ABSTRACT
Reproduction is characterized by a series of massive renovations at molecular, cellular, and tissue levels. Recent studies have strongly tended to reveal the involvement of basic molecular pathways such as autophagy, a highly conserved eukaryotic cellular recycling, during reproductive processes. This review comprehensively describes the current knowledge, updated to September 2022, of autophagy contribution during reproductive processes in males including spermatogenesis, sperm motility and viability, and male sex hormones and females including germ cells and oocytes viability, ovulation, implantation, fertilization, and female sex hormones. Furthermore, the consequences of disruption in autophagic flux on the reproductive disorders including oligospermia, azoospermia, asthenozoospermia, teratozoospermia, globozoospermia, premature ovarian insufficiency, polycystic ovarian syndrome, endometriosis, and other disorders related to infertility are discussed as well.
Abbreviations: AKT/protein kinase B: AKT serine/threonine kinase; AMPK: AMP-activated protein kinase; ATG: autophagy related; E2: estrogen; EDs: endocrine disruptors; ER: endoplasmic reticulum; FSH: follicle stimulating hormone; FOX: forkhead box; GCs: granulosa cells; HIF: hypoxia inducible factor; IVF: in vitro fertilization; IVM: in vitro maturation; LCs: Leydig cells; LDs: lipid droplets; LH: luteinizing hormone; LRWD1: leucine rich repeats and WD repeat domain containing 1; MAP1LC3: microtubule associated protein 1 light chain 3; MAPK: mitogen-activated protein kinase; MTOR: mechanistic target of rapamycin kinase; NFKB/NF-kB: nuclear factor kappa B; P4: progesterone; PCOS: polycystic ovarian syndrome; PDLIM1: PDZ and LIM domain 1; PI3K: phosphoinositide 3-kinase; PtdIns3P: phosphatidylinositol-3-phosphate; PtdIns3K: class III phosphatidylinositol 3-kinase; POI: premature ovarian insufficiency; ROS: reactive oxygen species; SCs: Sertoli cells; SQSTM1/p62: sequestosome 1; TSGA10: testis specific 10; TST: testosterone; VCP: vasolin containing protein.
KEYWORDS: Apoptosis, autophagy, fertility, follicles, granulosa cells, infertility
Introduction
Autophagy is composed of two Greek words auto (that means self) and phagein (that means to eat), which refers to a highly conserved catabolic pathway crucially essential to maintain cellular homeostasis. Although the process was described for the first time in the 1960s, it took approximately 30 years for the identification of the involved ATG (autophagy related) genes to propel main breakthroughs in deciphering the mechanistic complexities of autophagy [1–3]. Autophagy can be divided into three major forms, including macroautophagy, microautophagy, and chaperone-mediated autophagy, all of which are responsible to deliver the cargo to the lysosomes regardless of their different pathways [4,5]. Despite the initial consideration of autophagy, particularly macroautophagy, as a nonselective bulk degrative process, it is currently represented a highly selective mechanism due to the discovery of autophagy receptors, among which SQSTM1/p62 (sequestosome 1) was the first [6,7]. The propound of selective cargo became the basis for further classification of autophagy, which includes glycophagy and lipophagy (macromolecules), mitophagy (mitochondria), reticulophagy (endoplasmic reticulum), nucleophagy (parts of the nucleus), xenophagy (pathogens), and lysophagy (lysosomes) [6].
Contrary to the fact that autophagy is often referred to as a degradative mechanism, it is appropriate to describe it as a recycling pathway to more accurately reflect its physiological function, since the end products of the autophagic flux are not discarded but serve either as energy sources or as building blocks in the synthesis of macromolecules. Notably, a plethora of evidence demonstrated that autophagy is remarkably involved in a variety of vital biological processes ranging from starvation to adaptation, cell development and differentiation, innate and adaptive immunity, aberrant structures degradation, damaged or excessive organelles turnover, tumor suppression, cell survival, and regulated cell death [8–10]. In fact, the beneficial role of this process is assumed to contribute to diverse aspects of human and other organisms’ physiology and pathology including but not limited to metabolism and energy homeostasis, development, proliferation, differentiation, and cell death [11,12]. Incontrovertibly, a number of biological processes such as fertility and infertility, due to their extreme dependence on these beneficial roles of autophagy, can be considered an exhibition of autophagy function, which ultimately determines the fate of human fecundity by its regulatory ability.
It is well-documented that pregnancies and live births are intricated multi-step processes that result from a variety of highly regulated molecular pathways’ interactions at several consecutive levels including the development of female and male gametes, zygote formation and proliferation, and the development of embryo prior to, during, and after implantation. All of these biomolecule-mediated mechanisms require the further interaction of biomolecules with the surrounding environment such as male and female gonads, and subsequently after pregnancy in the female genital tract from tubes to the uterus and endometrium [13,14]. Notably, accumulative evidence has demonstrated that autophagy functions in a plethora number of cellular events during processes involved in both genders’ fertility. Interestingly, knowledge of the association between autophagy and the human reproductive system has been paramount to understanding physiology (fertility) and pathology (infertility), with the conclusive purpose of favoring describing and treating female and male infertility.
Indeed, numerous pieces of evidence have revealed the indispensable role of autophagy in maintaining the reproductive ability of both sexes, in which any disruption will lead to subsequent diseases [15,16]. The present review attempts to comprehensively describe the function of autophagy during reproductive processes in two separate chapters, including “male reproduction” and “female reproduction”, and ultimately illustrates the reproductive disorders/disruptions associated with autophagic flux interruption. For this purpose, all conducted studies until September 2022, without any initial exclusion, were prepared by searching for related keywords such as autophagy, ovary, uterus, prostate, fertility, infertility, sperm, spermatid, spermatogonia, spermatogenesis, testosterone (TST), hormone, estrogen (E2), progesterone (P4), follicle-stimulating hormone (FSH), luteinizing hormone (LH), ovum, zygote, embryo, decidualization, implantation, fertilization, germ cell, follicle, sexual cycle, etc. in Google Scholar, PubMed, and Scopus search engines. In the next step, studies that were not in English, the abstract lacked relevant material, were not original research, or the findings were not available were excluded, and what remained were included in this review.
