Abstract
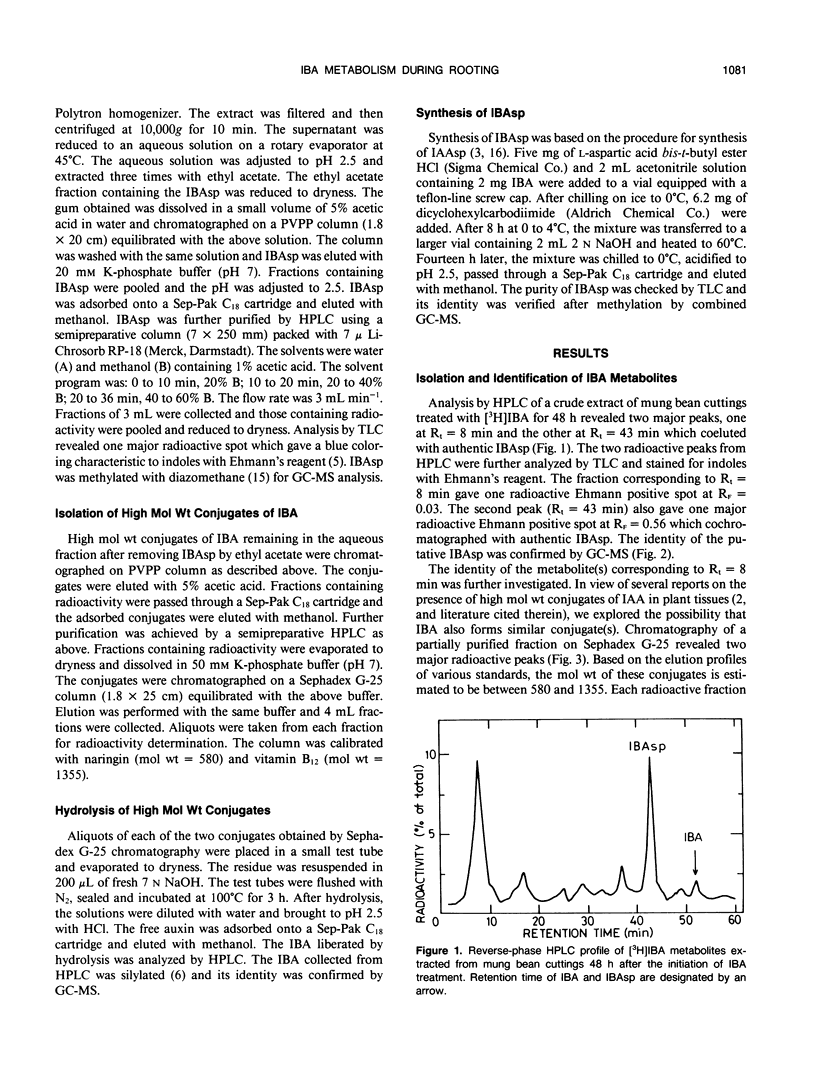
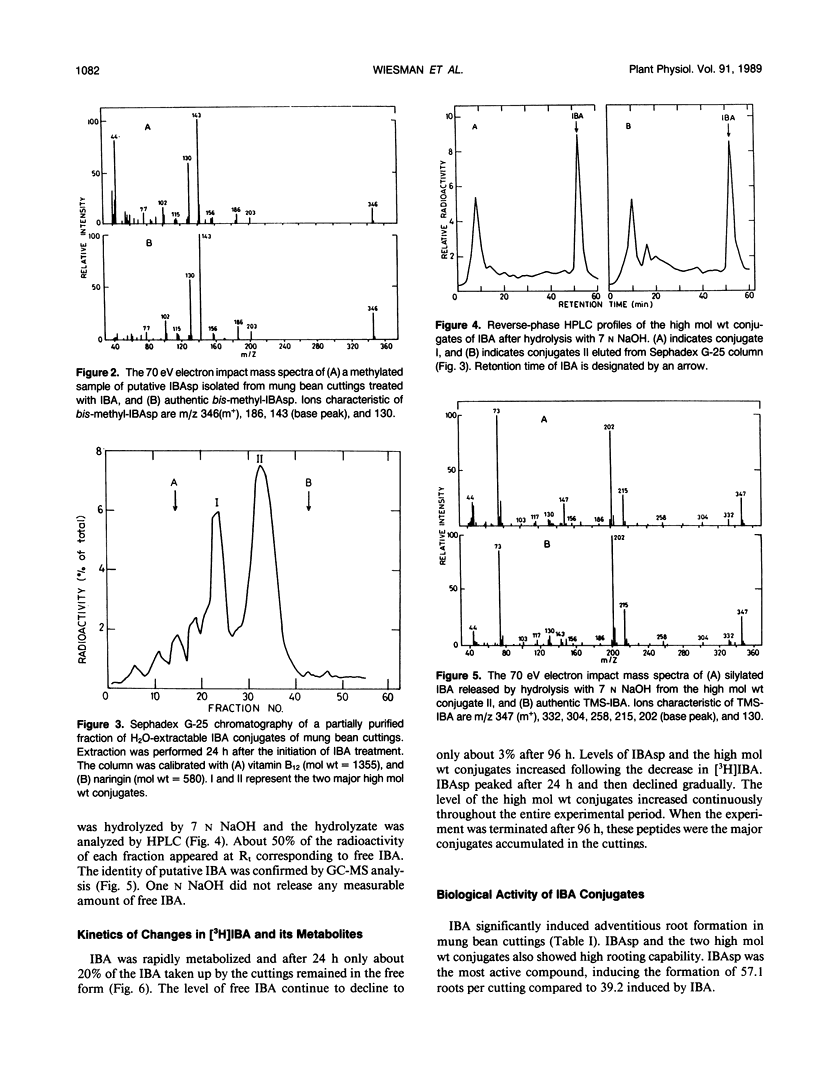
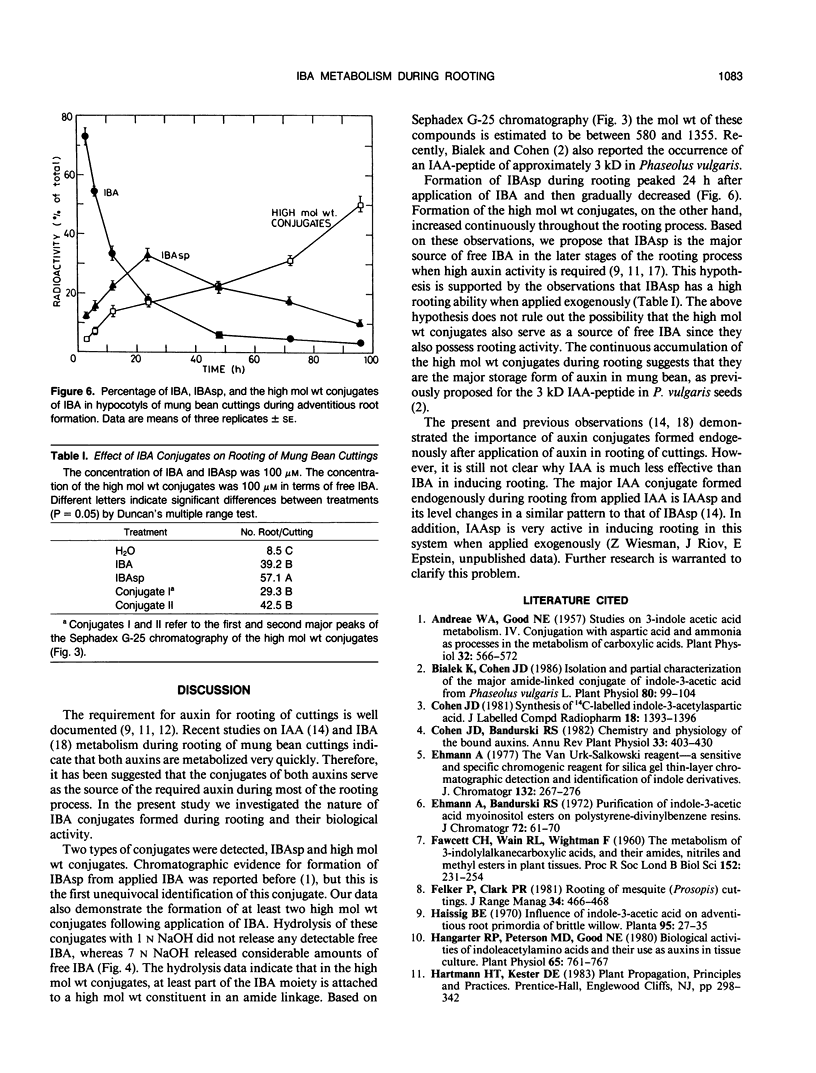
Indole-3-butyric acid (IBA) is rapidly metabolized by mung bean cuttings during rooting. Twenty-four hours after application, less than 20% of the applied IBA remained in the free form and its level decreased continuously in the later stages of rooting. Indole-3-butyrylaspartic acid (IBAsp) and at least two high molecular weight conjugates were the major metabolites in IBA-treated cuttings. In the latter conjugates, at least part of the IBA moiety is attached to a high molecular weight constituent in an amide linkage. IBAsp level peaked 24 hours after application of IBA to the cuttings and then declined. The level of the high molecular weight conjugates increased continuously throughout the rooting process. The conjugates were active in inducing rooting of cuttings, with IBAsp being superior to free IBA. It is suggested that IBA conjugates, and particularly IBAsp, serve as the source of auxin during the later stages of rooting.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreae W. A., Good N. E. Studies on 3-Indoleacetic Acid Metabolism. IV. Conjugation with Aspartic Acid and Ammonia as Processes in the Metabolism of Carboxylic Acids. Plant Physiol. 1957 Nov;32(6):566–572. doi: 10.1104/pp.32.6.