Abstract
Background
Non-alcoholic fatty liver disease (NAFLD) is easily neglected in the non-obese population. TyG index (triglyceride glucose Index) and TG/HDL-c (triglyceride to high-density lipoprotein cholesterol) are new indicators to evaluate insulin resistance (IR). Fibroscan is a non-invasive way to assess hepatic steatosis [by control attenuation parameters (CAP)] and fibrosis [by liver stiffness measurement (LSM)].The purpose of this study was to explore the correlation of TyG and its combination with obesity indicators [TyG-waist circumference (WC), TyG-body mass index (BMI)] and TG/HDL-c with CAP and LSM.
Method
One thousand seven hundred seventy-six adults (age ≥ 20 years, BMI < 30 kg/m2) in the National Health and Nutrition Examination Survey (NHANES) 2017–2018 were included. The correlations of CAP and LSM to the indexes were assessed by generalized linear models.. Receiver operating characteristic (ROC) curve was performed to evaluate the diagnostic capability of the indicators on NAFLD and liver stiffness.
Results
Survey-weighted percentage of NAFLD in non-obese was 38.6%. In the fully adjusted models, there were positive associations of TyG, TyG-BMI, TyG-WC and TG/HDL-c to CAP, with the βs of 24.810, 0.704, 0.29 and 2.983 (all p < 0.05), respectively. There were positive associations of TyG, TyG-BMI, TyG-WC, and TG/HDL-c to NAFLD, with ORs of 3.387, 1.03, 1.010 and 1.281 ((all p < 0.05)).The positive association was detected for TG/HDL-c and TyG-WC and LSM with βs of 0.057 and 0.004(p = 0.021 and p = 0.003).TyG-WC were positively associated with liver stiffness with OR of 1.006(95%CI = 1.002, 1.012). Furthermore, the TyG-WC had the strongest diagnostic capability (ROC = 0.806; 95%CI: 0.785–0.826) on NAFLD in non-obese participants, with a specificity of 0.737 and sensitivity of 0.746.
Conclusion
In US non-obese population, the TyG, TyG-BMI, TyG-WC, and TG/HDL-c are positively correlated with CAP and NAFLD. TyG-WC has clinical importance in identifying NAFLD in the non-obese population.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12876-023-03007-8.
Keywords: NAFLD, Non-obese, TyG, TG, HDL-c, Control attenuation parameters
Introduction
NAFLD, which is supposed to be one component of metabolic syndrome, affects approximately 25% of adults worldwide [1]. NAFLD will become the main cause of end-stage liver disease in the coming decades and exert great economic pressure on the global health care system [2, 3]. Therefore, early identification of NAFLD is of vital importance for the intervention. NAFLD usually goes like the shadow following obesity. However, it also insidiously occurs in non-obese individuals [4]. The occurrence of NAFLD in non-obese subjects is easy to be neglected. Globally, the prevalence of NAFLD is 3%—30% among non-obese subjects, defined as BMI < 25 kg/m2 in Asian and < 30 kg/m2 in the Occident [5]. Therefore, simple and convenient indicators are required to identify NAFLD in non-obese individuals.
TyG and its combination with other obesity indicators (TyG-BMI, TyG-WC) and TG/HDL-c are emerging indicators for evaluating insulin resistance (IR) in obese or type 2 diabetes mellitus (T2DM) individuals [6]. IR closely correlates with NAFLD, and the prevalence of NAFLD in T2DM is fivefold higher than those without diabetes [7]. However, in the non-obese population, the relationship between the above emerging indicators and NAFLD has not been investigated.
Due to the asymptomatic nature of NAFLD, early detection has proven to be challenging, particularly in non-obese individuals. Liver biopsy has traditionally been regarded as the definitive method for diagnosing NAFLD, but its invasiveness and high cost have hindered its widespread use [8]. Large-scale epidemiological studies mainly use liver enzymes and ultrasound to screen NAFLD. However, these indexes have low sensitivity and cannot identify high-risk NAFLD [9, 10]. Vibration-controlled transient elastography (VCTE) can quantify liver fat by using the controlled attenuation parameter (CAP), with a sensitivity of 87% and a specificity of 91% for detecting hepatic steatosis [11]. Liver steatosis (CAP) calculated by transient elastography (FibroScan) is an evidence-based noninvasive method to evaluate NAFLD [12]. Meanwhile, liver stiffness measurement (LSM) using VCTE has emerged as a commonly employed technique in gastroenterology and hepatology clinics [13]. While there are simple indicators available, such as the AST to-platelet ratio index (APRI) and Fibrosis-4 (FIB-4) index, to assess liver fibrosis, there remains a dearth of effective and straightforward indicators to identify liver steatosis specifically in non-obese populations [14].
This investigation was conducted to evaluate the prevalence of NAFLD using FibroScan in non-obese individuals. The relationships between TyG, TyG-BMI, TyG-WC, TG /HDL-c, and CAP were investigated. Furthermore, the diagnostic capabilities of the indexes on NAFLD and liver stiffness in the non-obese US population were also detected.
Methods
Data sources
The NHANES (National Health and Nutrition Examination Survey) is a population-based cross-sectional survey designed to collect information on the health and nutrition status of adults and children in the United States of America. Most of the data in NHANES is freely accessible to researchers worldwide. This investigation extracted data from 2017 to 2018.
