Abstract
Lichens play crucial roles in the ecosystems, contributing to soil formation and nutrient cycling, and being used in biomonitoring efforts to assess the sustainability of ecosystems including air quality. Previous studies on heavy metal accumulation in lichens have mostly relied on manipulated environments, such as transplanted lichens, leaving us with a dearth of research on how lichens physiologically respond to heavy metal exposure in their natural habitats. To fill this knowledge gap, we investigated lichens from two of South Korea’s geographically distant regions, Gangwon Province and Jeju Island, and examined whether difference in ambient heavy metal concentrations could be detected through physiological variables, including chlorophyll damage, lipid oxidation, and protein content. The physiological variables of lichens in response to heavy metals differed according to the collection area: Arsenic exerted a significant impact on chlorophyll degradation and protein content. The degree of fatty acid oxidation in lichens was associated with increased Cu concentrations. Our research highlights the value of lichens as a bioindicator, as we found that even small variations in ambient heavy metal concentrations can be detected in natural lichens. Furthermore, our study sheds light on which physiology variables that can be used as indicators of specific heavy metals, underscoring the potential of lichens for future ecology studies.
Keywords: Lichen, Heavy metals, Bioindicator, Physiology variables
1. Introduction
Lichen is a unique organism that consists of a symbiotic relationship between a fungus and algae or cyanobacteria. The fungus provides a structure and protection for the photobionts, which in turn provides food for the fungus through photosynthesis. This relationship is so close that lichen is considered a single organism, rather than two separate organisms living together. Lichens are highly prevalent in approximately 8% of terrestrial ecosystems [1], and are commonly found thriving in environments subjected to extreme temperatures, limited water availability, and low nutrient levels [2]. Due to their ability to tolerate a wide range of environmental conditions, lichens are key components of many ecosystems and contribute significantly to the sustainability of natural systems. Lichens play essential roles in the ecosystems such as soil formation, habitat creation for plants and insects, food source, and nutrient cycling, indicating that they can contribute to the overall health of an ecosystem. Some species of lichens can fix nitrogen from the atmosphere, making it available to other plants in the ecosystem. Other species can absorb heavy metals from the soil, helping to detoxify polluted areas, one of how they can contribute to environmental sustainability. Therefore, it can be said that studying lichens can provide significant assistance in assessing the health of ecosystems. The study of lichens not only helps us understand the state of the environment but also provides valuable insights into the intricate interplay between different organisms and their surroundings, promoting a deeper appreciation of the complexity and beauty of our natural world.
Lichens are widely recognized as bioindicators of environmental pollution because of their sensitivity to various pollutants, including heavy metals [3]. Therefore their health and distribution can be used to monitor air quality. Heavy metal pollution is a significant environmental problem, with detrimental effects on ecosystems and human health, [4] that accumulate in lichens and cause multiple physiological changes. The surface of lichens and the intercellular spaces of their medulla can serve as sites for the deposition of fine particles containing heavy metals that can remain unaltered for prolonged periods [5]. Their ability to accumulate particles allows them to retain significant quantities of heavy metals. Therefore, lichens have become highly regarded as bioindicators because of their capacity to retain these metals over time, which means that they are studied to assess the health of an ecosystem. The presence or absence of certain species of lichens can indicate the overall health of an ecosystem, and changes in lichen populations can be an early warning sign of environmental problems. Therefore, by studying lichens, scientists can gain valuable insights into the health of ecosystems, and take measures to ensure their sustainability.
Lichens are used in the field of biomonitoring, which involves monitoring environmental pollution using living organisms. Lichen biomonitoring involves measuring the concentrations of pollutants in lichen tissue and using that data to assess the levels of pollution in the surrounding environment. There are other research approaches on how the health of lichens within a particular area is negatively affected by the level of environmental pollution in that area. The accumulation of heavy metals inside lichen thallus can have adverse effects on their physiology. Heavy metals accumulated in lichen thallus can lead to the disruption of their metabolic pathways and the inhibition of photosynthesis. One of the most commonly studied physiological responses of lichens to heavy metal pollution is the degradation of chlorophyll [6], which can lead to decreased photosynthetic efficiency and ultimately affect their overall health. Heavy metal accumulation can cause oxidative stress in lichens, leading to the production of reactive oxygen species and subsequent lipid peroxidation [7]. Malondialdehyde (MDA) is a commonly used marker of lipid peroxidation [8] and is often used to assess oxidative stress in lichens exposed to heavy metals [9]. Furthermore, heavy metal accumulation can also affect the protein content of lichens, potentially leading to a decrease in overall metabolic activity [10]. As a result, the protein content, chlorophyll levels, and lipid oxidation in lichens can be negatively impacted. However, there is not much detailed research on which heavy metals specifically damage which physiology variable of lichen thalli.
Although considerable research has been conducted on the physiological responses of lichens to heavy metal pollution, most studies have been conducted in manipulated environments, including transplanted lichens [11–13] and artificial exposure to heavy metals in laboratories [14,15]. Therefore, little research has been conducted on how naturally occurring lichens react to heavy metals in different environments and whether lichen physiological variables can be used as indicators of different heavy metal concentrations in these environments. Further investigations are necessary to gain a more comprehensive understanding of the physiological effects of heavy metal exposure in natural lichen populations.
To bridge this knowledge gap, we collected lichens from four different natural habitats and explored whether their physiological variables, including chlorophyll degradation, MDA content, and protein content, could reveal slight differences in ambient heavy metal concentrations. The Gangwon Province and Jeju Island [16] in South Korea, known for their relatively pristine air quality, are suitable sites for our research. If lichens can accurately reflect fine variations in heavy metal levels in clean environments, their potential as bioindicators would be remarkable. Furthermore, we aimed to investigate the impact of seven heavy metals (As, Cu, Fe, Mn, Ni, Pb, and Zn) on lichens by observing physiological variables.
2. Materials and methods
2.1. Lichen collection
Our investigation included four different sites: two on Jeju Island (A1: 33.2753 N, 126.6623 E and A2: 33.4591 N, 126.5584 E) and two in Gangwon Province (B1: 7.8015 N, 128.9033 E and B2: 38.1691 N, 128.5211 E). Jeju Island is a large subtropical island off the southern coast of South Korea, whereas Gangwon Province is a mountainous province in the northeastern part of South Korea [16,17].