Autophagy overview
The preserved mechanism of autophagic flux is regulated by a series of proteins. MTOR (mechanistic target of rapamycin kinase) consists of two complexes including MTORC1 and MTORC2 each of which represents a different function and location. This kinase, which is involved in vital cellular processes such as proliferation and response to stimuli, is considered the main negative regulator of autophagy as it is able to directly phosphorylate the ULK1 (unc-51 like autophagy activating kinase 1) complex (ULK1-ULK2, ATG13, RB1CC1/FIP200, and ATG101) and inactivate autophagy. The anabolic inputs such as energy, nutrients, amino acids, growth factors, and hypoxia are considered regulators of MTOR activity [17,18]. In addition, AMP-activated protein kinase (AMPK) is a key regulator of autophagy, which controls the autophagic process by two distinct mechanisms, either through phosphorylation of MTORC1 and its inactivation or direct phosphorylation of ULK1 and its activation [19,20]. TFEB (transcription factor EB) is another positive regulator that controls autophagy and lysosomal biogenesis whose nuclear translocation leads to the activation of autophagy by interaction with both MTOR and AMPK [21,22]. Furthermore, the class I phosphoinositide 3-kinase (PI3K) could regulate the kinases that modulate autophagy. The incorporation of BECN1 (beclin 1), one of the class III phosphatidylinositol 3-kinase (PtdIns3K) subunits and an essential stimulator of phosphatidylinositol-3-phosphate (PtdIns3P) biosynthesis, into class I PI3K is controlled by its association with other proteins and kinases [23,24].
The process of macroautophagy, which is considered the major part of the autophagy process and the most evidenced type of it, could be assumed to consist of two total stages, the formation of the autophagosome and the delivery of the cargo to the lysosome. In a state where MTORC1 is inhibited, the ULK complex is dephosphorylated and thereby activated subsequently playing a pivotal role in autophagy initiation by phosphorylating several downstream factors. It is documented that the activated ULK localizes to the phagophore and induces the activation of PtdIns3K. Indeed, two distinct BECN1-PtdIns3K complexes cooperate to produce PtdIns3P. The produced PtdIns3P contributes to autophagosome nucleation or endolysosomal and autolysosomal maturation [25,26]. Subsequently, the ATGs are responsible for the elongation of autophagosome formation. The membrane of autophagosomes is supplied by vesicles containing ATG9A, which is described as the only transmembrane core ATG protein. Moreover, ATG2A and ATG2B, in cooperation with other proteins called WIPI (WD repeat domain, phosphoinositide interacting), participate in the early stages of membrane elongation at the site of PtdIns3P generation [27,28]. ATG12 conjugates to ATG5, which binds ATG16L1 (forming the ATG12–ATG5-ATG16L1 complex) and acts in the lipidation of MAP1LC3A (microtubule associated protein 1 light chain 3 alpha), all of which contribute to the expansion of the phagophore and completion of the autophagosome membrane [29]. Upon the activation of MAP1LC3A, it interacts with phosphatidylethanolamine, ATG3, and ATG7 resulting in the conversion of MAP1LC3A to MAP1LC3B [30,31]. This protein could locate in both the inner and outer membrane of the autophagosome and enable the autophagosome to bind its selected substrates [31,32]. During the final stage, the mature autophagosome containing the cargo fuses with the lysosome and forms the autolysosome, which is followed by the degradation of selected macromolecules or organelles [33].
Autophagy and the male reproductive system
The male reproduction process is completely depending on the testis function consisting of two fundamental parts including the mesenchyme and seminiferous tubules. Seminiferous tubules are made of Sertoli cells (SCs) and several germ cells. An appropriate interaction between SCs and germ cells within seminiferous tubules is essential for healthy spermatogenesis [34]. In fact, the tight connection between SCs and the blood-testis barrier assists SCs in providing nourishing and spatial support and makes them the main determinant of the number of germ cells. Meanwhile, Leydig cells (LCs) are the major functional part of mesenchyme which are essential for regulating spermatogenesis through the endocrine terms [35,36].
A plethora of research has revealed various important physiological roles of autophagy essential for testicular cell functions. Degradation of unnecessary components in seminiferous tubules, regulating the normal cytoskeletal organization, and adjusting the biosynthesis of sex steroids are the main autophagic activities in SCs [37,38]. Additionally, it is evidenced that autophagy could modulate the fundamental steps of the spermatogenesis process and any disruption in autophagic flux could end up in infertility. Herein, we will clarify the role of autophagy in this important process.
Autophagy and spermatogenesis
The spermatogenesis process includes mitotic amplification of spermatogonia, meiosis of spermatocytes to form spermatids, and spermiogenesis, the process of spermatid maturation, in which round haploid spermatids transform to become elongated spermatids [36,39]. Recent studies have demonstrated the engagement of autophagy in major parts of spermatogenesis, including the removal of residual bodies, acrosome biogenesis, cytoskeleton organization, and ectoplasmic specialization assembly [40].
Although autophagy has been assumed as a regulator of important processes involved in spermatogenesis, various factors and genes act as superior regulators of this process during spermatogenesis. The initiation of autophagy during spermatogenesis is mediated by Chtop/srag (chromatin target of PRMT1) through a two-way strategy including its promoter region that binds to Sox9 (SRY-box transcription factor 9), a transcription factor for male sex determination, and interaction with preexisting Becn1 [41]. The epg5 gene encodes one of the most vital components of autophagy and its deficiency leads to impaired autophagic flux. The maturation or processing of autophagosomes is one of the possible roles of this gene. The lack of expression or mutation in this gene is associated with selectively impaired spermatogonia. The presence of epg5 in fish and its homolog in the mouse testis (Epg5) provides a conserved function during vertebrate spermatogenesis [42]. The rapid increase in the expression of TFEB during the development and migration of germ cells from the basal membrane toward seminiferous tubules may indicate the regulatory role of this factor on autophagy during the differentiation of spermatogonia [43].