Study population
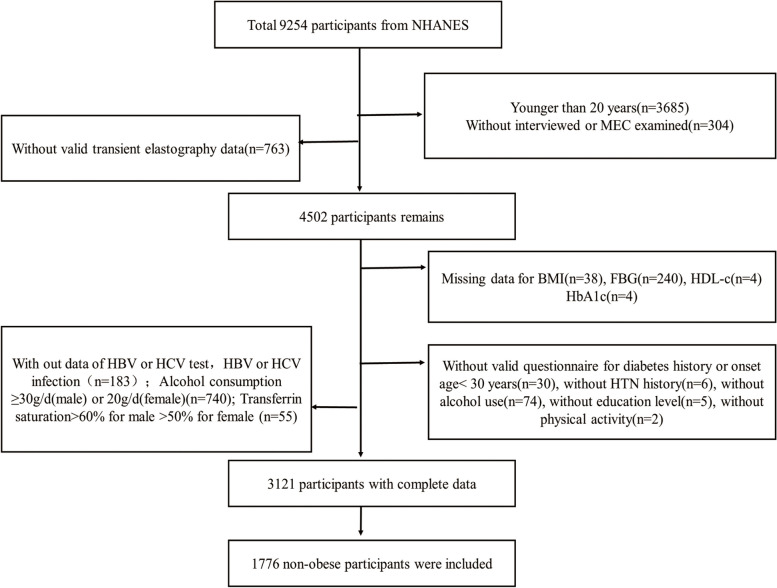
A total of 9254 persons were enrolled in NHANES from 2017 to 2018. Among them, 3685 persons were excluded for younger than 20 years and 304 participants were excluded for incomplete survey data. Another 763 persons were excluded for lack of transient elastography data. A total of 403 individuals were excluded for lack of laboratory examination data (FBG, HDL-C, HbA1c), anthropometric data (BMI), invalid questionnaire data (history of diabetes, history of HTN, alcohol use, education level, physical activity), and individuals with diabetes onset age less than 30 years old (to minimize the confounding factor for type 1 diabetes mellitus). Individuals (n = 183) were excluded because of no data of HBV or HCV test and with HBV or HCV infection (positive serum hepatitis B surface antigen and positive serum hepatitis C antibody). Individuals with alcohol consumption ≥ 30 g/d (male) or 20 g/d (female) were also excluded (n = 740). Participants with transferrin saturation > 60% for male and > 50% for female were also excluded (n = 55). Finally, 1776 individuals (BMI < 30 kg/m2) were included for investigation. See details in Fig. 1.
Fig. 1.
Flow chart for the population included in the study
Study variables
The index for our investigation was TyG, TyG-BMI, TyG-WC, and TG/HDL-c. TyG was calculated by formula of Ln[TG (mg/dL) × FPG (mg/dL)/2]. TyG-BMI was calculated by TyG × BMI and TyG-WC was calculated by TyG × WC. TG/HDL-c was defined as the ratio of TG to HDL-c. T2DM was defined by self-report or by HbA1c ≥ 6.5%. Hypertension was defined by self-report. Physical activity status was defined as a continuously vigorous and intensive activity for at least 10 min in a typical week. Education level was stratified according to whether he or she had attended high school. BMI < 30 kg/m2 was defined as non-obese. CAP ≥ 248 dB/m was defined as NAFLD [15]. The examination was considered reliable when a minimum of 10 valid measurements were acquired, with an interquartile range/median LSM ratio of less than 30%, following a fasting period of at least 3 h. 24-Hour Food Recalls were conducted to get data on alcohol intake. Sociodemographic variables such as age, gender, and race were extracted from the file named demographic variables. BMI and WC were extracted from the file named body measures. FBG, HbA1c, Total cholesterol (TC), TG, HDL-c, Alanine aminotransferase (ALT), transferrin saturation, hepatitis B, and hepatitis C were extracted from the laboratory data. Alcohol intake was extracted from dietary data.
Statistical analyses
Analysis was performed by R packages and Empower Stats (http://www.empowerstats.com). Weighted analysis was performed using survey weights of NHANES. The association of TyG, TyG-BMI, TyG-WC, and TG/HDL-c with NAFLD (reflected by CAP) and liver stiffness (reflected by LSM) was evaluated by the generalized linear regression model. The models were adjusted for age, gender, race, alcohol use, education level, physical activity, BMI, HTN, diabetes status, and TC. The diagnosis indicators and determine the threshold value were evaluated by ROC curve analysis. Analysis of Variance (ANOVA) was conducted for evaluating the prevalence of NAFLD in different quantiles of TyG-WC groups. P-value < 0.05 is defined as a significant difference.
Results
Participants were divided into two groups according to NAFLD or not. The survey-weighted percentage for NAFLD was 38.6% (95%CI: 33.9%, 43.3%). The mean CAP was 291.7(287.7, 295.6) dB/m in the NAFLD group. The mean LSM was 5.3 (5.0,5.6) kpa in NAFLD group. Compared with the non-NAFLD group, the TyG, and its combination indicators with obesity (TyG-BMI and TyG-WC) and TG/HDL-c indexes were higher than the NAFLD group (all p < 0.001). See details for weighted characteristics of participants included in the study in Table 1.
Table 1.
