVID-19-related medically attended hospital visits and COVID-19-related complications. With a tolerable safety profile, including no evidence of teratogenic or mutagenic effects, and low potential for drug–drug interactions or resistance [25,26,29,37–39], bemnifosbuvir may provide a safer option compared with other small-molecule inhibitors for COVID-19 treatment, and a follow-up phase III trial is now in progress [58].
Summary points.
Hospitalization due to COVID-19 has placed a significant burden on healthcare systems. With the advent of new SARS-CoV-2 variants, vaccines and other treatment approaches have shown diminished efficacy. As the pandemic continues to evolve, safe, effective, direct-acting and convenient antiviral agents with broad utility are needed.
Bemnifosbuvir is an oral, antiviral guanosine analog that inhibits viral RNA polymerase via a dual mechanism of action.
This phase III study of bemnifosbuvir was terminated early, enrolling only 216 of the planned 1386 patients. It did not achieve its primary end point, with patients who received bemnifosbuvir experiencing longer median time to symptom alleviation/improvement than those who received placebo.
Compared with those in the placebo group, patients who received bemnifosbuvir experienced fewer hospitalizations, with risk reduction for all evaluable patients (71%) and for those aged >40 years (82%).
Bemnifosbuvir treatment also led to fewer COVID-19-related medically attended hospital visits and COVID-19-related complications.
Bemnifosbuvir was well tolerated; most adverse events were mild to moderate, and no deaths occurred.
No difference in viral load was observed between the bemnifosbuvir and placebo groups.
With its dual mechanism of action, in vitro activity against all reported SARS-CoV-2 strains, favorable safety profile, and low potential for drug–drug interactions, orally-administered bemnifosbuvir may address some of the limitations of existing COVID-19 treatments. A global phase III study is currently underway.
Supplementary Material
Footnotes
Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.futuremedicine.com/doi/suppl/10.2217/fvl-2023-0115
Author contributions
All authors contributed to study protocol development, data collection, analysis and interpretation and clinical study report compilation. All authors were involved in the decision to submit this manuscript for publication.
Acknowledgments
The authors thank the patients and their families as well as the clinical trial site investigators for their contributions to the MORNINGSKY study.
Financial disclosure
This work was supported by Atea Pharmaceuticals, Inc and F. Hoffman-La Roche Ltd. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Competing interests disclosure
A Horga, K Pietropaolo, L Ishak and B Belanger are employees of Atea. R Saenz is an employee and owns stock for Genentech (a member of the Roche group). A Simón-Campos is a speaker and advisory board member for Pfizer, AstraZeneca, Roche and Regeneron. WJ Stubbings, N Collinson, C Granier, AC Hurt and S Wildum are employees and shareholders of F. Hoffman-La-Roche Ltd. B Zrinscak is an employee of F. Hoffman-La-Roche Ltd. K Lin is a former employee of Atea. X-J Zhou and J Hammond are employees and shareholders of Atea. The authors have no other competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript apart from those disclosed.
Writing assistance
Atea Pharmaceuticals, Inc. (MA, USA) enlisted and funded medical writing support from PRECISIONscientia (PA, USA), which was provided by R Myers and N Infarinato.
Ethical conduct of research
The study protocol and amendments were approved by an Institutional Review Board/Independent Ethics Committee, and written informed consent was obtained. The study was performed in accordance with the Declaration of Helsinki, Council for International Organizations of Medical Sciences International Ethical Guidelines, and International Conference for Harmonization Good Clinical Practice Guidelines.
