Abstract
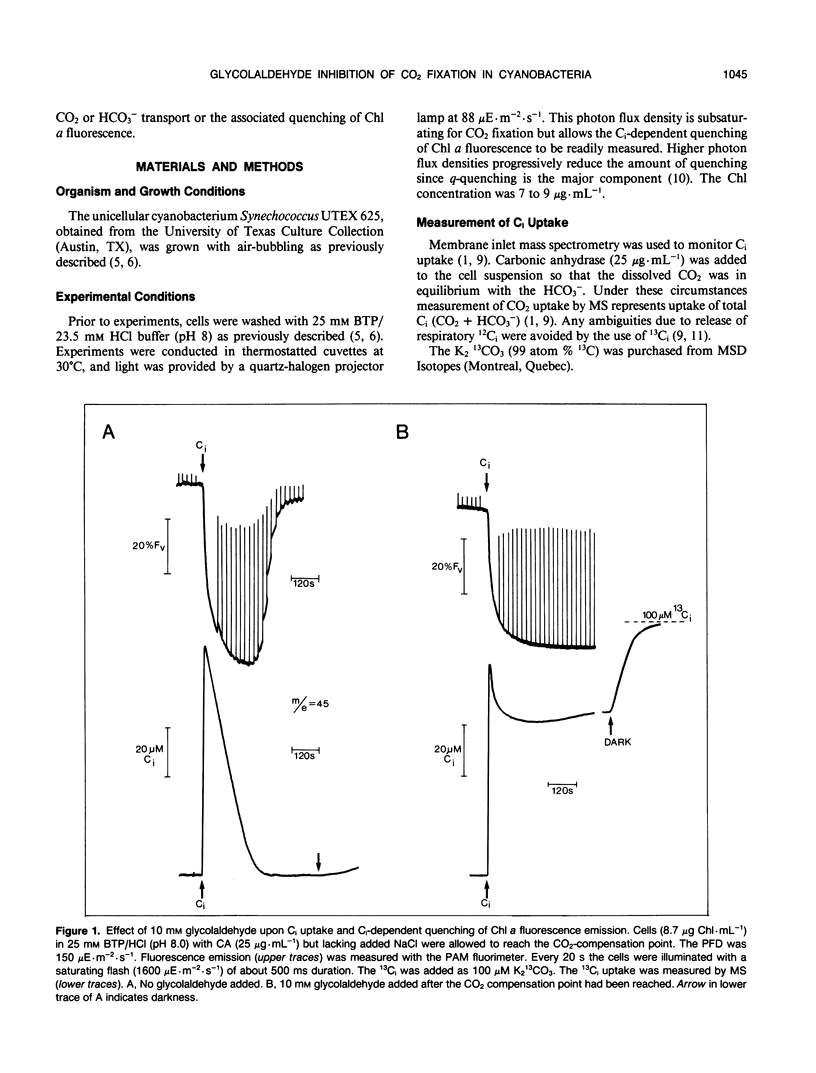
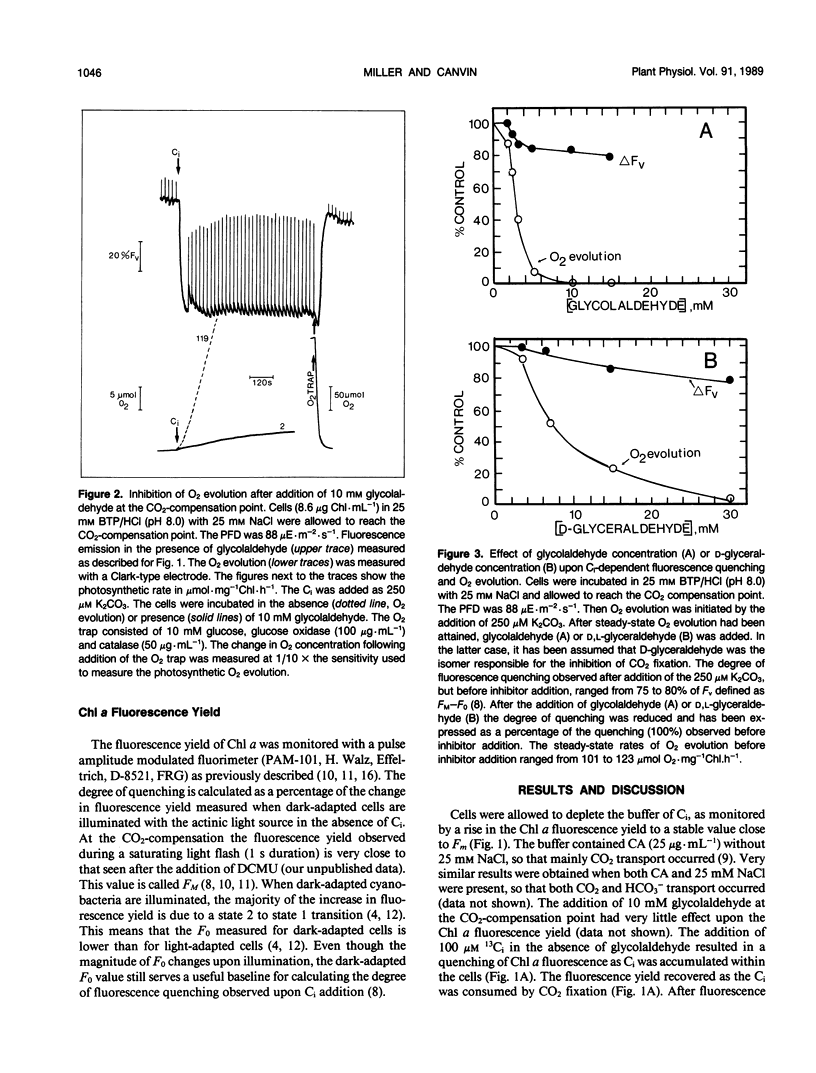
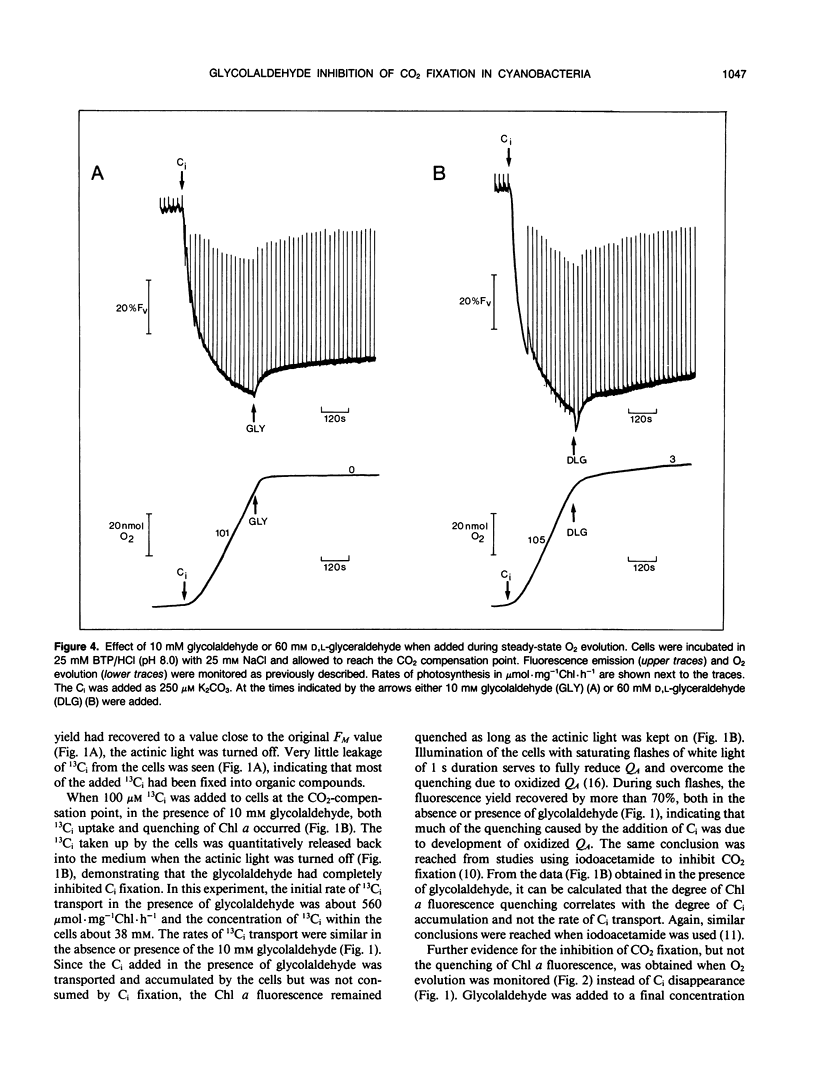
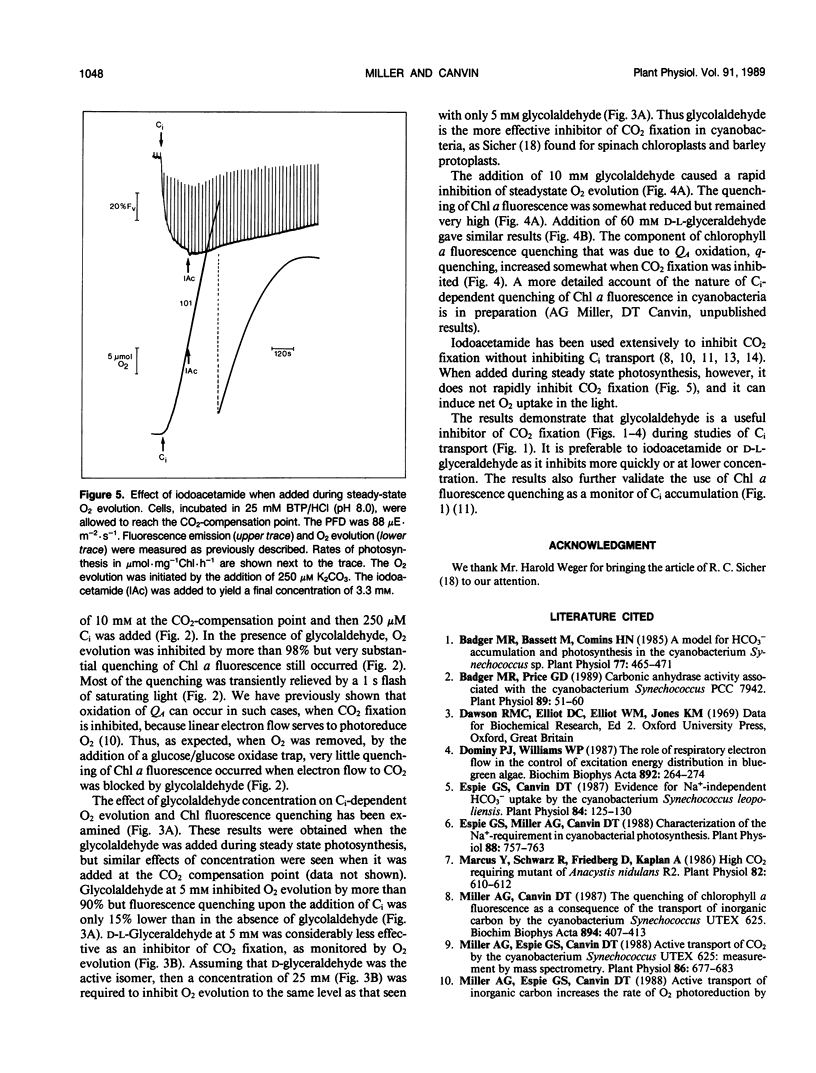
When studying active CO2 and HCO3− transport by cyanobacteria, it is often useful to be able to inhibit concomitant CO2 fixation. We have found that glycolaldehyde was an efficient inhibitor of photosynthetic CO2 fixation in Synechococcus UTEX 625. Glycolaldehyde did not inhibit inorganic carbon accumulation due to either active CO2 or HCO3− transport. When glycolaldehyde (10 millimolar) was added to rapidly photosynthesizing cells, CO2 fixation was stopped within 15 seconds. The quenching of chlorophyll a fluorescence remained high (≤ 82% control) when CO2 fixation was completely blocked by glycolaldehyde. This quenching was relieved upon the addition of a glucose oxidase oxygentrap. This is consistent with our previous finding that q-quenching in the absence of CO2 fixation was due to O2 photoreduction. Photosynthetic CO2 fixation was also inhibited by d,l,-glyceraldehyde but a