Weighted characteristics of participants included in the study
| Items | Total (n = 1776) | Non-NAFLD (n = 992) | NAFLD (n = 784) | p-value |
|---|---|---|---|---|
| Age (years) | 48.6 (46.9,50.3) | 44.1 (42.3,46.0) | 55.8 (53.9,57.7) | < 0.001 |
| Gender = male (%) | 48.8 (44.8,52.8) | 42.8 (36.6,49.2) | 58.3 (52.4,64.0) | < 0.001 |
| Ethic = Non-Hispanic White (%) | 63.1 (56.2,69.5) | 65.2 (58.8,71.1) | 59.9 (50.1,68.9) | 0.114 |
| Education = Less than high school (%) | 10.4 (7.9,13.5) | 8.9 (6.8,11.4) | 12.9 (9.0,18.0) | 0.008 |
| Alcohol use (%) | 90.7 (87.8,92.9) | 91.0 (88.2,93.1) | 90.2 (85.3,93.6) | 0.681 |
| Diabetes (%) | 9.1 (7.9,10.5) | 3.7 (2.6,5.3) | 17.7 (14.8,21.0) | < 0.001 |
| HTN (%) | 24.2 (21.0,27.7) | 16.5 (12.9,20.8) | 36.5 (32.2,40.9) | < 0.001 |
| Physical activity (%) | 25.2 (20.9,30.1) | 25.9 (20.3,32.3) | 24.2 (19.3,29.9) | 0.603 |
| TG (mg/dL) | 124.1 (118.3,129.9) | 101.1 (97.1,105.1) | 160.7 (151.1,170.4) | < 0.001 |
| TC (mg/dL) | 189.0 (185.4,192.6) | 185.9 (181.3,190.6) | 193.9 (189.4,198.4) | 0.017 |
| HDL-c (mg/dL) | 55.8 (55.0,56.6) | 58.7 (58.0,59.5) | 51.1 (49.4,52.8) | < 0.001 |
| BMI (kg/m2) | 25.0 (24.7,25.2) | 24.0 (23.7,24.3) | 26.5 (26.1,26.9) | < 0.001 |
| WC (cm) | 89.8 (89.0,90.5) | 85.9 (85.0,86.8) | 95.9 (95.1,96.8) | < 0.001 |
| HbA1c (%) | 5.6 (5.5,5.6) | 5.4 (5.4,5.4) | 5.9 (5.8,5.9) | < 0.001 |
| CAP (dB/m) | 238.2 (234.3,242.1) | 204.6 (201.5,207.8) | 291.7 (287.7,295.6) | < 0.001 |
| CAP categorical = NAFLD (%) | 38.6 (33.9, 43.4) | 0 | 100 | < 0.001 |
| TyG | 8.5 (8.5,8.6) | 8.3 (8.3,8.3) | 8.8 (8.8,8.9) | < 0.001 |
| TG/HDL | 2.6 (2.5,2.8) | 1.9 (1.8,2.0) | 3.7 (3.4,4.1) | < 0.001 |
| TyG-BMI | 213.1 (210.4, 215.8) | 199.8 (196.7, 203.0) | 234.2 (230.2, 238.2) | < 0.001 |
| TyG-WC | 767.2 (758.2, 776.1) | 716.0 (707.2, 724.8) | 848.9 (837.4, 860.5) | < 0.001 |
| LSM (Kpa) | 4.9 (4.7, 5.0) | 4.6 (4.4, 4.9) | 5.3 (5.0, 5.6) | < 0.001 |
| LSM categorical = liver stiffness (%) | 6.4 (4.7, 8.7) | 3.8 (2.8, 5.1) | 10.6 (6.5, 16.6) | < 0.001 |
For continuous variables: survey-weighted mean (95% CI), P-value was by survey-weighted linear regression. For categorical variables: survey-weighted percentage (95% CI), P-value was by survey-weighted Chi-square test
HTN hypertension, TG Triglyceride, TC Total cholesterol, HDL-c High density lipoprotein cholesterol, BMI body mass index, WC waist circumference, HbA1c glycosylated hemoglobin, CAP Controlled attenuation parameter, NAFLD Non-alcoholic fatty liver disease, TyG Ln[TG (mg/dL) × FPG (mg/dL)/2], TyG-BMI TyG × BMI, TyG-WC TyG × WC, TG/HDL-c ratio of TG to HDL-c
Generalized linear regression models were performed to evaluate the relationship between CAP and NAFLD and the above indexes. After fully adjusting for age, BMI, gender, race, education level, alcohol use, diabetes, HTN, physical activity, and TC, TyG and its combination indexes TyG-BMI and TyG-WC were positively correlated with CAP with the βs (95%CI) of 24.810 (95%CI 21.339, 28.280) (P < 0.001), 0.704 (95% CI 0.597, 0.811) (P < 0.001) and 0.219 (95%CI 0.149, 0.289) (P < 0.001), respectively. TG/HDL-c also had a positive relationship with CAP [β = 2.983(95%CI 1.433, 4.533), P = 0.004]. See details in Table 2. After fully adjusting for age, BMI, gender, race, education level, alcohol use, diabetes, HTN, physical activity, and TC, a positive relationship between TyG, TyG-BMI, TyG-WC, and NAFLD was found with ORs of 3.387 (95%CI 2.328, 4.928) (P < 0.001), 1.032 (95% CI 1.019, 1.045) (P = 0.001) and 1.010 (95%CI 1.007, 1.013) (P = 0.002), respectively. TG/HDL-c also had a positive correlation with NAFLD with the β = 1.281 (95%CI 1.169, 1.403) (P = 0.006). See details in Table 2.
Table 2.