Data sharing statement
The authors attest that this manuscript reports original clinical trial data. The study protocol and deidentified, individual data for this manuscript (text, tables, figures and appendices) will be available indefinitely for anyone wishing to access them. The study protocol and Statistical Analysis Plan are provided in the Supplementary Material.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
References
Papers of special note have been highlighted as: •• of considerable interest
- 1.Salian VS, Wright JA, Vedell PT et al. COVID-19 transmission, current treatment, and future therapeutic strategies. Mol Pharm. 18(3), 754–771 (2021). [DOI] [PubMed] [Google Scholar]
- 2.Di Fusco M, Shea KM, Lin J et al. Health outcomes and economic burden of hospitalized COVID-19 patients in the United States. J. Med. Econ. 24(1), 308–317 (2021). [DOI] [PubMed] [Google Scholar]
- 3.Iuliano AD, Brunkard JM, Boehmer TK et al. Trends in disease severity and health care utilization during the early omicron variant period compared with previous SARS-CoV-2 high transmission periods - United States, December 2020-January 2022. MMWR Morb. Mortal. Wkly Rep. 71(4), 146–152 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mathieu E, Ritchie H, Ortiz-Ospina E et al. A global database of COVID-19 vaccinations. Nat. Hum. Behav. 5(7), 947–953 (2021). [DOI] [PubMed] [Google Scholar]
- 5.Steele MK, Couture A, Reed C et al. Estimated number of COVID-19 infections, hospitalizations, and deaths prevented among vaccinated persons in the US, December 2020 to September 2021. JAMA Netw. Open. 5(7), e2220385 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jang EJ, Choe YJ, Yun GW et al. Reinfection with SARS-CoV-2 in general population, South Korea; nationwide retrospective cohort study. J. Med. Virol. 94(11), 5589–5592 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andrews N, Tessier E, Stowe J et al. Duration of protection against mild and severe disease by COVID-19 vaccines. N. Engl. J. Med. 386(4), 340–350 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pang X, Xu W, Liu Y, Li H, Chen L. The research progress of SARS-CoV-2 main protease inhibitors from 2020 to 2022. Eur. J. Med. Chem. 257, 115491 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. Therapeutics and COVID-19: living guideline by World Health Organization (WHO) (2021). https://apps.who.int/iris/handle/10665/345356
- 10.Kuriakose S, Singh K, Pau AK et al. Developing treatment guidelines during a pandemic health crisis: lessons learned from COVID-19. Ann. Intern. Med. 174(8), 1151–1158 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li G, Hilgenfeld R, Whitley R, De Clercq E. Therapeutic strategies for COVID-19: progress and lessons learned. Nat. Rev. Drug Discov., 1–27 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization. Therapeutics and COVID-19: Living Guideline (2022). https://apps.who.int/iris/handle/10665/353403 [PubMed]
- 13.VEKLURY. Prescribing information. Gilead Sciences, Inc; (2022). [Google Scholar]
- 14.Wang Z, Yang L, Song XQ. Oral GS-441524 derivatives: Next-generation inhibitors of SARS-CoV-2 RNA-dependent RNA polymerase. Front. Immunol. 13, 1015355 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hogan JI, Duerr R, Dimartino D et al. Remdesivir resistance in transplant recipients with persistent COVID-19. Clin. Infect. Dis., (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.LAGEVRIO. Emergency Use Authorization Fact Sheet. Merck Sharp & Dohme LLC; (2022). [Google Scholar]
- 17.PAXLOVID. Emergency Use Authorization Fact Sheet. Pfizer Inc; (2022). [Google Scholar]
- 18.Wen W, Chen C, Tang J et al. Efficacy and safety of three new oral antiviral treatment (molnupiravir, fluvoxamine and Paxlovid) for COVID-19: a meta-analysis. Ann. Med. 54(1), 516–523 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pepperrell T, Ellis L, Wang J, Hill A. Barriers to worldwide access for Paxlovid, a new treatment for COVID-19. Open Forum Infect. Dis. 9(9), ofac174 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Molnupiravir. NIH (2023). www.covid19treatmentguidelines.nih.gov/therapies/antivirals-including-antibody-products/molnupiravir/
- 21.Sanderson T, Hisner R, Donovan-Banfield IA, Peacock T, Ruis C. Identification of a molnupiravir-associated mutational signature in SARS-CoV-2 sequencing databases. medRxiv (2023) (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vuorio A, Kovanen PT, Raal F. Cholesterol-lowering drugs for high-risk hypercholesterolemia patients with COVID-19 while on Paxlovid™ therapy. Future Virol. 17(10), 761–765 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Batiha GE, Al-Kuraishy HM, Al-Gareeb AI et al. Targeting of neuroinflammation by glibenclamide in COVID-19: old weapon from arsenal. Inflammopharmacology. 31(1), 1–7 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Batiha GE, Al-Kuraishy HM, Al-Gareeb AI, Youssef FS, El-Sherbeni SA, Negm WA. A perspective study of the possible impact of obeticholic acid against SARS-CoV-2 infection. Inflammopharmacology. 31(1), 9–19 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Atea to advance global phase 3 registrational study of bemnifosbuvir in high-risk non-hospitalized patients with COVID-19. Press Release. Atea Pharmaceuticals; (2022). https://ir.ateapharma.com/news-releases/news-release-details/atea-advance-global-phase-3-registrational-study-bemnifosbuvir [Google Scholar]
- 26.Shannon A, Fattorini V, Sama B et al. A dual mechanism of action of AT-527 against SARS-CoV-2 polymerase. Nat. Commun. 13(1), 621 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This article describes the mechanism of action of AT-527 in more detail.