Interestingly, recent studies have demonstrated that autophagy was only activated during late meiotic spermatocytes but not in spermatogonia and early spermatocytes (Figure 1). In other words, autophagy must be inhibited for the initiation of the meiotic cell cycle. The transition from the mitotic phase to the meiotic cell cycle during spermatogenesis coincides with the suppression of autophagy by the STRA8 (stimulated by retinoic acid gene 8), a meiotic gatekeeper, which is mediated by repression of Nr1d1 (nuclear receptor subfamily 1, group D, member 1) expression, a nuclear hormone receptor gene [44]. Localization of MAP1LC3B at different stages of spermatid differentiation with the development of a large number of autophagosomes, and transfer of numerous endoplasmic reticulum (ER) to a Chrysanthemum flower center, which formed and expanded several double-layer membranes, indicates active autophagy in spermiogenesis in Chinese soft-shelled turtle [45]. Autophagy appears to be exacerbated by the development of male germ cells because the ATG7 and MAP1LC3 localization is poor in the early stages of germ cells near the basal membrane, but intensifies toward seminiferous tubules in the round to elongated spermatids. Furthermore, there is an increase in the number of lysosomes and autophagosomes concordant with the progression of spermiogenesis, which was not found in diploid cells. The transfer of the ER to the Chrysanthemum flower, involved in the removal of the cytoplasm, indicates the essential role of autophagy [46]. Similarly, ATG7 is moderately expressed in cytoplasmic extensions of SCs during inactive spermatogenesis, whereas it is strongly expressed during active spermatogenesis [47]. Along with that, MAP1LC3 demonstrated elevated expression from basal to the luminal compartment of the seminiferous tubule, as well as a higher expression during active than inactive spermatogenesis [47]. These findings, in addition to emphasizing the increase of autophagic flux in the late stages of spermatogenesis, determine the crosstalk between autophagy with other molecular pathways and cellular processes which in turn any interruption is followed by male fertility complications [48].
Figure 1.
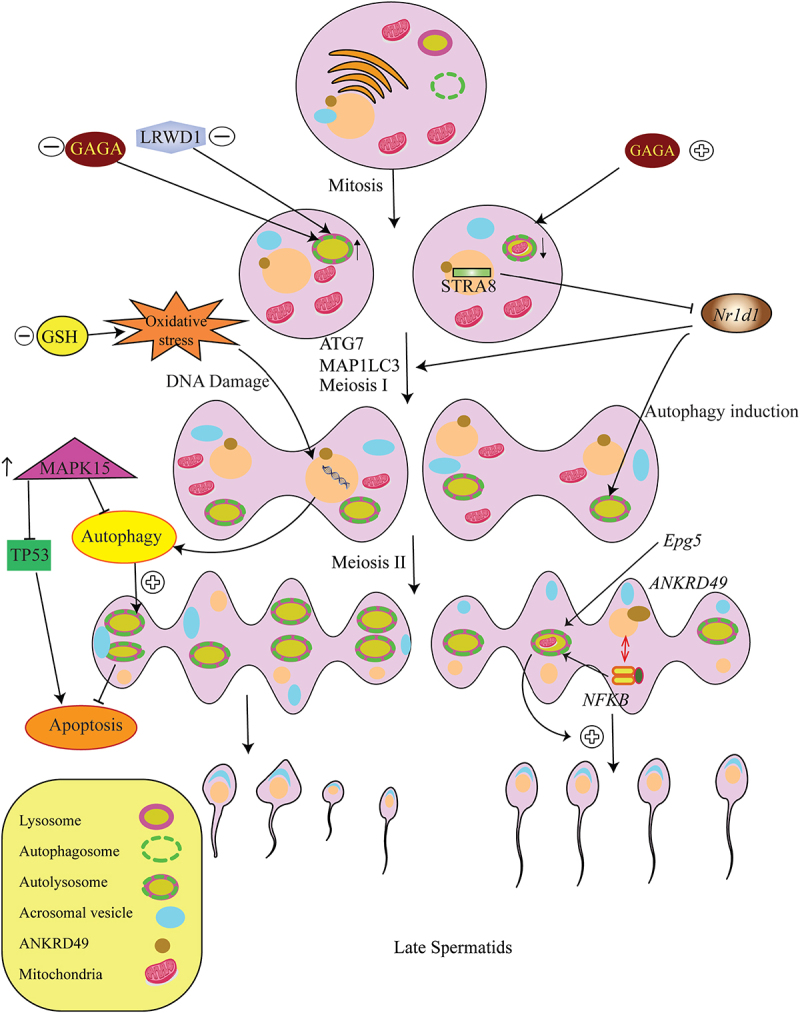
The role of autophagy during spermatogenesis. The induced overproduction of GAGA in the second or third mitosis in early spermatogenesis leads to an increase in the presence of autophagosomes, an increased autophagic flux, and a significant increase in mitophagy in spermatogonia. Moreover, the expression of Epg5 is involved in the augmentation of autophagy and mitochondrial clearance during spermatogenesis. Interestingly, the Nr1d1-mediated suppression of autophagy by STRA8 is necessary for mitotic to miotic transition. The rate of autophagy in spermatogenesis increases after meiosis I. Conversely, decreased levels of GSH increase the levels of MAP1LC3 proteins showing that autophagy is induced under oxidative stress, leading to the development and survival of male germ cells with more DNA damage and sperm deformations followed by azoospermia and oligozoospermia. ANKRD49, along with the NFKB pathway, increases the spermatogenesis rate via autophagy-dependent survival. In addition, overexpression of MAPK15 disrupts autophagy supportive function that controls the prevention of DNA damage and the activation of the TP53, hence causing malignant transformation of germ cells.
