Abstract
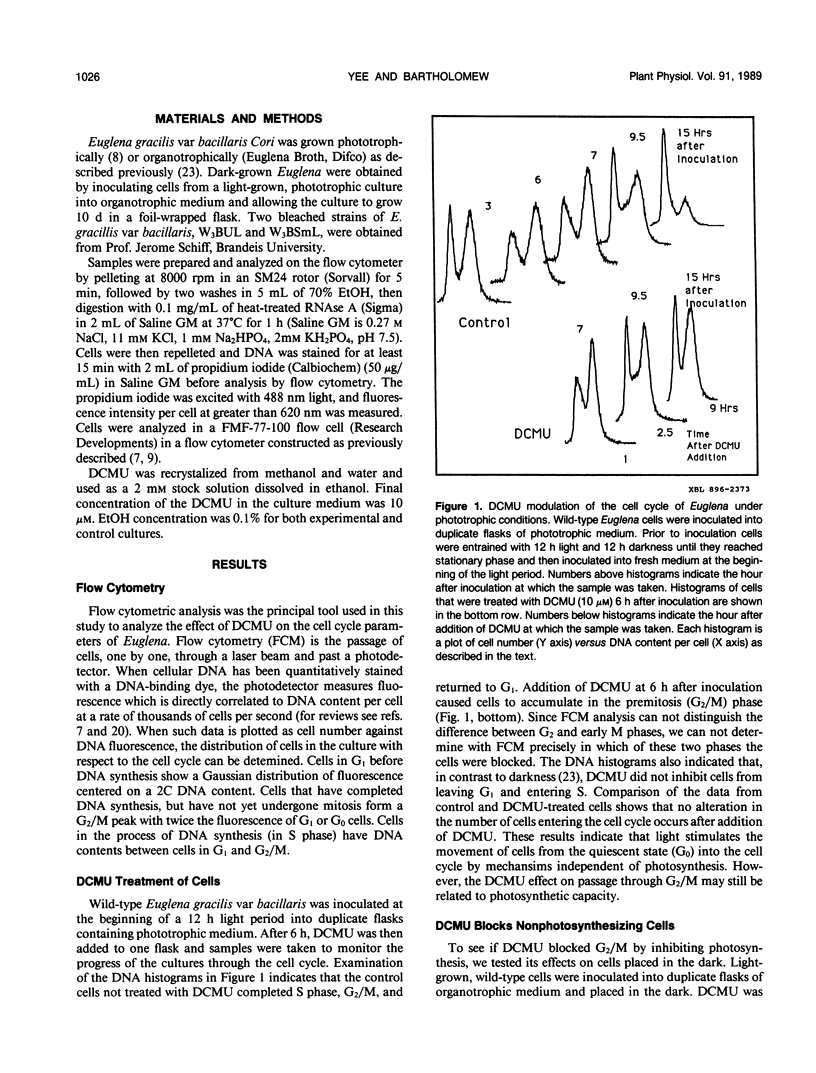
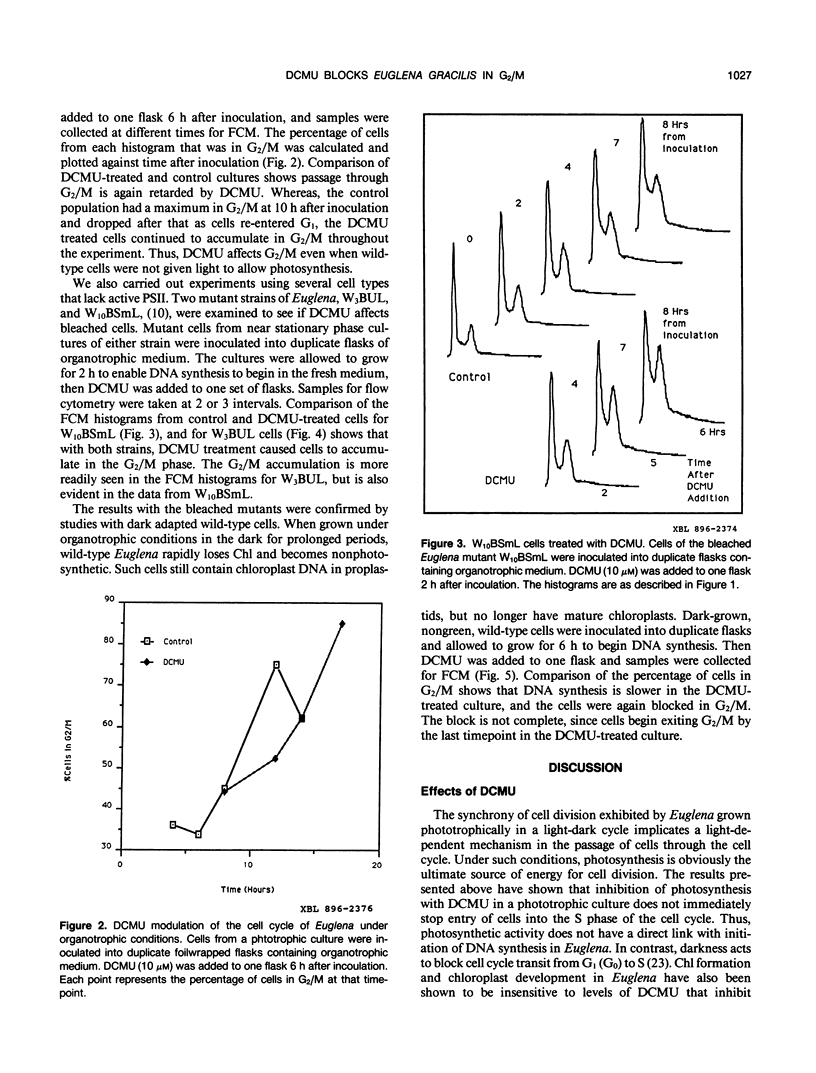
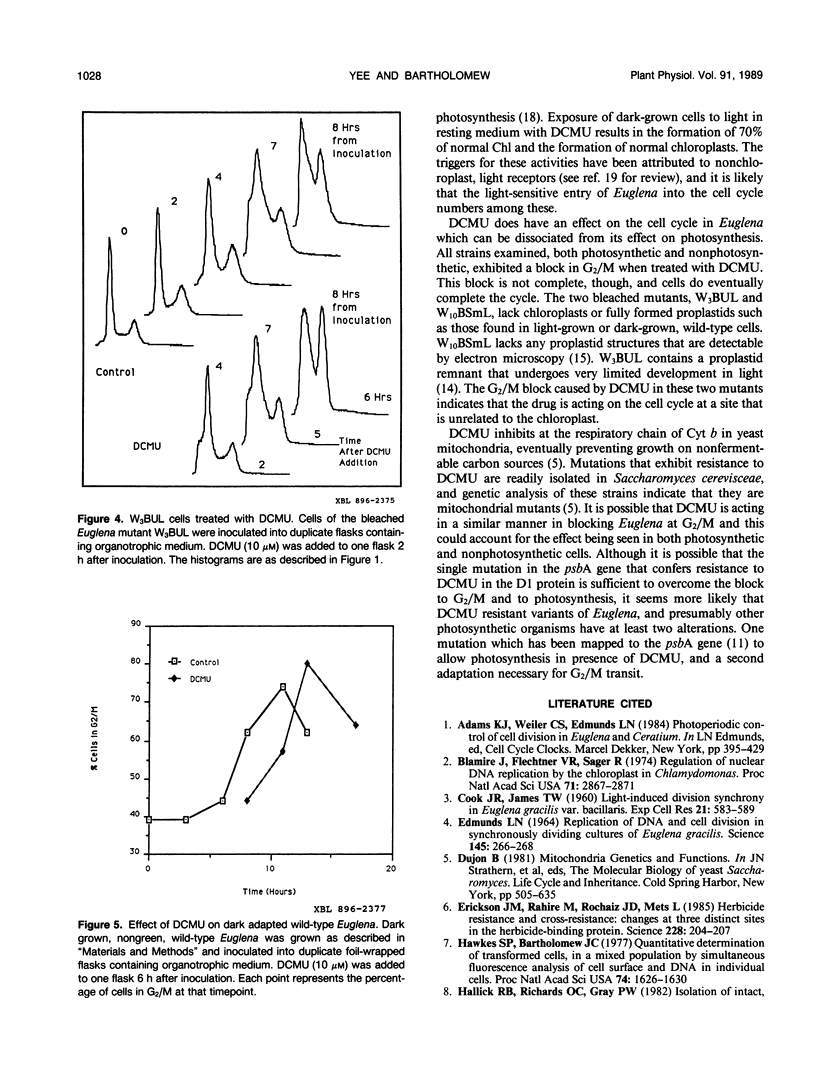
The cell cycle of the photosynthetic unicellular alga Euglena gracilis growing in phototrophic medium is regulated by light. To investigate the relationship of this cell cycle response to light stimulated photosynthesis, we have tested the effect of the photosynthesis inhibitor 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU) on Euglena cell cycle transit. While DCMU does not block light stimulated cells from entering the S phase of the cell cycle, it does inhibit the transit through G2/M. The specificity of this response and its relationship to photosynthesis was studied by looking at the effect of DCMU on dark grown wild-type cells, and on two bleached variants of Euglena (W3BUL and W10BSmL) that lack chloroplasts. The drug does block G2/M in these cells, but not entrance into the cell cycle. Our studies show that entrance of cells into the cell cycle from a quiescent state does not require active photosynthesis, and that DCMU has effects on G2/M transit that are independent of the photosynthetic capacity of the cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blamire J., Flechtner V. R., Sager R. Regulation of nuclear DNA replication by thechloroplast in Chlamydomonas. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2867–2871. doi: 10.1073/pnas.71.7.2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOK J. R., JAMES T. W. Light-induced division synchrony in Euglena gracills var. bacillaris. Exp Cell Res. 1960 Dec;21:583–589. doi: 10.1016/0014-4827(60)90292-5. [DOI] [PubMed] [Google Scholar]