The relationship between TyG, TyG-BMI, TyG-WC, TG/HDL-c and CAP and NAFLD (CAP ≥ 248 dB/m)
| Exposure | CAP (dB/m) [β(95%CI) p-value] | NAFLD [OR (95%CI) p-value] |
|---|---|---|
| TyG | 24.810 (21.339,28.280) < 0.001 | 3.387 (2.328, 4.928) < 0.001 |
| TG/HDL | 2.983 (1.433, 4.533) 0.004 | 1.281 (1.169, 1.403) 0.006 |
| TyG-BMI | 0.704 (0.597, 0.811) < 0.001 | 1.032 (1.019, 1.045) 0.001 |
| TyG-WC | 0.219 (0.149, 0.289) < 0.001 | 1.010 (1.007, 1.013) 0.002 |
Age, BMI, gender, ethic, education level, alcohol use, diabetes, HTN, physical activity and TC were adjusted. For the index of TyG-BMI, BMI was not adjusted
TyG Ln[TG (mg/dL) × FPG (mg/dL)/2], TyG-BMI TyG × BMI, TyG-WC TyG × WC, TG/HDL-c ratio of TG to HDL-c
Generalized linear regression models were used to assess the relationships between LSM and various indexes. After adjusting for age, BMI, gender, race, education level, alcohol use, diabetes, HTN, physical activity, and TC, we observed a positive association between TG/HDL-c and TyG-WC with LSM. The estimated βs (95%CI) were 0.057 (0.009, 0.105) and 0.004 (-0.000, 0.008) (P = 0.021 and 0.003), respectively. Additionally, TyG-WC showed a positive association with liver stiffness, with an OR (95% CI) of 1.006 (1.002, 1.012) (P = 0.013). Please refer to Table 3 for further details.
Table 3.
The relationship between TyG, TyG-BMI, TyG-WC, TG/HDL-c and LSM and liver stiffness (LSM ≥ 7 kpa)
| Exposure | LSM (kpa) [β(95%CI) p-value] | Liver stiffness [OR (95%CI) p-value] |
|---|---|---|
| TyG | 0.382 (-0.115, 0.878) 0.071 | 1.555 (0.875, 2.763) 0.071 |
| TG/HDL | 0.057 (0.009, 0.105) 0.021 | 1.052 (0.998, 1.131) 0.112 |
| TyG-BMI | 0.004 (-0.004, 0.011) 0.234 | 1.004 (0.988, 1.020) 0.567 |
| TyG-WC | 0.004 (-0.000, 0.008) 0.003 | 1.006 (1.002, 1.012) 0.013 |
Age, BMI, gender, ethic, education level, alcohol use, diabetes, HTN, physical activity and TC were adjusted. For the index of TyG-BMI, BMI was not adjusted. TyG = Ln[TG (mg/dL) × FPG (mg/dL)/2]
TyG-BMI TyG × BMI, TyG-WC TyG × WC, TG/HDL-c ratio of TG to HDL-c
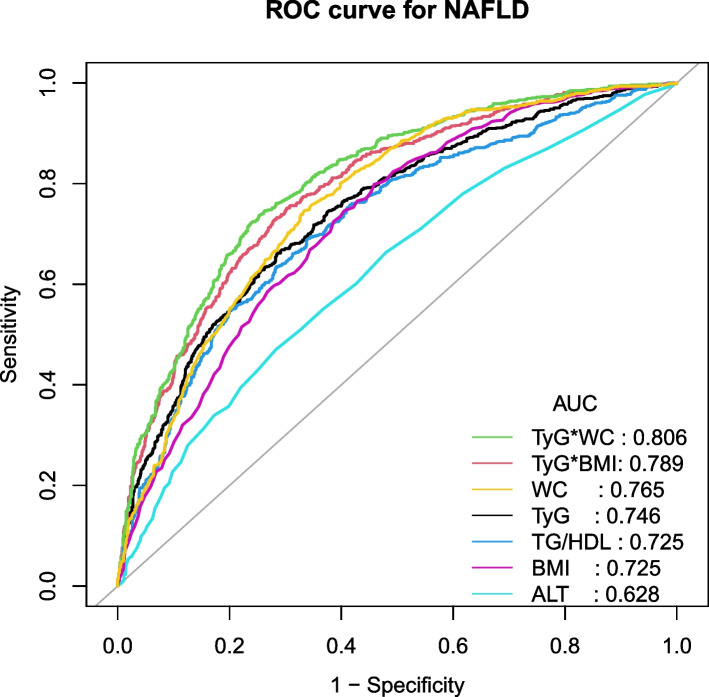
ROC curve analysis was performed to clarify the diagnostic capabilities of TyG, TyG-BMI, TyG-WC, TG/HDL-c, BMI, WC, and ALT for NAFLD in the non-obese population. Table 4 shows the diagnostic capability for NAFLD. In ascending order for AUC, the values of indexes were shown as followings: ALT [AUC = 0.628 (95%CI: 0.602, 0.654)], BMI [AUC = 0.725 (95%CI: 0.702, 0.748)], TG/HDL[AUC = 0.725 (95%CI: 0.701, 0.749)], TyG [AUC = 0.746 (95%CI: 0.723, 0.769)], WC[AUC = 0.765 (95%CI: 0.743, 0.787)], TyG-BMI [AUC = 0.789 (95%CI: 0.768, 0.810)] and TyG-WC [AUC = 0.806 (95%CI: 0.785, 0.826)]. TyG-WC index could be used to predict NAFLD with a specificity of 0.737, a sensitivity of 0.746, and the best threshold of 791.135. The ROC curves were shown in Fig. 2. TyG-WC was divided into 5 quantile groups (quantiles 1–5). The incidence of NAFLD in different quartile groups was 9.5, 18.4, 34.7, 58.5, and 83.1% (P-anova < 0.001). See details in Table 5. Supplementary Table 1 shows the diagnostic capability for liver stiffness. The TyG-WC were shown the highest value of AUC of 0.655(95%CI = 0.605, 0.706) compared with other indexes.