Because of the substantial quantity of thalli required for our subsequent experiments, we decided to exclude lichen colonies with a lower biomass, such as crustose lichens. We gathered lichens from the entire sampling area, except crustose lichens, and found that all of them were of the foliose lichens. Thirty foliose lichen thalli belonging to Candelaria, Cetrelia, Dirinaria, Heterodermia, Myelochroa, Parmotrema, Phaeophyscia, and Punctelia were collected from four distinct locations in South Korea (Figure 1) in 2021. The collected lichens were identified using morphological analysis in the field. A total of 30 foliose lichens were experimentally sampled and utilized for subsequent analyses, with three replicates (Table 1).
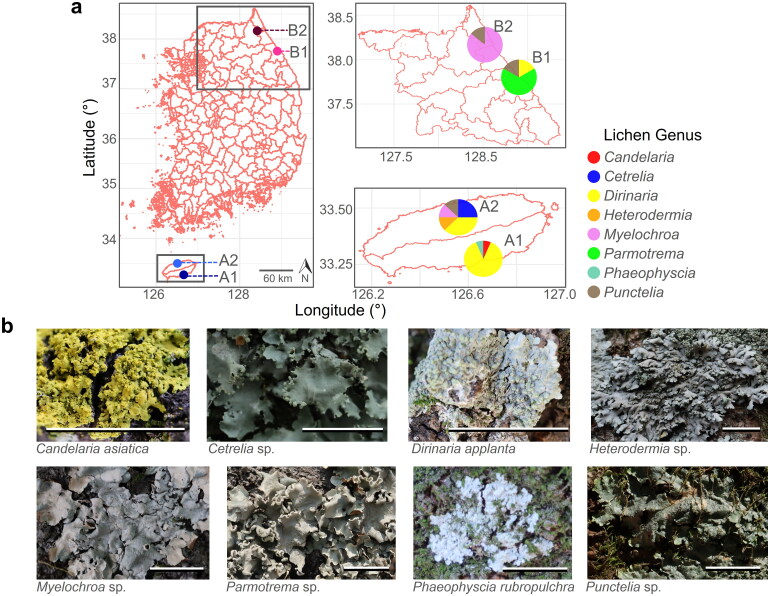
Figure 1.
Lichen collection information. The figure displays information on the lichen samples used in this study. Panel (a) shows the collection mapping, with different colors indicating the location and lichen genus variation. Panel (b) shows the morphological features of the lichens collected at a scale of 1 cm.
Table 1.
Average heavy metals concentration in lichen thalli.
| No | Sample | Genus | Site | As | Cu | Mn | Ni | Pb | Zn | Fe |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | LS01 | Dirinaria | A01 | 1.58 | 69.69 | 48.11 | 5.82 | 13.68 | 91.07 | 5177.83 |
| 2 | LS02 | Dirinaria | A01 | 0.99 | 54.65 | 30.53 | 2.27 | 7.59 | 137.47 | 2632.18 |
| 3 | LS03 | Candelaria | A01 | 1.25 | 33.81 | 68.49 | 5.68 | 11.4 | 228.44 | 5728.81 |
| 4 | LS04 | Dirinaria | A01 | 1.1 | 17.16 | 35.13 | 5.35 | 10.26 | 209.08 | 5558.86 |
| 5 | LS05 | Dirinaria | A01 | 1.1 | 24.36 | 19.54 | 2.27 | 5.84 | 164.62 | 3633.27 |
| 6 | LS06 | Dirinaria | A01 | 1.4 | 41.91 | 48.67 | 4.31 | 4.78 | 75.99 | 3775.8 |
| 7 | LS07 | Dirinaria | A01 | 1.62 | 22.83 | 46.38 | 3.77 | 6.04 | 49.27 | 2918.28 |
| 8 | LS08 | Dirinaria | A01 | 1.64 | 63.27 | 53.64 | 4.88 | 8.62 | 52.88 | 3790.97 |
| 9 | LS09 | Dirinaria | A01 | 1.37 | 78.71 | 40.14 | 3.53 | 6.88 | 173.84 | 2689.01 |
| 10 | LS10 | Phaeophyscia | A01 | 1.51 | 53.35 | 64.51 | 5.79 | 12.21 | 54.9 | 4813.66 |
| 11 | LS11 | Dirinaria | A01 | 1.13 | 34.46 | 44.61 | 3.26 | 13.55 | 68.67 | 2827.58 |
| 12 | LS13 | Dirinaria | A01 | 1.85 | 19.29 | 39.73 | 3.76 | 17.16 | 76.15 | 2606.67 |
| 13 | LS15 | Heterodermia | A02 | 0.69 | 13.75 | 21.2 | 1.63 | 2.63 | 83.42 | 584.32 |
| 14 | LS23 | Punctelia | A02 | 0.81 | 13.24 | 50.88 | 2.14 | 2.07 | 32.01 | 1232.17 |
| 15 | LS26 | Dirinaria | A02 | 1.38 | 25.11 | 32.84 | 2.57 | 15.02 | 63.97 | 3747.22 |
| 16 | LS27 | Cetrelia | A02 | 0.85 | 32.22 | 35.67 | 2.57 | 14 | 83.73 | 1341.58 |
| 17 | LS28 | Dirinaria | A02 | 1.12 | 25.96 | 25.79 | 2.34 | 7.65 | 52.32 | 1027.54 |
| 18 | LS29 | Myelochroa | A02 | 0.98 | 19.51 | 43.61 | 2.83 | 6.28 | 66.03 | 1685.52 |
| 19 | LS33 | Cetrelia | A02 | 0.53 | 44.47 | 29.99 | 1.61 | 7.24 | 78.66 | 413.18 |
| 20 | LS36 | Parmotrema | B01 | 2.64 | 18.71 | 37.95 | 1.86 | 11.72 | 59.85 | 2270.27 |
| 21 | LS37 | Parmotrema | B01 | 2.7 | 13.22 | 40.78 | 1.68 | 7.65 | 54.58 | 1579.71 |
| 22 | LS40 | Dirinaria | B01 | 1.37 | 22.45 | 42.49 | 1.71 | 9.83 | 72.32 | 2100.07 |
| 23 | LS41 | Punctelia | B01 | 2.78 | 17.97 | 80.55 | 3.24 | 11.44 | 58.14 | 5255.39 |
| 24 | LS44 | Myelochroa | B02 | 2.76 | 21.45 | 47.72 | 2.47 | 9.57 | 71.01 | 2858.91 |
| 25 | LS45 | Punctelia | B02 | 1.87 | 11.46 | 43.12 | 1.08 | 3.38 | 66.89 | 944.96 |
| 26 | LS46 | Myelochroa | B02 | 2.91 | 25.58 | 59.77 | 2.1 | 13.24 | 76.24 | 3649.84 |
| 27 | LS47 | Myelochroa | B02 | 1.89 | 10.33 | 39.11 | 1.09 | 12.85 | 63.28 | 536.76 |
| 28 | LS48 | Myelochroa | B02 | 2.12 | 17.18 | 59.12 | 1.27 | 8.5 | 89.77 | 589.13 |
| 29 | LS49 | Myelochroa | B02 | 3.36 | 21.74 | 75.56 | 2.38 | 11.75 | 87.12 | 2113.63 |
| 30 | LS50 | Myelochroa | B02 | 2.3 | 19.26 | 81.35 | 2.3 | 7.69 | 68.99 | 5030.23 |
The unit is milligrams of the heavy metal per kilogram of lichen thallus.