Mitophagy and spermatogenesis
When mitochondria are defective, mitophagy, the specific degradation of mitochondria by autophagic flux, functions to eliminate the damaged mitochondria [49]. Thus, it could be claimed that mitophagy is the most powerful controller of mitochondrial quality. In case of any disruption in mitochondrial health, mitophagy initiates the recycling and producing small healthy mitochondria to save cellular hemostasis. Conversely, shreds of evidence suggested that the dynamic remodeling of mitochondria is a significant cellular event that occurs during spermatogenesis. The term dynamic refers to the fusion and fission of the mitochondria [50]. The alterations in the size, number, and shape of mitochondria in different stages of sperm growth and development are reported. For instance, undifferentiated spermatogonia generally contain tiny and fragmented mitochondria [50]. Whereas, to initiation of meiosis and differentiation of spermatogonia into spermatocytes, mitochondria undergo mitofusin-mediated fusion to supply the required energy. Subsequently, a rapid fragmentation occurs in the mitochondria to produce small and sphere ones and organize them in the central spiral pattern between the postmitotic spermatid. Finally, at the end of spermatid maturation, mitochondria are transformed into residual bodies in order to be recycled by heterotrophic degradation in SCs [51,52]. Therefore, it is pivotally necessary to elucidate whether mitophagy is involved in this dynamic restructure of mitochondria and what is the importance of mitophagy in male reproducibility.
Spermiogenesis, the end stage of spermatogenesis, requires various changes in cells including elongation and condensation of the sperm nucleus, acrosome biosynthesis, and flagella formation. Throughout this process, the elimination of excess mitochondria by mitophagy is an indispensable part of spermatozoa production [53]. Several studies have shown that any defect, mutation, or conditional knocking out of genes serving as key molecules of autophagy, led to mitochondrial dynamic disruption and male fertility complications. For example, ATG7-null mice exhibit an intense coiled flagellum and dislocated poorly condensed mitochondria [53]. Furthermore, SPATA33 (spermatogenesis associated 33), a vital protein in localizing sperm calcineurin to the mitochondria and regulating sperm motility is also a receptor for mitophagy involved in the degradation processes in male germline cells. SPATA33 is activated under starvation stress and promotes mitophagy via directly mediating ATG16L1 interaction with VDAC2 (voltage dependent anion channel 2), an outer mitochondrial membrane protein [54]. Therefore, the findings indicate the probability of crucial involvement of mitophagy in degrading mitochondria during spermiogenesis which is extremely vital for sperm motility too, probably in terms of energy supply, which will be discussed later.
Autophagy and removal of residual bodies
The first ultrastructural studies described the TST-independent phagocytic function of SCs, which suggested the removal of residual bodies and germ cell debris in an autophagy-lysosome-mediated pathway [55]. In nematodes, although engulfment and degradation of residual bodies in gonadal sheath cells involve the genes related to apoptotic cell clearance, the lack of essential genes to activate apoptosis did not affect the removal of residual bodies. Thereby, the “eat me” signal and phagocytosis are more critical [56]. In mammals, the segregation of the dominant part of the cytoplasm into residual bodies is involved in the generation of spermatozoa, which are detached and removed from the spermatids [57,58]. Moreover, the UPS, which is responsible for Parkin-mediated mitophagy, is extremely active during mammalian spermiogenesis [59,60]. Some ATGs such as ATG7 are highly involved in the removal of extra cytoplasm [61].
Autophagy and acrosome biogenesis
Autophagy can be considered one of the main regulators of organelles required for the development and differentiation of germ cells (Table 1). The acrosome is a lysozyme-dependent specialized fertilization organelle that covers the anterior of the sperm nucleus and contains enzymes that penetrate the outer membrane of an egg cell, allowing the egg to be fertilized. Autophagy could be considered an inducer of acrosome biogenesis since germ cell-specific atg7 knockout mice have a defect in the biogenesis of this organelle and exhibited a phenotype similar to human globozoospermia. ATG7 appears to play a role in the secondary stages of spermatogenesis, during the Golgi phase in which preacrosomal vesicles fuse with a single acrosome vesicle, leading to irregular or round-headed spermatozoa [64]. ATG5 is an autophagic core protein that is involved in acrosome biogenesis and normal male fertility by contributing to elongating spermatid development and sperm individualization [63]. A recent study revealed that acrosome biogenesis and manchette development during mouse spermiogenesis is dependent on autophagic flux as well as PFN4 (profilin family member 4) expression, a protein that localizes to the acrosome-acroplaxome-manchette complex and is highly expressed during spermiogenesis [65]. The downregulation of this protein is accompanied by upregulated PI3K-AKT/PKB (AKT serine/threonine kinase) pathway and reduced AMPK levels all of which lead to autophagic blockage and thereby inhibition of acrosome formation [65].
Table 1.
Autophagy is required for spermatogenesis.
| Process | Autophagy role | Refs |
|---|---|---|
| The progress of spermatogenesis |
|
[45–48,55,62] |
| Mitophagy |
|
[42,53,54] |
| Removal of residual bodies |
|
[46,55,63] |
| Acrosome biogenesis |
|
[64,65] |
| Cytoskeleton reorganization |
|
[40,61] |
| Lisophagy and stress |
|
[66–68] |
Note: Autophagy possesses a crucial conserved role in progressing germ cell maturation, removal of residual bodies, maintaining homeostasis, the survival of SCs, the biogenesis of required organelles, and advancing spermatogenesis in cooperation with other factors.
Cytoskeleton organization
The cytoskeleton as the main mechanical support for the cell consists of two major systems: F-actin and microtubules [65]. The communication between SCs and germ cells is vital to support the growth and maturation of germ cells that require ectoplasmic specialization. These connections are regulated by the cytoskeleton. ATG7 is an essential factor for F-actin organization in mouse embryonic fibroblast cells. Autophagy causes the breakdown of PDLIM1 (PDZ and LIM domain1), a negative cytoskeleton organization regulator in SCs, which is necessary for the proper accumulation of ectoplasmic specialization [40]. ATG7, and perhaps MAP1LC3, are highly involved in the breakdown of PDLIM1, and defect in these ATGs leads to pathological alterations such as cytoskeleton disorganization, lack of extra cytoplasm removal, and drop in sperm motility [61].