Table 4.
AUC of TyG-BMI, TyG-WC, TyG, BMI, WC, ALT, and TG/HDL-C for diagnosing NAFLD
| Variables | AUC | 95%CI low | 95%CI upp | Best threshold | Specificity | Sensitivity |
|---|---|---|---|---|---|---|
| TyG | 0.746 | 0.723 | 0.769 | 8.598 | 0.714 | 0.662 |
| TyG-BMI | 0.789 | 0.768 | 0.810 | 219.810 | 0.698 | 0.751 |
| TyG-WC | 0.806 | 0.785 | 0.826 | 791.135 | 0.737 | 0.746 |
| TG/HDL | 0.725 | 0.701 | 0.749 | 2.184 | 0.716 | 0.635 |
| ALT | 0.628 | 0.602 | 0.654 | 19.500 | 0.718 | 0.470 |
| BMI | 0.725 | 0.702 | 0.748 | 25.050 | 0.595 | 0.746 |
| WC | 0.765 | 0.743 | 0.787 | 90.650 | 0.666 | 0.746 |
TyG Ln[TG (mg/dL) × FPG (mg/dL)/2], TyG-BMI TyG × BMI, TyG-WC TyG × WC, TG/HDL-c ration of TG to HDL-c, BMI body mass index, WC waist circumference, ALT Alanine aminotransferase, AUC area under curve
Fig. 2.
ROC curves for NAFLD
Table 5.
The prevalence of NAFLD in 5 quantiles of TyG-WC
| Groups for TyG-WC | Survey-weighted percentage for distribution (95%CI) (%) | Survey-weighted percentage for the prevalence of NAFLD (95%CI) (%)a |
|---|---|---|
| Quantiles 1 (476.1–661.4) | 22.4 (19.3,25.8) | 9.5 (5.1,16.9) |
| Quantiles 2 (661.4–747.1) | 21.4 (18.8,24.2) | 18.7 (15.0,23.1) |
| Quantiles 3 (747.1–812.9) | 21.2 (17.9,24.9) | 34.7 (25.5,45.3) |
| Quantiles 4 (812.9–884.4) | 16.6 (14.5,19.0) | 58.5 (51.1,65.5) |
| Quantiles 5 (884.4–1273.4) | 18.4 (15.4,21.8) | 83.1 (72.3,90.3) |
aindicated P-anova < 0.001. P-anova for the prevalence of NAFLD in 5 quantiles groups was calculated by weighted linear regression model
Discussion
Searching for a noninvasive and simple way to screen NAFLD has always been a hot topic for large-scale population studies. In this study, the relationship between NAFLD and the indexes of TyG, TyG-BMI, TyG-WC, and TG/HDL-c in the non-obese population was investigated; It was found that each of them was positively correlated with CAP and NAFLD. Among them, TyG-WC was the most effective in screening NAFLD in the non-obese population. This study also explored the relationship between liver cirrhosis and TyG and its combined indicators. Although TG/HDL-c and TyG-WC were positively correlated with LSM, only Tyg-WC and were independent risk factors for liver cirrhosis in non-obese population.
Due to different patient selections, diagnostic methods, BMI threshold, and lifestyles of the assessed population, the prevalence of non-obese NAFLD vary widely, ranging from 3 to 30% worldwide. A meta-analysis (2020) showed that around 40% of NAFLD occurs was non-obese population, and nearly 20% of NAFLD seemed to be lean individuals [16]. It emphasized that obesity should not be the only criterion for NAFLD screening. In this investigation, the prevalence of NAFLD, diagnosed by FibroScan, was 38.6% in the non-obese population, and it was 56.7% in the whole population diagnosed by the same cut-off value [17]. The estimated prevalence of non-alcoholic fatty liver disease (NAFLD) has been reported to vary widely, ranging from 7 to 26% [18]. It is worth noting that the prevalence of NAFLD in our study is relatively high. This could be attributed to the relatively low cutoff point used in the diagnostic criteria for NAFLD (CAP ≥ 248 db/m), as well as the relatively high cutoff point for non-obese diagnostic (BMI < 30 kg/m2). The high prevalence rate emphasizes the importance of identifying non-obese NAFLD through a simple and convenient method.
The pathophysiological mechanisms of non-obese NAFLD are similar to that of obese NAFLD. However, there are unique characteristics in the pathogenesis of non-obese NAFLD [19]. The key process is the overload of free fatty acids (FFAs), which makes the disposal mechanisms overwhelmed, thus leading to liver steatosis [20]. IR also contributed to NAFLD in both obese and non-obese populations, via increasing de novo lipogenesis in liver; Besides, IR can also indirectly promote the occurrence of NAFLD by reducing the inhibition of lipolysis in the fat depots and increasing the delivery of FFAs to the liver [21, 22]. It was reported visceral adiposity contributed as much as 50% to the level of FFAs in the portal vein [23]; Visceral fat accumulation in non-obese NAFLD was more serious than that in non-obese non-NAFLD; Therefore, visceral fat may be a promoting factor in the development of non-obese NAFLD. Since lean body mass is responsible for glucose disposal mediated by insulin, sarcopenia may cause impaired glucose tolerance and IR. In that case, sarcopenia was also reported to trigger non-obese NAFLD [19]. Genetic polymorphisms in PNPLA3, TM6SF2, GCKR, and MBOAT7, may also contribute to the occurrence of NAFLD in non-obese [24–26]. NAFLD in non-obese individuals could also develop into the decompensated end stage of cirrhosis [19]. Therefore, it is of great importance to identify NAFLD in non-obese individuals.