At each site, all foliose lichen samples were collected from the branches of trees, using a sterilized scalpel. To ensure consistency in sample collection, the following criteria were used: 1) Only lichen samples with a diameter greater than 1 cm were collected; 2) Lichen samples were collected from the outer edges of the thallus to avoid contamination from surrounding material; 3) Only healthy-looking lichen samples were collected. Each lichen sample was placed in a separate sterilized plastic bag, labeled with the date and location of collection, and transported to the laboratory for analysis. Upon arrival at the laboratory, lichen samples were carefully washed with deionized water to remove any extraneous material. Samples were then air-dried in a dust-free environment for a day, before being analyzed for lichen physiology variables and heavy metal content.
2.2. Investigation of lichen physiology variables and accumulated heavy metal concentration
The effects of heavy metal accumulation on lichens were evaluated by measuring three lichen physiological variables: chlorophyll degradation, malondialdehyde (MDA) levels, and aqueous soluble protein contents. Chlorophyll degradation was quantified using the method described by Ronen and Galun [18]. Grinded lichen powder (50 mg) was immersed in 2 ml of dimethyl sulfoxide and incubated in the dark for one day. The absorption spectrum of the supernatant was measured at 415 and 435 nm, and the degree of chlorophyll degradation was calculated as the ratio of the optical density at 435 nm to that at 415 nm. MDA levels were measured as a marker of lipid peroxidation using the method described by Heath and Packer [8]. We homogenized 50 mg of powdered lichen thalli in 1 ml of distilled water and added 1 ml of 20% trichloroacetic acid (TCA) containing 0.5% 2-thiobarbituric acid. The resulting reagent was incubated at 95 °C for 30 min, after which the absorption spectrum of the supernatant was measured at 532 and 600 nm. MDA levels were calculated using the extinction coefficient [19], by subtracting the optical density for absorbance (OD) at 532 nm from that at 600 nm. The soluble protein content in the lichen samples was determined using the method described by Arb and Brunold [20]. First, 50 mg of powdered lichen thalli was immersed in a solution of 0.1 M Tris-HCl buffer (pH 8.0) containing 15 mM MgCl2. 100 µl of 10% TCA. To the concentrated protein sample, we subjected the resulting mixture to centrifugation at 12,000 rpm for 1 min. The supernatant (50 µl) was then subjected to protein quantification using the Bradford method [21], and a standard curve generated with bovine serum albumin was used to calculate the protein concentration.
Approximately 0.1 g of homogenized lichen thalli was weighed and transferred to a digestion tube. The digestion procedure was performed following EPA method 3051 A [22]. Briefly, samples were digested with a mixture of HNO3 and HCl at a 3:1 ratio using a microwave-assisted digestion system. The digested samples were diluted with deionized water to a final volume of 50 mL. Heavy metal analyses were performed using an inductively coupled plasma mass spectrometer (MultiWave 7000; Anton Paar, USA). Calibration standards for each metal were prepared by serial dilution of a certified multi-element stock solution (PerkinElmer, USA). All samples were analyzed in triplicate, and mean values were used for data analysis. Blank samples and certified reference materials were analyzed alongside the samples to monitor potential contamination and ensure the accuracy and precision of the analyses. The concentration of each heavy metal in the lichen samples was calculated based on a calibration curve obtained from standards. The total concentration of heavy metals in the lichen samples was then calculated by summing the concentrations of all detected metals. The concentration is reported in milligrams per kilogram of lichen tissue.
2.3. Statistics and visualization
The following analyses were performed using software R v.3.5.3 [23]. To check for data normality, we first conducted a Shapiro test [24]. Because the normality validation of acquired data was violated, we used the non-parametric Mann–Whitney U test [25] for pairwise comparisons and the Kruskal-Wallis test [26] followed by the Bonferroni correction [27] for multiple comparisons. All graphs in this study were visualized using “ggplot2 [28]” and “ggpubr [29]” packages. Collection mapping was constructed with “rgdal” and “scatterpie [30]” packages based on GPS data. Non-metric multidimensional scaling was ordinated using “vegan” [31] based on Bray-Curtis distance [32]. To assess the relationship between lichen physiology variables, we examined the distribution patterns of the data and Spearman correlation coefficients [33], utilizing the “GGally” package. To confirm the statistical significance of this relationship, we performed a simple linear regression analysis [34]. Multiple linear regression analyses were conducted to identify correlations between heavy metal concentrations and lichen physiological variables. We checked for multicollinearity among factors found to be statistically significant using variance inflation factors (VIF) [35]. We attempted to exclude variables with a VIF value above 10 in the subsequent multiple regression analysis as they were considered to have multicollinearity, and all types of heavy metals showed a VIF below 10. We performed a simple linear regression analysis, excluding unexplainable factors, and utilized the “ggplot2” and “reshape2” packages for visualizing the correlations between variables.