Ectoplasmic specialization is an actin microfilament-rich anchoring junction that consists of an apical side which is a facilitator of spermatid development, and a basal side which is the structural part of the blood-testis-barrier. Some early studies showed that the apical ectoplasmic specialization participates in sperm head shaping [69]. The remodeling of ectoplasmic specialization is closely connected to cytoskeleton organization. The molecular mechanism underlying this process is still not clear, however considering the dependency of ectoplasmic specialization assembly on the cytoskeleton, the effect of autophagy on it might be indirectly and through modulating the cytoskeleton. For instance, the knocking down of ATG7 and ATG5 influenced the apical ectoplasmic specialization structure [40]. In fact, the absence of ATG7 and ATG5 and the lack of autophagy leads to the accumulation of PDLIM1 and disruption of the F-actin hoops of the apical ectoplasmic specialization.
Lipophagy and stress
Colocalization of MAP1LC3 and lipid droplets (LDs) in SCs during the proliferation of spermatogonia indicates the role of lipophagy in germ cell development [66]. Autophagy is involved in the consumption of LDs by Chinese soft-shelled turtle cells, which is a key process in steroidogenesis [67]. Deficiency of GSH, by altering the levels of MAP1LC3A and MAP1LC3B proteins, showed that autophagy is triggered under oxidative stress, independent of the AMPK pathway, and affects the antioxidant capacity of seminiferous tubules, therefore determining the development and survival of male germ cells [68]. Overexpression of MAPK15 (mitogen-activated protein kinase 15) causes malignant transformation of germ cells by disrupting an autophagic stress support pathway that controls DNA damage prevention and consequent activation of the TP53/p53 tumor suppressor [70]. In addition, downregulation in the TSGA10 (testis specific 10) during spermatid differentiation/maturation is accompanied by disrupted autophagy and overloaded reactive oxygen species (ROS) production leading to teratospermia [59]. Although under physiological conditions autophagy causes cell survival, upon exposure to the stress stimuli, the inhibition of apoptosis (upregulated BCL2) and autophagy (non-alteration of MAP1LC3B and SQSTM1 levels) lead to prolonged survival of SCs [71] (Figure 2).
Figure 2.
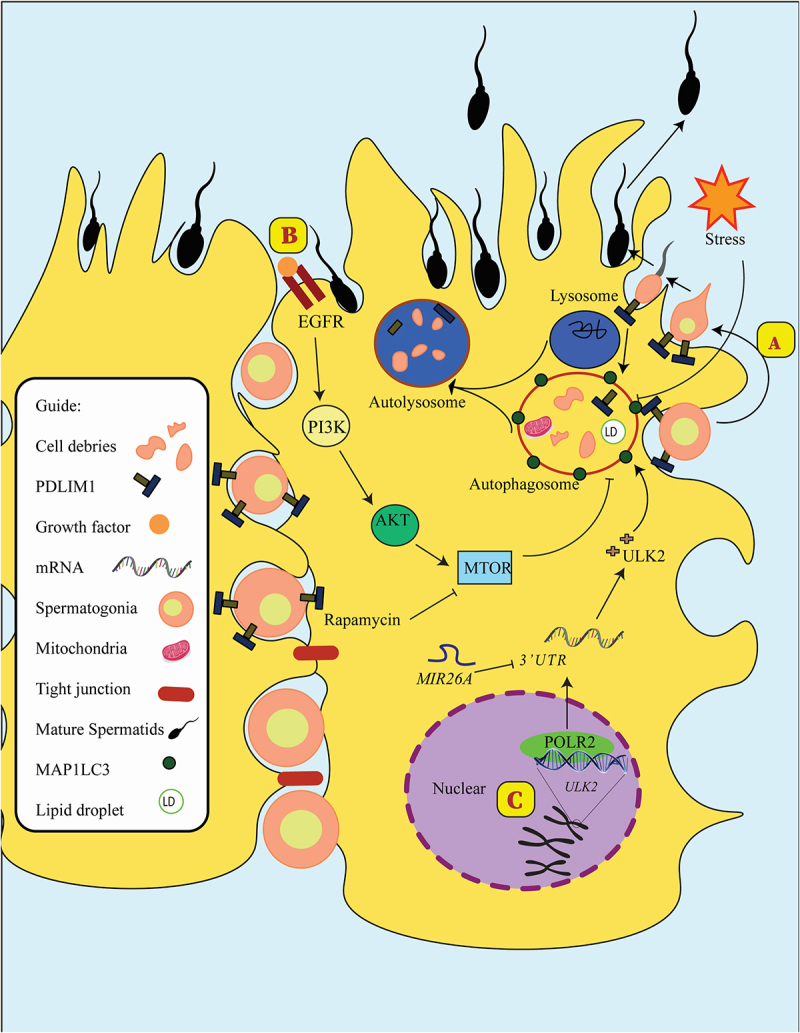
Autophagy in Sertoli cells. (a) Mature spermatids released after PDLIM1 breakdown via autophagy in SCs. (b) Activating PI3K-AKT-MTOR signaling could diminish autophagosome formation and reduce ULK2, the homolog of Atg1 in yeast. In addition, rapamycin as an MTOR inhibitor could positively affect autophagy flux. (c) MIR26A could reduce the expression of ULK2 in SCs and decrease autophagy flux in these cells.
By putting the found pieces of the puzzle together, including the involvement of mitochondria as the main energy supplier and dynamic changes in this organelle during spermatogenesis, the participation of autophagy in the spermatogenesis process as well as mitophagy in the recycling of defective mitochondria, and the autophagy-dependent removal of residual bodies, a clear picture will be determined. Taking all together, findings reveal the importance of autophagic flux during spermatogenesis and in different types of testicular cells. Hence, it could be concluded that autophagy is a quality control center for the sperm production process in both a nonspecific (bulk autophagy) or specific (mitophagy) manner. In addition, either overactive or deactivated autophagy has irreparable consequences for the fate of male fertility.