- 27.Takashita E, Kinoshita N, Yamayoshi S et al. Efficacy of antibodies and antiviral drugs against COVID-19 omicron variant. N. Engl. J. Med. 386(10), 995–998 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Atea Pharmaceuticals reports third quarter 2022 financial results and provides business update. Press Release. Atea Pharmaceuticals; (2022). https://ir.ateapharma.com/news-releases/news-release-details/atea-pharmaceuticals-reports-third-quarter-2022-financial [Google Scholar]
- 29.Atea Pharmaceuticals reports first quarter 2023 financial results and provides business update. News release. Atea Pharmaceuticals; (2023). https://ir.ateapharma.com/news-releases/news-release-details/atea-pharmaceuticals-reports-first-quarter-2023-financial [Google Scholar]
- 30.Huang Q. Bemnifosbuvir (BEM, AT-527), a potent inhibitor of SARS-CoV-2 variants of concern (VOC), and a promising oral antiviral with a high resistance barrier for treatment of COVID-19 and other coronavirus infections. Lecture presented at: International Conference on Antiviral Research. Lyon, France: (March 13–17 2023). [Google Scholar]; •• Provides further evidence of efficacy of AT-527 for the treatment of COVID-19.
- 31.Zhou XJ, Horga A, Puri A et al. AT-527 achieves antiviral concentrations in the human lung. Poster presented at: the 2021 ISIRV-WHO Conference. Virtual; (October 19–21, 2021). [Google Scholar]; •• Includes important pharmacokinetic data for AT-527.
- 32.Atea Pharmaceuticals provides update and topline results for phase 2 MOONSONG trial evaluating AT-527 in the outpatient setting. Press Release. Atea Pharmaceuticals; (2021). https://ir.ateapharma.com/news-releases/news-release-details/atea-pharmaceuticals-provides-update-and-topline-results-phase-2 [Google Scholar]
- 33.Atea Pharmaceuticals reports first quarter 2022 financial results and provides business update. Press Release. Atea Pharmaceuticals; (2022). https://ir.ateapharma.com/news-releases/news-release-details/atea-pharmaceuticals-reports-first-quarter-2022-financial [Google Scholar]
- 34.Clinicaltrials.Gov. Safety and efficacy of AT-527 in subjects with moderate coronavirus disease (COVID-19) in a hospital setting. NCT04396106; (2020). https://clinicaltrials.gov/ct2/show/NCT04396106 [Google Scholar]
- 35.Clinicaltrials.Gov. Study to evaluate the effects of AT-527 in non-hospitalized adult patients with mild or moderate COVID-19. NCT04709835; (2021). https://clinicaltrials.gov/ct2/show/NCT04709835 [Google Scholar]
- 36.Horga A, Kuritzkes DR, Kowalczyk JJ et al. Phase 2 study of bemnifosbuvir in high-risk participants in a hospital setting with moderate COVID-19. Future Virol. (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Includes the results of the phase II clinical trial of AT-527 for the treatment of high-risk participants in a hospital setting with moderate COVID-19.