TyG, its combination with obesity and TG/HDL-c are novel indicators to evaluate IR in epidemiological studies. IR is closely related to NAFLD. TyG emerged as a new force, which was shown to be superior to HOMA-IR in evaluating IR [27–29]. A previous study demonstrated that the TyG index could be used to predict a diagnosis of NAFLD with AUC 0.763 in children [30]. TyG is also positively related to the development of liver fibrosis evaluated by fibrosis score and fibrosis-4 (Fib-4) in NAFLD patients [31]. In Chinese adults, a study found that TyG can effectively identify NAFLD evaluated by ultrasound scan, with AUC 0.782 and threshold 8.5 [32]. For TG/HDL-c, a study demonstrated that the TG/HDL-C ratio is associated with NAFLD with an AUC of 0.675 [33]. Waist circumference, as an index of visceral fat accumulation, is an important parameter to identify fatty liver in non-obese people. NAFLD patients had a greater WC than non-NAFLD in non-obese [34, 35]. Our study found that the combination of TyG and waist circumference (TyG-WC) was superior to other indicators in assessing NAFLD, which might be because the index reflects the combination of IR and visceral fat. This indicator is easily available and of great significance in screening NAFLD in a large-scale population study.
As far as we know, this is the first time to explore the relationship between liver steatosis (indicated by CAP) with TyG, TyG-BMI, TyG-WC, and TG / HDL-C in non-obese people. For every 1 unit increase in TyG, TyG-BMI, TyG-WC, and TG/HDL-c, the corresponding increases in CAP were 24.810, 2.983, 0.704, and 0.219 dB/m. It is observed that for non-obese NAFLD, lifestyle modification had little effect on reducing hepatic steatosis. However, this study reminds us that revising the above indexes may help to improve liver steatosis. Since the risk of NAFLD is closely associated with TyG-WC, interventions aiming to reduce triglyceride, glucose control, and abdominal obesity may be beneficial for decreasing the risk of NAFLD.
Liver biopsy remains the gold standard for diagnosis of NAFLD. However, due to the limitations of sampling variability, invasiveness, and high cost, liver biopsy cannot be used in large-scale epidemiological investigations [36]. Currently, ultrasound (US) B-mode imaging is the first-line method for diagnosing NAFLD, which allows subjective evaluation of fatty infiltration. However, it has low efficiency in detecting mild steatosis [37]. Magnetic resonance imaging-derived proton density fat fraction (MRI-PDFF) seems to be the most accurate noninvasive diagnostic method for NAFLD. However, MRI-PDFF cannot be widely used in the general population for high requirements for equipment. The controlled attenuation parameter (CAP) is a parameter based on ultrasonic signals, which is measured with M or XL probe by FibroScan ®. NAFLD could be diagnosed by CAP with an AUC of 0.82 at the cut-off value of 248 dB/m [15]. CAP represents the first-line method to screen NAFLD in the general population [38]. However, few studies have evaluated the accuracy of CAP in diagnosing NAFLD based on liver biopsy. More population-based studies are needed to clarify the optimal cut-off point for the diagnosis of NAFLD by CAP.
When exploring the relationship between LSM and TyG related indicators, we found that the diagnostic ability of TyG-WC in liver cirrhosis was not very prominent (AUC = 0.655) in non-obese individuals. This remains that TyG-WC was insufficient to predict liver fibrosis in the non-obese individuals. Previous studies have found that although TyG is associated with liver fibrosis in patients with NAFLD [31], it does not demonstrate sufficient diagnostic efficacy for liver fibrosis (AUC = 0.589) [39]. In obese and overweight without diabetes, the TyG-WC in diagnosis liver fibrosis was also relatively limited (AUC = 0.618) [40]. However, it was also found TyG index was independently associated with nonalcoholic steatohepatitis with AUC of 0.75 [41].
Cirrhosis has placed a significant economic and healthcare burden on numerous countries, particularly in the past three decades [14]. The influence of hepatitis B and C is projected to diminish in the near future, giving way to the prominence of non-alcoholic steatohepatitis (NASH) [42]. To achieve early detection of NAFLD, particularly in low-income countries, cost-effective interventions are essential. Fasting plasma glucose, triglycerides, and waist circumference are three easily obtainable physiological indicators. The TyG-WC, which incorporates these three indicators, can facilitate primary healthcare professionals in assessing participants' risk of NAFLD. The identification of this indicator holds valuable implications for the early diagnosis and treatment of NAFLD in non-obese individuals.
This study has several limitations. Firstly, the influence of medications used for glycemic control and dyslipidemia on TyG levels could not be accounted for in this research, which may introduce a confounding factor. Secondly, liver biopsy is considered the gold standard for diagnosing NAFLD, but it was not feasible due to the high risk and large sample size involved in this study. Therefore, alternative non-invasive methods were used to assess liver stiffness. Thirdly, the study participants were predominantly Americans, and caution should be exercised when extrapolating the findings to other ethnic populations worldwide. Different ethnic groups may have varying obesity standards, and this study did not conduct further subgroup analysis based on ethnicity.
Conclusion
In the non-obese population, the indexes of TyG, TyG-BMI, TyG-WC, and TG/HDL-c were positively correlated with CAP and NAFLD. Among these indexes, TyG-WC was superior to others in identifying NAFLD.
Supplementary Information
Additional file 1: Supplementary Table 1. AUC of TyG-BMI, TyG-WC, TyG, BMI, WC, ALT, and TG/HDL-C for diagnosing liver stiffness.