Autophagy and sperm motility and viability
Sperm Motility is a decisive factor for the qualification of male fertility, which is under the regulation of various crucial components, mainly through modulating Ca2+ and cAMP levels [72]. In 1998, Hargreaves et al. demonstrated that treatment with chloroquine reduced the motility and viability of human spermatozoa, however, the main reason was not elucidated [73]. Later studies introduced chloroquine as a strong autophagy inhibitor, and thus the relationship between sperm motility with autophagy was considered [74,75].
The predominant localization of MAP1LC3 and ATG7 in elongating and round spermatids may indicate the regulatory role of autophagy in spermiogenesis. Sperm motility is inevitably associated with flagella synthesis. ATG7 is essential for the biogenesis of spermatozoa flagella and the elimination of cytoplasm, as the removal of this autophagy-related protein has led to pathological changes such as a remarkable drop in sperm motility. These defects can be attributed to the impairment in the organization of the cytoskeleton, because, as mentioned earlier, the breakdown of PDLIM1 in an autophagy-lysosome pathway is essential for the development and maturation of germ cells [61]. ATG16L1, a core autophagic protein, is involved in intraflagella transport, a protective mechanism required for the assembly and maintenance of both cilia and flagella. Indeed, this appears to be an auxiliary function of autophagy and the IFT20 gene plays the key role, because other major autophagy markers such as MAP1LC3 and ubiquitin have apparently no role, and in addition, the knockout of this gene in male germ cells causes infertility, abnormality of spermatids, reduced sperm count and motility, and even declined ATG16L1 levels [76]. ATGs such as MAP1LC3, ATG5, ATG16L1, BECN1, SQSTM1, etc. are highly active in sperm and are involved in the viability and motility of spermatozoa. Autophagy activation induces increased sperm motility, reduced TOMM20 (translocase of outer mitochondrial membrane 20) and PINK1 expression, inhibited CASP3 (caspase 3)-CASP7 activation, and decreased apoptosis leading to cell survival [77]. Interestingly, sperm quality and motility increase after supplementation with amino acids, especially leucine, by affecting autophagy and inhibiting the fusion of autophagosomes with lysozyme, in a manner dependent on activating the Pi3k-Akt signaling pathway [78].
The current study in the “Autophagy and Spermatogenesis” section dealt with the effect of autophagy-dependent mitochondrial degradation and other organelle removal processes on sperm production and quality. Indeed, post-fertilization degradation of sperm mitochondria which is known as mitophagy is seldom reported in mammals and is mediated by the interaction of the autophagic pathway with the ubiquitin-proteasome system [79]. In this regard, the ubiquitin-binding autophagy receptor, SQSTM1, along with the proteasome-interacting ubiquitinated protein dislocase called VCP (valosin containing protein) are considered key factors during post-fertilization sperm mitophagy in both porcine and primates [80]. A recent study revealed that SQSTM1 is expressed in the midpiece/mitochondrial sheath of the sperm tail only after coincubation with oocytes, whereas VCP is expressed both prior to and upon co-incubation in the sperm mitochondria sheath. Moreover, subsequent to the binding of these proteins to sperm mitochondria, two sperm-borne pro-mitophagy proteins including SPATA18 (spermatogenesis associated 18) and PACRG (parkin coregulated) underwent alterations in the localization and degradation [81]. Accordingly, the palmitoylation of Vac8, or its homolog in mice called ARMC3 (armadillo repeat containing 3), mediates autophagic flux initiation through recruitment of Pik3c3-c1 which in turn promotes PtdIns3P generation at the phagophore assembly site leading to the recruitment of Atg18-Atg2 and subsequently autophagic degradation of cytosolic ribosomes. Importantly, this manner of cytosolic ribosome elimination by autophagy during spermiogenesis is highly required for providing energy for flagellar and sperm motility [82]. As mentioned earlier, ATG5 is involved in acrosome biogenesis and thereby affects sperm morphology, motility, and quality [63]. Consequently, autophagy is considered a novel biomarker of sperm quality as any alteration in related genes expression is followed by low sperm quality [83].
Autophagy and male sex hormones
Hector Chemes, for the first time, explained that autophagy-mediated phagocytosis by SCs is TST-independent [55]. Although no link between autophagy and TST had been described here, it could be considered a spark for further studies. In 1995, researchers found that autophagy had a high frequency in steroid-producing cells in which the levels of autophagy coincided with fluctuations in steroid secretion. Therefore, they suggested the regulatory role of autophagy in steroid secretion [84]. Decreased TST production by LCs occurs in late-onset hypogonadism with a decrease in autophagic activity. In autophagy inhibition, the expression of STAR (steroidogenic acute regulatory protein), which is responsible for transporting cholesterol into mitochondria and subsequent steroidogenesis, is disrupted, which may be the reason for the decrease in TST production. Interestingly, autophagy stimulation with rapamycin treatment increases steroidogenesis in LCs of old, not young, rats. Therefore, the reduction of steroidogenesis in old LCs can be caused by the decline of autophagy [85]. Recently, the regulatory role of Sirt1 on TST biosynthesis is believed to be mediated through autophagy [86]. Accordingly, autophagy is documented to be involved in the production of TST by transporting cholesterol to the LCs. Autophagic disruption in steroidogenic cell-specific, which is primarily the site of TST production, is associated with decreased serum TST levels due to defects in cholesterol uptake and causes symptoms similar to late‐onset hypogonadism [65]. By disrupting autophagy, NHERF2/Na+/H+ exchanger regulatory factor 2 accumulates in the LCs and subsequently degrades SCARB1 (scavenger receptor class B member 1), eventually leading to insufficient cholesterol supply [87]. Lipophagy is involved in modulating the breakdown of cholesteryl esters into free cholesterol which is the TST biosynthesis substrate [88].
Mitophagy, mitochondrial biogenesis and dynamics, normal ATP production, physiological levels of TST, and low levels of NO-cGMP pathway could be considered characteristics of healthy LCs as any disruption in mentioned factors leads to aging-related hypogonadism [89]. On the contrary, in patients with non-obstructive azoospermia, the level of BECN1 is negatively correlated with serum TST levels suggesting this autophagic marker is a predictor of lower chances of positive sperm retrieval [90].