- 37.Vo A, Good SS, Agrawal N, Sommadossi JP. Low risk of drug-drug interactions (DDIs) for bemnifosbuvir (BEM) based upon in vitro metabolism and transporter interaction studies. Poster presented at: International Conference on Antiviral Research. Lyon, France: (March 13–17, 2023). [Google Scholar]
- 38.Zhou X-J, Morelli G, Montrond M et al. Bemnifosbuvir has low potential to interfere with P-gp, BCRP, and OATP1B1-mediated transport. Poster presented at: Conference on Retroviruses and Opportunistic Infections. Seattle, Washington: (February 19–22, 2023). [Google Scholar]
- 39.Zhou XJ, Morelli G, Montround M et al. No dose adjustments for CYP3A4 substrates when co-administered with bemnifosbuvir. Poster presented at: Conference on Retroviruses and Opportunistic Infections. Seattle, Washington: (February 19–22, 2023). [Google Scholar]
- 40.ClinicalTrials.gov. Study to evaluate the effects of RO7496998 (AT-527) in non-hospitalized adult and adolescent participants with mild or moderate COVID-19 (MORNINGSKY). NCT04889040; (2021). https://clinicaltrials.gov/ct2/show/NCT04889040 [Google Scholar]
- 41.US Food and Drug Administration. COVID-19: developing drugs and biological products for treatment or prevention: guidance for industry (2021). www.fda.gov/drugs/guidance-compliance-regulatory-information/guidances-drugs
- 42.US Food and Drug Administration. Assessing COVID-19-related symptoms in outpatient adult and adolescent subjects in clinical trials of drugs and biological products for COVID-19 prevention or treatment. Guidance for Industry; (2020). www.fda.gov/regulatory-information/search-fda-guidance-documents/assessing-covid-19-related-symptoms-outpatient-adult-and-adolescent-subjects-clinical-trials-drugs [Google Scholar]
- 43.Bernal J, Gomes Da Silva MM, Musungaie DB et al. Molnupiravir for oral treatment of COVID-19 in nonhospitalized patients. N. Engl. J. Med. 386(6), 509–520 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hammond J, Leister-Tebbe H, Gardner A et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with COVID-19. N. Engl. J. Med. 386(15), 1397–1408 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vallejos J, Zoni R, Bangher M et al. Ivermectin to prevent hospitalizations in patients with COVID-19 (IVERCOR-COVID19) a randomized, double-blind, placebo-controlled trial. BMC Infect Dis. 21(1), 635 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marquez C, Kerkhoff AD, Schrom J et al. COVID-19 symptoms and duration of direct antigen test positivity at a community testing and surveillance site, January 2021–2022. MedRxiv, 2022.2005.2019.22274968 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gottlieb RL, Vaca CE, Paredes R et al. Early remdesivir to prevent progression to severe COVID-19 in outpatients. N. Engl. J. Med. 386(4), 305–315 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ganatra S, Dani SS, Ahmad J et al. Oral nirmatrelvir and ritonavir in non-hospitalized vaccinated patients with Covid-19. Clin. Infect. Dis., (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pfizer reports additional data on Paxlovid supporting upcoming new drug application submission to U.S. FDA. Business Wire; (2022). www.businesswire.com/news/home/20220613005755/en/ [Google Scholar]
- 50.Centers for Disease Control and Prevention. COVID-19 rebound after Paxlovid treatment (2022). https://emergency.cdc.gov/han/2022/pdf/CDC_HAN_467.pdf
- 51.Atea's AT-527, an oral antiviral drug candidate, reduces viral replication in hospitalized patients with COVID-19 in phase 2 interim analysis. Atea Pharmaceuticals; (2021). https://ir.ateapharma.com/news-releases/news-release-details/ateas-527-oral-antiviral-drug-candidate-reduces-viral [Google Scholar]
- 52.AstraZeneca. Evusheld significantly prevented COVID-19 disease progression or death in TACKLE phase III treatment trial. Press release; (2022). www.astrazeneca.com/media-centre/press-releases/2022/evusheld-significantly-prevented-covid-19-disease-progression-or-death-in-tackle-phase-iii-treatment-trial.html [Google Scholar]
- 53.Adagio Therapeutics announces ADG20 (adintrevimab) is the first monoclonal antibody to meet primary endpoints with statistical significance across pre- and post-exposure prophylaxis and treatment for COVID-19 and plans to seek U.S. emergency use authorization. Globe Newswire; (2022). https://investors.adagiotx.com/news-releases/news-release-details/adagio-therapeutics-announces-adg20-adintrevimab-first [Google Scholar]
- 54.Montgomery H, Hobbs FDR, Padilla F et al. Efficacy and safety of intramuscular administration of tixagevimab-cilgavimab for early outpatient treatment of COVID-19 (TACKLE): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Respir. Med., (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Desai A, Gyawali B. Endpoints used in phase III randomized controlled trials of treatment options for COVID-19. EClinicalMedicine. 23, 100403 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Beigel JH, Tomashek KM, Dodd LE et al. Remdesivir for the treatment of COVID-19 – final report. N. Engl. J. Med. 383(19), 1813–1826 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Atea Pharmaceuticals announces U.S. FDA fast track designation granted to bemnifosbuvir, an investigational oral antiviral, for the treatment of COVID-19. News release. Atea pharmaceuticals; (2023). https://ir.ateapharma.com/news-releases/news-release-details/atea-pharmaceuticals-announces-us-fda-fast-track-designation-0 [Google Scholar]
- 58.Clinicaltrials.Gov. SUNRISE-3: efficacy and safety of bemnifosbuvir in high-risk outpatients with COVID-19. NCT05629962; (2022). https://clinicaltrials.gov/ct2/show/NCT05629962 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