Acknowledgements
The authors thank the staff and the participants of the NHANES study for their valuable contributions.
Authors’ contributions
Shuying Li and Li Feng contributed to data collection, statistical analysis, and writing and revising of the manuscript. Jie Ding contributed to the statistical analysis. Jiangfeng Mao, Tangbin Yuan and Weihong Zhou supervised the study and contributed to the polishing and reviewing of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Beijing Municipal Natural Science Foundation (No. 7212080) and the Health Science and Technology Development Major Project of Nanjing (No. ZDX21001).
Availability of data and materials
The data that support the findings of this study are available from the corresponding author Dr. Mao upon reasonable request.
Declarations
Ethics approval and consent to participate
The study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving research study participants were approved by the National Center for Health Statistics Research Ethics Review Board. Written informed consent was obtained from all subjects.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Shuying Li and Li Feng contributed equally to this work.
Contributor Information
Weihong Zhou, Email: njzhouwh@126.com.
Tangbin Yuan, Email: 2655130265@qq.com.
Jiangfeng Mao, Email: maojiangfeng88@vip.sina.com.
References
- 1.Younossi ZM, et al. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 2.Younossi Z, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15(1):11–20. doi: 10.1038/nrgastro.2017.109. [DOI] [PubMed] [Google Scholar]
- 3.Estes C, et al. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67(1):123–133. doi: 10.1002/hep.29466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agorastos A, Bozikas VP. Gut microbiome and adaptive immunity in schizophrenia. Psychiatriki. 2019;30(3):189–192. doi: 10.22365/jpsych.2019.303.189. [DOI] [PubMed] [Google Scholar]
- 5.Kim D, Kim WR. Nonobese fatty liver disease. Clin Gastroenterol Hepatol. 2017;15(4):474–485. doi: 10.1016/j.cgh.2016.08.028. [DOI] [PubMed] [Google Scholar]
- 6.Sanchez-Garcia A, et al. Diagnostic accuracy of the triglyceride and glucose index for insulin resistance: a systematic review. Int J Endocrinol. 2020;2020:4678526. doi: 10.1155/2020/4678526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khan RS, et al. Modulation of insulin resistance in nonalcoholic fatty liver disease. Hepatology. 2019;70(2):711–724. doi: 10.1002/hep.30429. [DOI] [PubMed] [Google Scholar]
- 8.Khalifa A, Rockey DC. The utility of liver biopsy in 2020. Curr Opin Gastroenterol. 2020;36(3):184–191. doi: 10.1097/MOG.0000000000000621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwenzer NF, et al. Non-invasive assessment and quantification of liver steatosis by ultrasound, computed tomography and magnetic resonance. J Hepatol. 2009;51(3):433–445. doi: 10.1016/j.jhep.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 10.Torres DM, Harrison SA. Diagnosis and therapy of nonalcoholic steatohepatitis. Gastroenterology. 2008;134(6):1682–1698. doi: 10.1053/j.gastro.2008.02.077. [DOI] [PubMed] [Google Scholar]
- 11.Pu K, et al. Diagnostic accuracy of controlled attenuation parameter (CAP) as a non-invasive test for steatosis in suspected non-alcoholic fatty liver disease: a systematic review and meta-analysis. BMC Gastroenterol. 2019;19(1):51. doi: 10.1186/s12876-019-0961-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oeda S, et al. Diagnostic accuracy of fibroscan and factors affecting measurements. Diagnostics. 2020;10(11):940. doi: 10.3390/diagnostics10110940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mozes FE, et al. Diagnostic accuracy of non-invasive tests for advanced fibrosis in patients with NAFLD: an individual patient data meta-analysis. Gut. 2022;71(5):1006–1019. doi: 10.1136/gutjnl-2021-324243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lomonaco R, et al. Advanced liver fibrosis is common in patients with type 2 diabetes followed in the outpatient setting: the need for systematic screening. Diabetes Care. 2021;44(2):399–406. doi: 10.2337/dc20-1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karlas T, et al. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J Hepatol. 2017;66(5):1022–1030. doi: 10.1016/j.jhep.2016.12.022. [DOI] [PubMed] [Google Scholar]
- 16.Ye Q, et al. Global prevalence, incidence, and outcomes of non-obese or lean non-alcoholic fatty liver disease: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5(8):739–752. doi: 10.1016/S2468-1253(20)30077-7. [DOI] [PubMed] [Google Scholar]
- 17.Zhang X, et al. Prevalence and factors associated with NAFLD detected by vibration controlled transient elastography among US adults: results from NHANES 2017–2018. PLoS One. 2021;16(6):e0252164. doi: 10.1371/journal.pone.0252164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahadi M, et al. A review of non-alcoholic fatty liver disease in non-obese and lean individuals. J Gastroenterol Hepatol. 2021;36(6):1497–1507. doi: 10.1111/jgh.15353. [DOI] [PubMed] [Google Scholar]
- 19.Phipps M, Wattacheril J. Non-alcoholic fatty liver disease (NAFLD) in non-obese individuals. Frontline Gastroenterol. 2020;11(6):478–483. doi: 10.1136/flgastro-2018-101119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuchay MS, Choudhary NS, Mishra SK. Pathophysiological mechanisms underlying MAFLD. Diabetes Metab Syndr. 2020;14(6):1875–1887. doi: 10.1016/j.dsx.2020.09.026. [DOI] [PubMed] [Google Scholar]