3. Results
3.1. Lichen diversity of the collection
The collected foliose lichens belonged to eight different genera: Candelaria, Cetrelia, Dirinaria, Heterodermia, Myelochroa, Parmotrema, Phaeophyscia, and Punctelia (Figure 1). The taxonomic composition of lichens varied by collection site. For example, at sites A1 and A2, Dirinaria applanta was the most abundant species, Parmotrema was strongly dominant at site B1, and Myelochroa occupied the largest proportion at site B2. Although D. appellant was found in all collection sites except B2, Heterodermia, and Cetrelia were only found in site A2. Although the collected areas contained a greater variety of lichens, the analysis excluded crustose lichens such as Lepraria due to their relatively low biomass, as outlined in the Materials and Methods section. In summary, three, five, three, and two foliose lichen genera were collected in sites A1, A2, A3, and A4, respectively.
3.2. Significant variation in heavy metal concentrations within lichen thalli as a function of location
We tested whether there were significant lichen physiological responses related to heavy metals across different lichen genera at a single site or the presence in other sites regardless of the lichen genus. Regarding MDA levels, significant differences were observed among the genera (Fig. S1). At Site A1, no significant differences were found among the three genera: Candelaria, Dirinaria, and Phaeophyscia. At Site A2, numerical differences were significant among five genera: Cetralia, Dirinaria, Heterodermia, Myelochroa, and Punctelia. At Site B1, Parmotrema exhibited higher MDA levels compared to Punctelia. Punctelia showed higher levels than Myelochroa at Site B2. Overall, we observed higher MDA levels in three genera: Parmotrema, Punctelia, and Phaeophyscia. However, no significant differences in MDA levels were found across sites when considering all collected genera collectively.
Regarding chloroplast damage, except for Site A2, there were no significant differences observed among genera (Figure S2). In contrast, when considering all genera collectively, significant differences in chlorophyll damage were observed across the sites. The content of soluble proteins seemed to be influenced by both the lichen genus and the site (Fig. S3). While there were some differences in the three lichen physiology values among genera, we aggregated the genus data by site for subsequent analyses due to limitations in the number of collected samples (Figure 2a). Interestingly, chlorophyll damage was higher in Site B compared to Site A, whereas soluble protein content was lower in Site B compared to Site A.
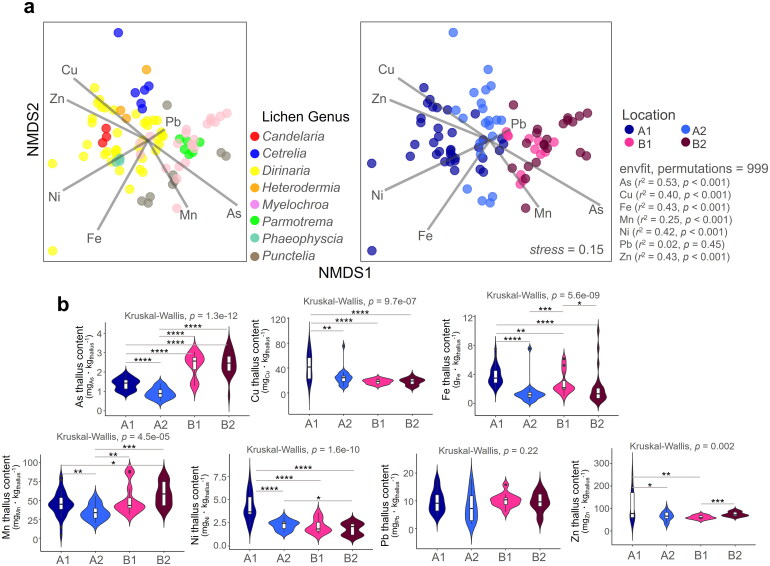
Figure 2.
Accumulated heavy metals concentration in lichen thalli. We utilized non-metric multidimensional scaling to examine variation in heavy metal concentrations among different lichen genera and locations (a) and then displayed the results using a violin plot to highlight the differences in concentration levels across locations (b). ****p < 0.001, ***p < 0.005, **p < 0.01, *p < 0.05
Heavy metals including those of copper (Cu), iron (Fe), nickel (Ni), and zinc (Zn) concentrations in lichens collected from site A1 were generally higher than those in other sites. Lichens collected from sites B1 and B2 showed higher levels of arsenic (As) accumulation compared to those from A1 and A2. Lichens collected from site A2 had relatively lower levels of accumulated heavy metals than those collected from the remaining three sites. Except for lead (Pb), all other heavy metal concentrations showed significant differences among collection sites (Figure 2b). In addition, lichens collected from site B2 exhibited higher levels of manganese (Mn) accumulation than the remaining three sites. The concentrations of Cu, Fe, Ni, and Zn were highest in lichens collected from site A1. The largest difference in heavy metal concentrations within lichens between Jeju Island and Gangwon Province was found for As, with higher concentrations observed in Gangwon.
3.3. Linkage between three lichen physiological variables
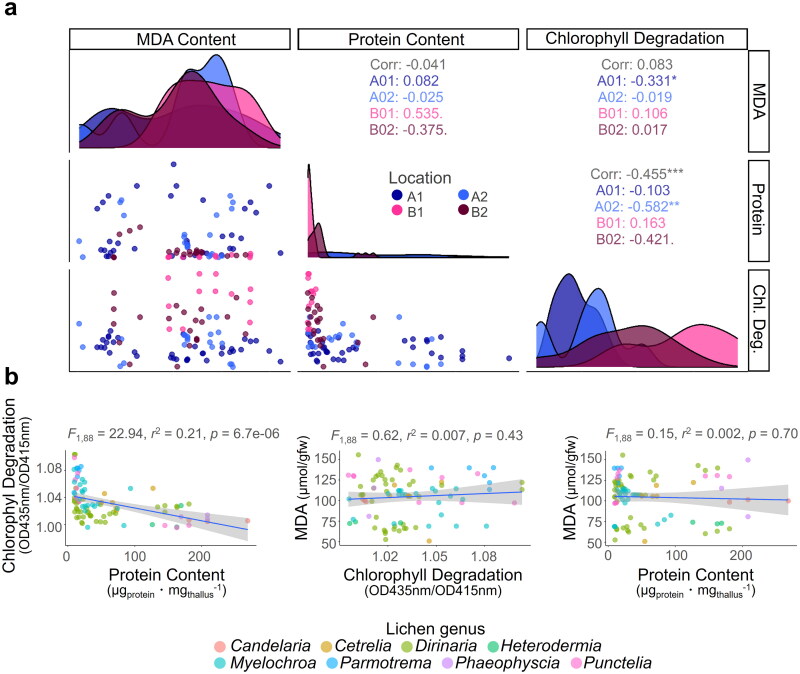
Three physiological variables of lichens, namely malondialdehyde (MDA) content representing the degree of lipid oxidation, soluble protein content, and degree of chlorophyll damage, differed significantly depending on collection location (Figure 3a, Table 2). In particular, MDA content was higher, and soluble protein content was lower in lichens from Gangwon Province than in Jeju Island. Large differences in the degree of chlorophyll damage between lichens collected from Jeju Island and those collected from Gangwon Province, were apparent, with lichens from the latter showing the greatest damage. We used the Spearman correlation to examine the correlation between the three health indicators of lichens and found a significant negative correlation between soluble protein content and the degree of chlorophyll damage. The MDA content did not have a significant relationship with other two physiology parameters, chlorophyll degradation, and protein content.