sixfold higher concentration was required. Glycolaldehyde acted much more rapidly than iodoacetamide (15 seconds versus 300 seconds) and did not cause the onset of net O2 evolution often observed with iodoacetamide. Glycolaldehyde will be a useful inhibitor when it is required to study CO2 and HCO3− transport without the complication of concomitant CO2 fixation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badger M. R., Bassett M., Comins H. N. A Model for HCO(3) Accumulation and Photosynthesis in the Cyanobacterium Synechococcus sp: Theoretical Predictions and Experimental Observations. Plant Physiol. 1985 Feb;77(2):465–471. doi: 10.1104/pp.77.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badger M. R., Price G. D. Carbonic Anhydrase Activity Associated with the Cyanobacterium Synechococcus PCC7942. Plant Physiol. 1989 Jan;89(1):51–60. doi: 10.1104/pp.89.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espie G. S., Canvin D. T. Evidence for Na-Independent HCO(3) Uptake by the Cyanobacterium Synechococcus leopoliensis. Plant Physiol. 1987 May;84(1):125–130. doi: 10.1104/pp.84.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espie G. S., Miller A. G., Canvin D. T. Characterization of the na-requirement in cyanobacterial photosynthesis. Plant Physiol. 1988 Nov;88(3):757–763. doi: 10.1104/pp.88.3.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus Y., Schwarz R., Friedberg D., Kaplan A. High CO(2) Requiring Mutant of Anacystis nidulans R(2). Plant Physiol. 1986 Oct;82(2):610–612. doi: 10.1104/pp.82.2.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. G., Espie G. S., Canvin D. T. Active Transport of CO(2) by the Cyanobacterium Synechococcus UTEX 625 : Measurement by Mass Spectrometry. Plant Physiol. 1988 Mar;86(3):677–683. doi: 10.1104/pp.86.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. G., Espie G. S., Canvin D. T. Active Transport of Inorganic Carbon Increases the Rate of O(2) Photoreduction by the Cyanobacterium Synechococcus UTEX 625. Plant Physiol. 1988 Sep;88(1):6–9. doi: 10.1104/pp.88.