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialek K., Cohen J. D. Isolation and Partial Characterization of the Major Amide-Linked Conjugate of Indole-3-Acetic Acid from Phaseolus vulgaris L. Plant Physiol. 1986 Jan;80(1):99–104. doi: 10.1104/pp.80.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehmann A. The van urk-Salkowski reagent--a sensitive and specific chromogenic reagent for silica gel thin-layer chromatographic detection and identification of indole derivatives. J Chromatogr. 1977 Feb 11;132(2):267–276. doi: 10.1016/s0021-9673(00)89300-0. [DOI] [PubMed] [Google Scholar]
- FAWCETT C. H., WAIN R. L., WIGHTMAN F. The metabolism of 3-indolylalkanecarboxylic acids, and their amides, nitriles and methyl esters in plant tissues. Proc R Soc Lond B Biol Sci. 1960 May 17;152:231–254. doi: 10.1098/rspb.1960.0035. [DOI] [PubMed] [Google Scholar]
- Hangarter R. P., Peterson M. D., Good N. E. Biological activities of indoleacetylamino acids and their use as auxins in tissue culture. Plant Physiol. 1980 May;65(5):761–767. doi: 10.1104/pp.65.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norcini J. G., Heuser C. W. Changes in the Level of [C]Indole-3-Acetic Acid and [C]Indoleacetylaspartic Acid during Root Formation in Mung Bean Cuttings. Plant Physiol. 1988 Apr;86(4):1236–1239. doi: 10.1104/pp.86.4.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]