Figure 3.
Relationship between three physiological variables in lichens. Our study utilized data distribution and Spearman correlations to explore the relationships between the three lichen physiological variables (a), and we conducted a linear regression to confirm the statistical significance of these associations (b). ***p < 0.005, **p < 0.01, *p < 0.05
Table 2.
Lichen physiology variables.
| No | Sample | Genus | Location | Chlorophyll | MDA | Protein |
|---|---|---|---|---|---|---|
| 1 | LS01 | Dirinaria | A01 | 1.02 | 130.16 | 67.37 |
| 2 | LS02 | Dirinaria | A01 | 1.03 | 139.87 | 147.39 |
| 3 | LS03 | Candelaria | A01 | 1.01 | 99.94 | 219.36 |
| 4 | LS04 | Dirinaria | A01 | 1.02 | 68.79 | 43.54 |
| 5 | LS05 | Dirinaria | A01 | 1.03 | 66.1 | 16.11 |
| 6 | LS06 | Dirinaria | A01 | 1.02 | 111.05 | 15.28 |
| 7 | LS07 | Dirinaria | A01 | 1.01 | 114.96 | 23.17 |
| 8 | LS08 | Dirinaria | A01 | 1 | 106.96 | 48.96 |
| 9 | LS09 | Dirinaria | A01 | 1.01 | 136.51 | 24.16 |
| 10 | LS10 | Phaeophyscia | A01 | 1.01 | 115.22 | 195.37 |
| 11 | LS11 | Dirinaria | A01 | 1.02 | 68.62 | 31.05 |
| 12 | LS13 | Dirinaria | A01 | 1.03 | 63.13 | 162.84 |
| 13 | LS15 | Heterodermia | A02 | 1.01 | 73.02 | 152.65 |
| 14 | LS23 | Punctelia | A02 | 1 | 130.07 | 162.84 |
| 15 | LS26 | Dirinaria | A02 | 1.03 | 100.39 | 11.34 |
| 16 | LS27 | Cetrelia | A02 | 1.04 | 97.29 | 84.62 |
| 17 | LS28 | Dirinaria | A02 | 1.03 | 119.15 | 15.45 |
| 18 | LS29 | Myelochroa | A02 | 1.03 | 107.13 | 40.75 |
| 19 | LS33 | Cetrelia | A02 | 1.03 | 105.58 | 63.26 |
| 20 | LS36 | Parmotrema | B01 | 1.07 | 139.72 | 14.46 |
| 21 | LS37 | Parmotrema | B01 | 1.08 | 121.64 | 14.95 |
| 22 | LS40 | Dirinaria | B01 | 1.08 | 113.31 | 10.19 |
| 23 | LS41 | Punctelia | B01 | 1.07 | 97.39 | 11.01 |
| 24 | LS44 | Myelochroa | B02 | 1.03 | 111.93 | 25.8 |
| 25 | LS45 | Punctelia | B02 | 1.05 | 132.58 | 14.95 |
| 26 | LS46 | Myelochroa | B02 | 1.06 | 68.85 | 9.86 |
| 27 | LS47 | Myelochroa | B02 | 1.02 | 77.1 | 83.8 |
| 28 | LS48 | Myelochroa | B02 | 1.02 | 108.21 | 25.8 |
| 29 | LS49 | Myelochroa | B02 | 1.06 | 101.4 | 21.69 |
| 30 | LS50 | Myelochroa | B02 | 1.05 | 111.38 | 21.2 |
The unit is OD435nm/OD415nm for the degree of chlorophyll damage, μmol/gfw for the amount of malondialdehyde, and μgproteinㆍmgthallus−1 for the amount of soluble protein.
A simple linear regression analysis of the three lichen health indicators was conducted to check statistical significance, and demonstrated a strong negative correlation between soluble protein content and the extent of chlorophyll damage (Figure 3b). Similar to the results of Spearman correlation analysis, the amount of MDA was not significantly related to chlorophyll degradation or protein content.
3.4. Correlations between accumulated heavy metals and lichen physiology
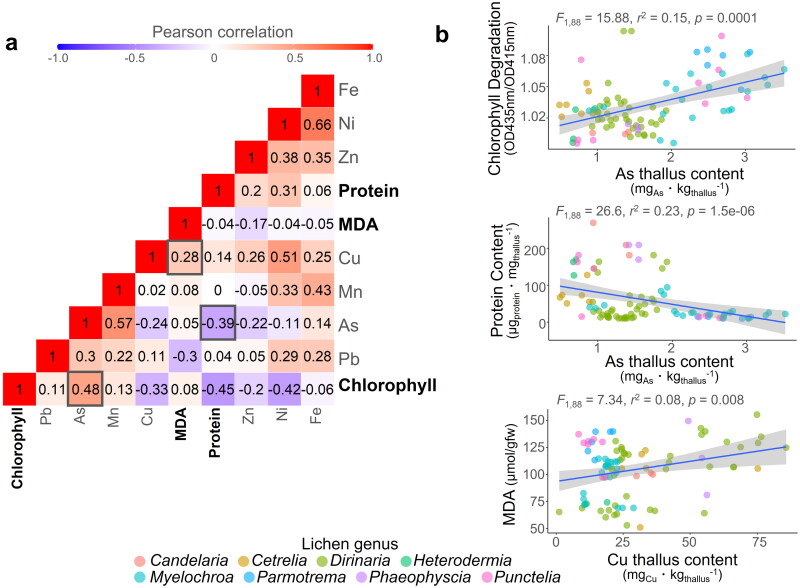
To investigate the potential impact of heavy metal accumulation on lichen physiology, we performed Pearson correlation analysis between various variables including heavy metals concentrations and lichen physiology variables (Figure 4a). Correlation analysis between the three physiological variables revealed that As was positively correlated with chlorophyll degradation and negatively correlated with soluble protein content. Moreover, as the Cu concentration in the lichen increased, there was a concomitant increase in MDA levels, indicating a heightened degree of lipid peroxidation. This supports the notion that different heavy metals lead to different physiological responses in lichens.