- EDMUNDS L. N., Jr REPLICATION OF DNA AND CELL DIVISION IN SYNCHRONOUSLY DIVIDING CULTURES OF EUGLENA GRACILIS. Science. 1964 Jul 17;145(3629):266–268. doi: 10.1126/science.145.3629.266. [DOI] [PubMed] [Google Scholar]
- Erickson J. M., Rahire M., Rochaix J. D., Mets L. Herbicide resistance and cross-resistance: changes at three distinct sites in the herbicide-binding protein. Science. 1985 Apr 12;228(4696):204–207. doi: 10.1126/science.228.4696.204. [DOI] [PubMed] [Google Scholar]
- Hawkes S. P., Bartholomew J. C. Quantitative determination of transformed cells in a mixed population by stimultaneous fluorescence analysis of cell surface and DNA an individual cells. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1626–1630. doi: 10.1073/pnas.74.4.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan P. K., Wheeless L. L., Jr Quantitative single cell analysis and sorting. Science. 1977 Oct 14;198(4313):149–157. doi: 10.1126/science.905822. [DOI] [PubMed] [Google Scholar]
- Johanningmeier U., Hallick R. B. The psbA gene of DCMU-resistant Euglena gracilis has an amino acid substitution at serine codon 265. Curr Genet. 1987;12(6):465–470. doi: 10.1007/BF00434825. [DOI] [PubMed] [Google Scholar]
- Monroy A. F., Schwartzbach S. D. Influence of photosynthesis and chlorophyll synthesis on polypeptide accumulation in greening euglena. Plant Physiol. 1985 Apr;77(4):811–816. doi: 10.1104/pp.77.4.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osafune T., Schiff J. A. Events surrounding the early development of Euglena chloroplasts. Light-induced changes in a proplastid remnant in mutant W3BUL. J Ultrastruct Res. 1980 Oct;73(1):64–76. doi: 10.1016/0022-5320(80)90116-1. [DOI] [PubMed] [Google Scholar]