Conversely, some autophagic degradation functions appear to be regulated by TST. For example, the clearance of androgen binding protein whose dysregulation causes endocrine and andrological diseases is mediated by autophagy, and TST has a negative effect on its degradation, as by selectively inhibiting autophagy, TST prolongs the half-life of this protein [91]. In addition, a higher level of TST is documented to cause growth inhibition of prostate cancer cells through two parallel autophagy-mediated processes including ferritinophagy and nucleophagy [92].
Autophagy and the female reproductive system
Autophagy and folliculogenesis
Folliculogenesis is the process of follicles growth, which is associated with the maturation, proliferation, and differentiation of oocytes, granulosa cells (GC), and theca cells and finally results in ovulation or atresia of follicles. Females are born with a non-expanding pool of primordial follicles of about 2 million, which declines to 400,000 by the time they reach menarche [93]. Primordial follicles consist of an oocyte arrested at prophase I and a layer of GCs surrounding the dormant oocyte [94,95]. Monthly, a certain number of primordial follicles are recruited which is accompanied by the maturation of the oocyte and differentiation of GC leading to the formation of primary follicles. The proliferation and differentiation of GC along with the maturation of oocytes continue to form secondary follicles [94,95]. By the recruitment of androgen-producer cells, known as theca cells, antral follicles are formed [96]. A plethora of evidence has determined that the autophagic flux is involved in the establishment of the follicular pool, the proliferation and differentiation of GC, and the survival of germ cells, oocytes, and follicles. In addition, the programmed death of GC and germ cells and follicular atresia are dependent on autophagy, which may be with or without the cooperation of the apoptotic cell death program.
Morphologically, in invertebrates and during ovarian differentiation, the death of germinative cells in the ovarioles occurs in a process similar to apoptosis, while autophagy is responsible for somatic cell death. Incidentally, when the primordial pool is established, autophagy under the influence of SIRT1‐involved epigenetic regulation prevents germ cell over-loss, which is associated with increased levels of H4K16ac and suppression of apoptosis [97]. Importantly, histone acetylation is considered the action site of endogenous and exogenous compounds on the autophagic flux. Melatonin, for example, could improve oocyte maturation and subsequent developmental competence of parthenogenetic embryos by decreasing the levels of H3K27ac and H4K16ac and the increase in histone acetylation and autophagy in metaphase II oocytes [98].
In Bactrocera oleae and Ceratitis capitata, two well-known fruit flies, during the late developmental stages of oogenesis, autophagy, not apoptosis, is responsible for follicular epithelium programmed death [99]. Furthermore, Atg3 is required for choriogenesis, the last stage of an insect’s oogenesis [100]. However, both autophagy and apoptosis mechanisms are involved in cell loss in the perinatal ovary of vertebrates, the allocation of each mechanism to one type of germ cell or somatic cell is not proposed, and both autophagy and apoptosis had a role in the abrupt disappearance of germ cells shortly after birth [101]. Oogenesis and communication between germ cells and follicle cells are mediated through starvation-induced autophagy via insulin/TOR signaling [102]. Indeed, the induction of autophagy by starvation is necessary for primordial follicle formation in neonatal mice [103]. During oogenesis, dysfunctional mitochondria surround the Balbiani body, a characteristic organelle in both invertebrate and vertebrate oocytes that is involved in critical functions such as the transport of germplasm and localized RNAs to a specific region of the oocyte and delivery of the mitochondria toward the germplasm, are eliminated by autophagy [104,105]. orb in invertebrates is the homolog of vertebrate CPEB which is involved in the maturation and polarization of oocytes via poly(A) tail elongation of several hundred mRNAs, as well as in early oogenesis through binding to atg mRNAs, which in collaboration with twin/Ccr4, suppresses Atg12 mRNA translation, leads to decreased autophagy and cell death in the Drosophila ovary [106]. Moreover, it is documented that autophagy is involved in cyst breakdown and primordial folliculogenesis in prenatal mouse ovaries by ROS clearance [107]. However, starvation in the mouse at birth causes impairment of germ cell cyst breakdown and follicle assembly by increasing autophagy, apoptosis, and oxidative stress [108].
The proliferation and differentiation of GC during folliculogenesis depend on the autophagic flux. A recent study revealed that ATG5 and BECN1 affect the expression of CYP19A1/Aromatase and FSHR, two genes associated with GC differentiation, E2 synthesis, and degradation of the WT1 transcription factor, which is a GC differentiation inhibitor, hence contributing to the differentiation of ovarian GC [109]. Concordantly, the inhibition of BECN1 by MIR30A-5p results in the survival of GCs [110]. Conversely, autophagy is suggested to be involved in GCs death, too [111]. The involvement of autophagy in a wide range of GC vital processes, although determines the unique importance of autophagy, leads to complications of targeting autophagy to confront pathological conditions as it increases the possibility of undesired effects. Therefore, conducting further studies are encouraged to determine how autophagy is involved in GC processes, the interaction of autophagy with other intracellular regulatory mechanisms, the impact of extracellular factors, the level of autophagy alterations in different stages of the cell cycle, and the possible consequences of its manipulation prior to the initiation of clinical interventions.
It is documented that both apoptosis and autophagy are involved in germ cell depletion and follicular atresia in the mammalian ovary (Figure 3). Increased autophagy and eventual ovarian reserve depletion may be due to a defect in the DNA repair system caused by transcription factor LHX8 (LIM homeobox 8) ablation [112]. Since the loss of BECN1 or ATG7 results in the premature loss of germ cells, autophagy appears to be a cell survival program for the maintenance of female germ cell endowment before establishing ovarian primordial follicle pools [113]. Besides, the ATG7 knockout is associated with POI and germ cell over-loss, and autophagy protects the oocytes against over-loss caused by apoptosis in neonatal ovaries under starvation [62]. In contrast, in oocytes deficient in MCL1 (myeloid cell leukemia sequence 1), a pro-survival factor, elevated autophagy and apoptosis, and mitochondrial dysfunction will be followed by POI [114]. Furthermore, autophagy, which acts genetically upstream of DNA fragmentation in the ovary, mediates cell death during early oogenesis in Drosophila melanogaster, another invertebrate [115]. In addition, in the Drosophila ovary, mitochondrial dynamics during programmed cell death are regulated by both autophagy and apoptosis mechanisms [116]. Unexpectedly, in Drosophila, caspase activity does not cause cell death in suppressed parterosomal activity due to Hsp83 loss but is essential in other important processes such as ensuing compensatory autophagy, female fertility, and organism viability [117].