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. G., Espie G. S., Canvin D. T. Chlorophyll a Fluorescence Yield as a Monitor of Both Active CO(2) and HCO(3) Transport by the Cyanobacterium Synechococcus UTEX 625. Plant Physiol. 1988 Mar;86(3):655–658. doi: 10.1104/pp.86.3.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T., Kaplan A. The Stoichiometry between CO(2) and H Fluxes Involved in the Transport of Inorganic Carbon in Cyanobacteria. Plant Physiol. 1987 Apr;83(4):888–891. doi: 10.1104/pp.83.4.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero J. M., Lara C., Guerrero M. G. Dependence of nitrate utilization upon active CO2 fixation in Anacystis nidulans: a regulatory aspect of the interaction between photosynthetic carbon and nitrogen metabolism. Arch Biochem Biophys. 1985 Mar;237(2):396–401. doi: 10.1016/0003-9861(85)90291-7. [DOI] [PubMed] [Google Scholar]
- Stokes D. M., Walker D. A. Photosynthesis by isolated chloroplasts. Inhibition by DL-glyceraldehyde of carbon dioxide assimilation. Biochem J. 1972 Aug;128(5):1147–1157. doi: 10.1042/bj1281147. [DOI] [PMC free article] [PubMed] [Google Scholar]