Figure 4.
Correlation between heavy metals concentration and three physiological variables in lichens. To investigate the relationships between heavy metal concentrations and the three physiological variables in lichens, we used the Pearson correlation coefficient (a) and confirmed the statistical significance of these associations using linear regression (b).
Multiple regression analysis was performed to assess the significance of the relationship between heavy metal concentrations in the lichens and their physiological variables. A significant correlation was observed between the extent of chlorophyll damage and the presence of the heavy metals As and Ni, with no evidence of multicollinearity between the two metals (Figure 4b, Table 3). In order to mitigate the effects of multicollinearity, we attempted to exclude variables with a variance inflation factor (VIF) greater than 10 from our multiple regression analysis, and observed that all heavy metal types had VIF values below this threshold. The absence of multicollinearity among heavy metals in the multiple regression analysis implies that the sources of heavy metal occurrence vary by type. The levels of soluble proteins were significantly correlated with the accumulation of two heavy metals, As and Mn. Statistical significance was found to be much greater for As than for Mn. Our results indicate a robust positive relationship between MDA levels and the accumulation of Cu and Pb, which is in line with the findings of the Pearson correlation analysis.
Table 3.
Multiple linear regression analysis results between heavy metals concentration and lichen physiology parameters.
| Chlorophyll | Estimate | p value | VIF* |
|---|---|---|---|
| (Intercept) | 1.03E + 00 | <2e-16 | |
| As | 1.28E-02 | 0.003 | 2.06 |
| Cu | −5.41E-05 | 0.70 | 1.44 |
| Mn | −5.04E-05 | 0.79 | 2.07 |
| Ni | −9.18E-03 | 0.0004 | 2.74 |
| Pb | 6.82E-04 | 0.28 | 1.27 |
| Zn | 9.77E-07 | 0.99 | 1.3 |
| Fe | 2.99E-06 | 0.07 | 2.1 |
| Protein | |||
| (Intercept) | 52.01 | 0.04 | |
| As | −45.2 | 0.0001 | |
| Cu | −0.44 | 0.24 | |
| Mn | 1.05 | 0.04 | |
| Ni | 12.19 | 0.07 | |
| Pb | 2.05 | 0.22 | |
| Zn | 0.12 | 0.38 | |
| Fe | −0.007 | 0.12 | |
| MDA | |||
| (Intercept) | 1.08E + 02 | <2e-16 | |
| As | 5.91E + 00 | 0.19 | |
| Cu | 6.15E-01 | 0.0001 | |
| Mn | 9.98E-02 | 0.62 | |
| Ni | −1.92E + 00 | 0.47 | |
| Pb | −2.42E + 00 | 0.0005 | |
| Zn | −1.00E-01 | 0.08 | |
| Fe | 4.47E-04 | 0.8 |
The heavy metals that showed statistical significance are indicated in bold font.
VIF: variance inflation factor.
4. Discussion
Lichens are remarkable organisms with the ability to respond sensitively to a wide range of environmental factors, making them valuable bioindicators of ecological health. Our study adopted a new approach by venturing into two of Korea’s most pristine regions, namely Gangwon Province and Jeju Island, to collect lichens and investigate their physiological responses to ambient heavy metal concentrations. Specifically, our study aimed to identify the physiological variables of lichens that could be used as indicators of heavy metal exposure, and to determine which types of heavy metals affect these variables.
An inverse relationship between protein content and the degree of chlorophyll damage in lichens was apparent in this study. In general, earlier studies have shown that exposure to pollutants can lead to a decline in lichen chlorophyll content, which in turn can affect their protein content [36, 37]. This is because chlorophyll is required for photosynthesis, which produces the fixed C required for subsequent protein synthesis. [38]. The reason for the inverse relationship between protein content and chlorophyll damage in lichens observed in this study may be due to the role that proteins play in protecting the photosynthetic machinery of lichens from various stresses, including oxidative stress caused by environmental factors such as heavy metals, UV radiation, and air pollution. Proteins are involved in various functions in lichens, including the synthesis of enzymes and pigments necessary for photosynthesis, as well as the repair and protection of cellular structures and membranes from oxidative damage. Therefore, higher levels of protein in lichens may confer greater protection against stressors such as heavy metals, which can induce oxidative damage to chlorophyll molecules and the photosynthetic machinery. In contrast, lower protein content may result in a greater susceptibility to oxidative stress, leading to chlorophyll damage and reduced photosynthetic efficiency. This may explain the inverse relationship between protein content and the degree of chlorophyll damage observed in the study. However, the exact relationship between chlorophyll degradation and protein content is influenced by several factors [39]. Further research is needed to better understand the underlying mechanisms and relationships between chlorophyll content, protein content, and lichen health.
A significant increase in MDA levels was observed in the present study, indicative of a decrease in membrane integrity, in lichens exposed to high Cu concentrations. This suggests that Cu accumulation inside lichen thallus can cause physical damage to the cell membrane, which may further exacerbate the negative effects of Cu on lichen health. Membrane integrity is essential for proper cellular physiological functioning [7] because it allows the selective transport of nutrients and water across the cell membrane. However, Cu can damage the membrane structure by generating reactive oxygen species (ROS) [40], which can cause damage to cellular components such as proteins, lipids, and DNA. ROS can also disrupt the integrity of the plasma membrane by oxidizing membrane lipids, leading to the leakage of MDA into the extracellular environment. Our findings are consistent with those of a previous study that reported the negative effects of Cu on membrane integrity in lichens [41], as well as with broader research indicating the deleterious effects of heavy metal pollution on ecosystems [42]. In addition to its direct impact on lichen health, Cu pollution can have cascading effects at other trophic levels, because lichens serve as an important food source for a variety of organisms [43].