- 21.Donnelly KL, et al. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115(5):1343–1351. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu X, et al. Liraglutide ameliorates non-alcoholic steatohepatitis by inhibiting NLRP3 inflammasome and pyroptosis activation via mitophagy. Eur J Pharmacol. 2019;864:172715. doi: 10.1016/j.ejphar.2019.172715. [DOI] [PubMed] [Google Scholar]
- 23.Nielsen S, et al. Splanchnic lipolysis in human obesity. J Clin Invest. 2004;113(11):1582–1588. doi: 10.1172/JCI21047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sookoian S, Pirola CJ. Systematic review with meta-analysis: risk factors for non-alcoholic fatty liver disease suggest a shared altered metabolic and cardiovascular profile between lean and obese patients. Aliment Pharmacol Ther. 2017;46(2):85–95. doi: 10.1111/apt.14112. [DOI] [PubMed] [Google Scholar]
- 25.Romeo S, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40(12):1461–1465. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mancina RM, et al. The MBOAT7-TMC4 variant rs641738 increases risk of nonalcoholic fatty liver disease in individuals of European descent. Gastroenterology. 2016;150(5):1219–1230.e6. doi: 10.1053/j.gastro.2016.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tao L, et al. Triglyceride-glucose index as a marker in cardiovascular diseases: landscape and limitations. Cardiovasc Diabetol. 2022;21(1):68. doi: 10.1186/s12933-022-01511-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simental-Mendía LE, Rodríguez-Morán M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. 2008;6(4):299–304. doi: 10.1089/met.2008.0034. [DOI] [PubMed] [Google Scholar]
- 29.Behiry EG, et al. Evaluation of TG-HDL ratio instead of HOMA ratio as insulin resistance marker in overweight and children with obesity. Endocr Metab Immune Disord Drug Targets. 2019;19(5):676–682. doi: 10.2174/1871530319666190121123535. [DOI] [PubMed] [Google Scholar]
- 30.Ye X, et al. Pentraxin 3 and the TyG Index as Two Novel Markers to Diagnose NAFLD in Children. Dis Markers. 2021;2021:1–7. doi: 10.1155/2021/8565943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tutunchi H, et al. Triglyceride glucose (TyG) index and the progression of liver fibrosis: a cross-sectional study. Clin Nutr ESPEN. 2021;44:483–487. doi: 10.1016/j.clnesp.2021.04.025. [DOI] [PubMed] [Google Scholar]
- 32.Zhang S, et al. The triglyceride and glucose index (TyG) is an effective biomarker to identify nonalcoholic fatty liver disease. Lipids Health Dis. 2017;16(1):15. doi: 10.1186/s12944-017-0409-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Catanzaro R, et al. Triglycerides to high-density lipoprotein cholesterol ratio for diagnosing nonalcoholic fatty liver disease. Minerva Gastroenterol (Torino) 2022;68(3):261–268. doi: 10.23736/S2724-5985.21.02818-X. [DOI] [PubMed] [Google Scholar]
- 34.Wattacheril J, Sanyal AJ. Lean NAFLD: an underrecognized outlier. Curr Hepatol Rep. 2016;15(2):134–139. doi: 10.1007/s11901-016-0302-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim D, et al. Body fat distribution and risk of incident and regressed nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2016;14(1):132–8.e4. doi: 10.1016/j.cgh.2015.07.024. [DOI] [PubMed] [Google Scholar]
- 36.Papatheodoridi M, Cholongitas E. Diagnosis of Non-alcoholic Fatty Liver Disease (NAFLD): current concepts. Curr Pharm Des. 2018;24(38):4574–4586. doi: 10.2174/1381612825666190117102111. [DOI] [PubMed] [Google Scholar]
- 37.Ferraioli G, Soares ML. Ultrasound-based techniques for the diagnosis of liver steatosis. World J Gastroenterol. 2019;25(40):6053–6062. doi: 10.3748/wjg.v25.i40.6053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Loomba R, et al. Multicenter validation of association between decline in MRI-PDFF and histologic response in NASH. Hepatology. 2020;72(4):1219–1229. doi: 10.1002/hep.31121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guo W, et al. The triglyceride-glucose index is associated with the severity of hepatic steatosis and the presence of liver fibrosis in non-alcoholic fatty liver disease: a cross-sectional study in Chinese adults. Lipids Health Dis. 2020;19(1):218. doi: 10.1186/s12944-020-01393-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khamseh ME, et al. Triglyceride Glucose Index and Related Parameters (Triglyceride Glucose-Body Mass Index and Triglyceride Glucose-Waist Circumference) identify nonalcoholic fatty liver and liver fibrosis in individuals with overweight/obesity. Metab Syndr Relat Disord. 2021;19(3):167–173. doi: 10.1089/met.2020.0109. [DOI] [PubMed] [Google Scholar]
- 41.Riviere B, et al. The triglycerides and glucose (TyG) index: A new marker associated with nonalcoholic steatohepatitis (NASH) in obese patients. Diabetes Metab. 2022;48(4):101345. doi: 10.1016/j.diabet.2022.101345. [DOI] [PubMed] [Google Scholar]
- 42.Tapper EB, et al. Erratum: cost-effective evaluation of nonalcoholic fatty liver disease with NAFLD fibrosis score and vibration-controlled transient elastography. Am J Gastroenterol. 2016;111(3):446–446. doi: 10.1038/ajg.2016.14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary Table 1. AUC of TyG-BMI, TyG-WC, TyG, BMI, WC, ALT, and TG/HDL-C for diagnosing liver stiffness.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author Dr. Mao upon reasonable request.