- Osafune T., Schiff J. A. W10BSmL, a mutant of Euglena gracilis var. bacillaris lacking plastids. Exp Cell Res. 1983 Oct 15;148(2):530–535. doi: 10.1016/0014-4827(83)90176-3. [DOI] [PubMed] [Google Scholar]
- Pfister K., Steinback K. E., Gardner G., Arntzen C. J. Photoaffinity labeling of an herbicide receptor protein in chloroplast membranes. Proc Natl Acad Sci U S A. 1981 Feb;78(2):981–985. doi: 10.1073/pnas.78.2.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff J. A. The development, inheritance, and origin of the plastid in Euglena. Adv Morphog. 1973;10:265–312. doi: 10.1016/b978-0-12-028610-2.50010-1. [DOI] [PubMed] [Google Scholar]
- Schiff J. A., Zeldin M. H., Rubman J. Chlorophyll Formation and Photosynthetic Competence in Euglena During Light-Induced Chloroplast Development in the Presence of 3, (3,4-dichlorophenyl) 1,1-Dimethyl Urea (DCMU). Plant Physiol. 1967 Dec;42(12):1716–1725. doi: 10.1104/pp.42.12.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shneyour A., Ben-Shaul Y., Avron M. Structural changes in Euglena gracilis grown autotrophically in the light with 3,(3,4-dichlorophenyl)-1, 1-dimethyl urea (DCMU). Exp Cell Res. 1969 Nov;58(1):1–9. doi: 10.1016/0014-4827(69)90110-4. [DOI] [PubMed] [Google Scholar]
- Spudich J. L., Sager R. Regulation of the Chlamydomonas cell cycle by light and dark. J Cell Biol. 1980 Apr;85(1):136–145. doi: 10.1083/jcb.85.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee M. C., Bartholomew J. C. Light regulation of the cell cycle in Euglena gracilis bacillaris. Cytometry. 1988 Jul;9(4):387–393. doi: 10.1002/cyto.990090417. [DOI] [PubMed] [Google Scholar]