Our results demonstrate that heavy metal accumulation has a significant negative impact on the health of lichens. Specifically, we observed a significant increase in damaged chlorophyll in lichens exposed to high levels of heavy metals, as previously reported [44], indicating that heavy metal pollution can interfere with normal metabolic processes in lichens [45]. Furthermore, the previously described levels of proteins and oxidized fatty acids are based on diverse underlying mechanisms, making it difficult to explain how heavy metals specifically harm these variables. Therefore, we recommend chlorophyll content as a suitable primary variable for lichens that respond to external pollutants.
5. Conclusions
Environmental sustainability is a critical issue in today’s world, with increasing levels of pollution and degradation of natural resources threatening the health of ecosystems and human populations. Our study highlights the potential of lichen studies in promoting environmental sustainability, particularly in the context of heavy metal pollution. Our findings demonstrate that lichens can serve as effective bioindicators of heavy metal pollution in the environment, providing a cost-effective and noninvasive way to monitor environmental health. By studying lichens, we can gain insight into the impact of human activities on the environment, and develop effective strategies for reducing heavy metal pollution. Furthermore, our study underscores the importance of sustainable practices in promoting environmental health. Sustainable practices, such as reducing greenhouse gas emissions, conserving natural resources, and minimizing waste, are essential for preserving the health of ecosystems and protecting human health. By promoting sustainable practices, we can reduce the impact of human activities on the environment and mitigate the effects of pollution.
Supplementary Material
Acknowledgements
The authors greatly appreciate and acknowledge the experimental support of the members of the Korean Lichen Research Institute and Jung Shin Park (postdoc position at Korea National Arboretum).
Funding Statement
This study was supported by a grant from the Korea National Arboretum under Grant [KNA1-1-27, 21-1].
Authors’ contributions
J.H.Y., S.-O.O. and J.-S.H. designed the study. S.-O.O. performed sample collection. J.H.Y. analyzed the data and visualized the figures. J. H. Y., S. O. O., and J. S. H. wrote and revised the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Not applicable.
References
- 1.Larson D. The absorption and release of water by lichens. Bibliotheca Lichenologica. 1987;25(35):351–360. [Google Scholar]
- 2.Kappen L. Response to extreme environments. In The lichens. Academic Press; 1973. p. 311–380. [Google Scholar]
- 3.Bačkor M, Loppi S.. Interactions of lichens with heavy metals. Biologia Plant. 2009;53(2):214–222. doi: 10.1007/s10535-009-0042-y. [DOI] [Google Scholar]
- 4.Roberts T, Goodman G.. Persistence of heavy metals in soils and natural vegetation following closure of a smelter. Trace Subst Environ Health. 1973;7(CONF-730613-). [Google Scholar]
- 5.Garty J, Galun M, Kessel M.. Localization of heavy metals and other elements accumulated in the lichen thallus. New Phytol. 1979;82(1):159–168. doi: 10.1111/j.1469-8137.1979.tb07571.x. [DOI] [Google Scholar]
- 6.Puckett K. The effect of heavy metals on some aspects of lichen physiology. Can J Bot. 1976;54(23):2695–2703. doi: 10.1139/b76-290. [DOI] [Google Scholar]
- 7.Tarhanen S, Metsärinne S, Holopainen T, et al. Membrane permeability response of lichen Bryoria fuscescens to wet deposited heavy metals and acid rain. Environ Pollut. 1999;104(1):121–129. doi: 10.1016/S0269-7491(98)00157-2. [DOI] [Google Scholar]
- 8.Heath RL, Packer L.. Photoperoxidation in isolated chloroplasts: II. Role of electron transfer. Arch Biochem Biophys. 1968;125(3):850–857. doi: 10.1016/0003-9861(68)90523-7. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez CM, Pignata ML.. The influence of air pollution on soluble proteins, chlorophyll degradation, MDA, sulphur and heavy metals in a transplanted lichen. Chem Ecol. 1994;9(2):105–113. doi: 10.1080/02757549408038568. [DOI] [Google Scholar]
- 10.Conti ME, Cecchetti G.. Biological monitoring: lichens as bioindicators of air pollution assessment—a review. Environ Pollut. 2001;114(3):471–492. doi: 10.1016/s0269-7491(00)00224-4. [DOI] [PubMed] [Google Scholar]
- 11.Białońska D, Dayan F.. Chemistry of the lichen Hypogymnia physodes transplanted to an industrial region. J Chem Ecol. 2005;31(12):2975–2991. doi: 10.1007/s10886-005-8408-x. [DOI] [PubMed] [Google Scholar]
- 12.Conti ME, Tudino M, Stripeikis J, et al. Heavy metal accumulation in the lichen Evernia prunastri transplanted at urban, rural and industrial sites in Central Italy. J Atmos Chem. 2004;49(1–3):83–94. doi: 10.1007/s10874-004-1216-9. [DOI] [Google Scholar]
- 13.Carreras HA, Pignata M.. Biomonitoring of heavy metals and air quality in Cordoba city, Argentina, using transplanted lichens. Environ Pollut. 2002;117(1):77–87. doi: 10.1016/s0269-7491(01)00164-6. [DOI] [PubMed] [Google Scholar]
- 14.Guner A, Turkez H, Aslan A.. The in vitro effects of Dermotocarpon intestiniforme (a lichen) extracts against cadmium induced genetic and oxidative damage. Ekoloji. 2012;21(84):38–46. doi: 10.5053/ekoloji.2012.845. [DOI] [Google Scholar]
- 15.Cabral JP. Copper toxicity to five Parmelia lichens in vitro. Environ ExpBot. 2003;49(3):237–250. doi: 10.1016/S0098-8472(02)00087-4. [DOI] [Google Scholar]
- 16.Jeong SH, Kim JH, Son BK, et al. Comparison of air pollution and the prevalence of allergy-related diseases in Incheon and Jeju city. Korean J Pediatr. 2011;54(12):501–506. doi: 10.3345/kjp.2011.54.12.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park S, Kim SJ, Yu H, et al. Developing an adaptive pathway to mitigate air pollution risk for vulnerable groups in South Korea. Sustainability. 2020;12(5):1790. doi: 10.3390/su12051790. [DOI] [Google Scholar]
- 18.Ronen R, Galun M.. Pigment extraction from lichens with dimethyl sulfoxide (DMSO) and estimation of chlorophyll degradation. Environ Exp Bot. 1984;24(3):239–245. doi: 10.1016/0098-8472(84)90004-2. [DOI] [Google Scholar]
- 19.Kwon TW, Menzel DB, Olcott J.. Reactivity of malondialdehyde with food constituents. J Food Sci. 1965;30:808–813. [Google Scholar]
- 20.Arb C, Brunold C.. Lichen physiology and air pollution. I. Physiological responses of in situ Parmelia sulcata among air pollution zones within Biel, Switzerland. Can J Bot. 1990;68(1):35–42. doi: 10.1139/b90-006. [DOI] [Google Scholar]
- 21.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1–2):248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 22.Link DD, Walter PJ, Kingston H.. Development and validation of the new EPA microwave-assisted leach method 3051A. Environ Sci Technol. 1998;32(22):3628–3632. doi: 10.1021/es980559n. [DOI] [Google Scholar]
- 23.Chambers JM. Software for data analysis: programming with R. vol. 2: Springer; 2008. doi: 10.1007/978-0-387-75936-4. [DOI] [Google Scholar]
- 24.Royston JP. An extension of Shapiro and Wilk’s W test for normality to large samples. J R Stat Soc: C Appl Stat. 1982;31(2):115–124. doi: 10.2307/2347973. [DOI] [Google Scholar]
- 25.Ruxton GD. The unequal variance t-test is an underused alternative to student’s t-test and the mann–whitney U test. Behav Ecol. 2006;17(4):688–690. doi: 10.1093/beheco/ark016. [DOI] [Google Scholar]
- 26.Breslow N. A generalized Kruskal-Wallis test for comparing K samples subject to unequal patterns of censorship. Biometrika. 1970;57(3):579–594. doi: 10.1093/biomet/57.3.579. [DOI] [Google Scholar]
- 27.Napierala MA. What is the Bonferroni correction? AAOS Now. 2012;6(4):40–42. [Google Scholar]
- 28.Wickham H, Chang W, Wickham MH.. Package ‘ggplot2. Create Elegant Data Visualisations Using the Grammar of Graphics Version. 2016;2(1):1–189. [Google Scholar]
- 29.Kassambara A. ggpubr: ‘ggplot2’ Based Publication Ready Plots. R package version 0.6.0, 2023. Available from: https://rpkgs.datanovia.com/ggpubr/. [Google Scholar]
- 30.Yu G. scatterpie: Scatter Pie Plot. [Online]. 2018. Available from: https://CRAN.R-project.org/package=scatterpie [Google Scholar]
- 31.Oksanen J, Blanchet FG, Kindt R, et al. Package ‘vegan. Community Ecology Package, Version. 2013;2(9):1–295. [Google Scholar]
- 32.Beals EW. Bray-Curtis ordination: an effective strategy for analysis of multivariate ecological data. Adv Ecol Res. 1984;14:1–55. [Google Scholar]
- 33.Wissler C. The spearman correlation formula. Science. 1905;22(558):309–311. doi: 10.1126/science.22.558.309. [DOI] [PubMed] [Google Scholar]
- 34.Quandt RE. Tests of the hypothesis that a linear regression system obeys two separate regimes. J Am Stat Assoc. 1960;55(290):324–330. doi: 10.1080/01621459.1960.10482067. [DOI] [Google Scholar]
- 35.Lallmahamood M. An examination of individual’s perceived security and privacy of the internet in Malaysia and the influence of this on their intention to use E-commerce: using an extension of the technology acceptance model. J Internet Banking Commer. 1970;12(3):1–26. [Google Scholar]
- 36.Garty J, Karary Y, Harel J.. The impact of air pollution on the integrity of cell membranes and chlorophyll in the lichen Ramalina duriaei (De not.) bagl. transplanted to industrial sites in Israel. Arch Environ Contam Toxicol. 1993;24(4):455–460. doi: 10.1007/BF01146161. [DOI] [Google Scholar]
- 37.von Arb C, Mueller C, Ammann K, et al. Lichen physiology and air pollution: II. Statistical analysis of the correlation between SO2, NO2, NO and O3, and chlorophyll content, net photosynthesis, sulphate uptake and protein synthesis of Parmelia sulcata Taylor. New Phytol. 1990;115(3):431–437. doi: 10.1111/j.1469-8137.1990.tb00468.x. [DOI] [PubMed] [Google Scholar]
- 38.Eckhardt U, Grimm B, Hörtensteiner S.. Recent advances in chlorophyll biosynthesis and breakdown in higher plants. Plant Mol Biol. 2004;56(1):1–14. doi: 10.1007/s11103-004-2331-3. [DOI] [PubMed] [Google Scholar]
- 39.Munzi S, Pirintsos SA, Loppi S.. Chlorophyll degradation and inhibition of polyamine biosynthesis in the lichen Xanthoria parietina under nitrogen stress. Ecotoxicol Environ Saf. 2009;72(2):281–285. doi: 10.1016/j.ecoenv.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 40.Vantová I, Bačkor M, Klejdus B, et al. Copper uptake and copper-induced physiological changes in the epiphytic lichen Evernia prunastri. Plant Growth Regul. 2013;69(1):1–9. doi: 10.1007/s10725-012-9741-z. [DOI] [Google Scholar]
- 41.Sujetovienė G. Copper induced physiological changes and oxidative damage in lichen Ramalina farinacea. Biologija. 2014;60(4):196–201. doi: 10.6001/biologija.v60i4.3039. [DOI] [Google Scholar]
- 42.Mohammed AS, Kapri A, Goel R.. Heavy metal pollution: source, impact, and remedies. Biomanagement of Metal-Contaminated Soils. 2011;:1–28. [Google Scholar]
- 43.Asplund J, Wardle DA.. How lichens impact on terrestrial community and ecosystem properties. Biol Rev Camb Philos Soc. 2017;92(3):1720–1738. doi: 10.1111/brv.12305. [DOI] [PubMed] [Google Scholar]
- 44.Pisani T, Munzi S, Paoli L, et al. Physiological effects of arsenic in the lichen Xanthoria parietina (L.) Th. Fr. Chemosphere. 2011;82(7):963–969. doi: 10.1016/j.chemosphere.2010.10.079. [DOI] [PubMed] [Google Scholar]
- 45.Van Kooten O, Snel JF.. The use of chlorophyll fluorescence nomenclature in plant stress physiology. Photosynth Res. 1990;25(3):147–150. doi: 10.1007/BF00033156. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.