Figure 3.
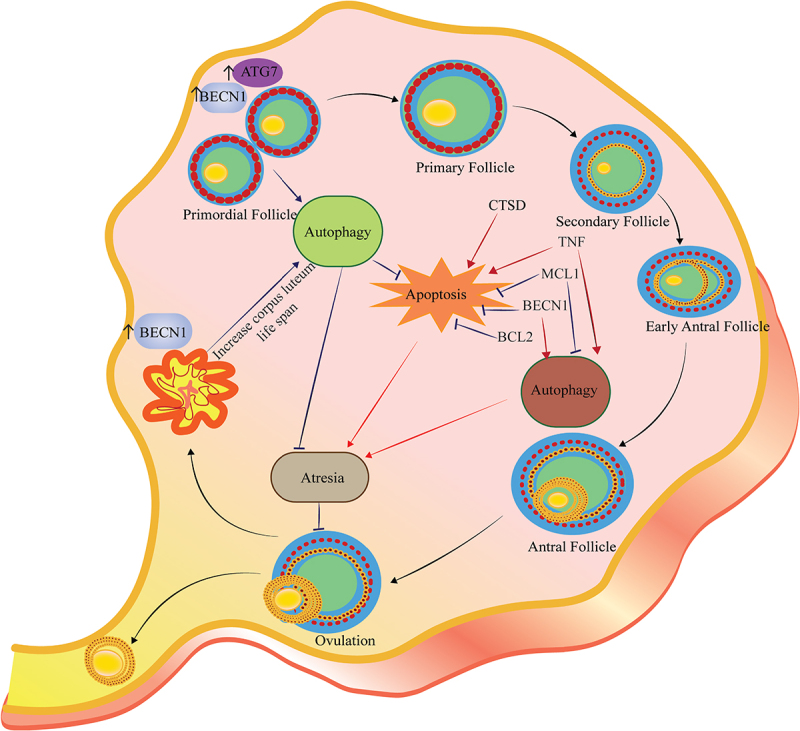
Autophagy regulates follicular atresia. Follicular atresia could occur in all steps of follicular development. Both autophagy and apoptosis play a crucial role in the follicular atresia in each step. The figure depicts the crosstalk between autophagy and apoptosis and the role of the BCL2 family, BECN1, and CTSD in follicular atresia and post-ovulatory complex. Autophagy appears to be a cell survival program for the maintenance of female germ cell endowment before establishing ovarian primordial follicle pools. Expression of ATG7 and BECN1 in primordial follicles protects the oocysts against over-loss caused by apoptosis. MCL1 is an ovarian pro-survival factor that inhibits autophagy and apoptosis to prevent POI. Also, inflammatory markers such as TNF suppress ovulation, which is associated with an increase in both apoptosis and autophagy. The presence of BECN1 in the corpus luteum during the luteal phase increases life span rather than cell death.
Ovarian reserve is considered the main source of female reproductive ability, which may be insulted by environmental factors (chemotherapy, environmental toxins) and genetic disorders (hypoestrogenism), which ultimately lead to POI, subfertility, and infertility [118–120]. The involvement of autophagy in the establishment of the follicular pool and promoting the survival of follicular cells may suggest autophagy as a novel target to confront ovarian failure in susceptible people (Table 2). However, augmentation of autophagy to promote the survival of ovarian follicles may cause undesired effects since this process also contributes to follicular atresia and granulosa cell death. Recent studies have clarified that although autophagy impairment is associated with ovarian failure, autophagy, considered a non-apoptotic cell death program, in cooperation with apoptosis, plays a role in reducing the ovarian reserve of follicles in females diagnosed with ovarian complications. Therefore, the dual role of autophagy in follicles sufficiently proves that introducing ATGs as a novel target to confront ovarian complications is in the preliminary stages of research, hence it vitally requires further studies.
Table 2.
Autophagy is a predominant mechanism that affects pre- and post-fertilization processes.
| Stages | Findings | Refs | |
|---|---|---|---|
| Pre-fertilization | Ovarian pool establishment |
|
[97,101,103,108,112,114] |
| GC differentiation |
|
[109,111] | |
| Follicular atresia |
|
[116,117,121,122] | |
| Oocytes and Oogenesis |
|
[99,102,106,107,113,123,124] | |
| Luteolysis |
|
[125–127] | |
| Fertilization | Fertilization |
|
[128,129] |
| Elimination of paternal organelles |
|
[80,130–134] | |
| Elimination of maternal organelles |
|
[134,135] | |
| Embryonic development | Embryogenesis |
|
[136–140] |
| Implantation |
|
[141,142] |
Note: Autophagic flux in cooperation with various signaling pathways as well as apoptosis can be considered the main regulator of the pre- and post-fertilization processes. Although most studies indicate the essential role of autophagy in germ cell survival, some have suggested that inhibition of autophagy at certain stages is involved in maintaining the ovarian reserve. Successful fertilization and embryonic development require precise regulation of autophagy.
By the formation of preantral follicles, GCs differentiate into cumulus cells, which are responsible for recruiting the theca cells [96]. Until this stage, folliculogenesis is not dependent on gonadotropins but is considered a paracrine unit dependent on the secretions of GC. Recruiting theca cells are from the progenitor pool at ovarian mesenchyme and mesonephros layers and theca cells are subsequently divided into theca interna and externa layers. Mature follicles, called antral follicles, display receptors for gonadotropins and thus respond first to FSH and then to LH. In the phase of response to gonadotropins during folliculogenesis, follicles act as sex steroid-producing units that produce E2 in response to FSH, while the response to LH causes completion of meiosis I, androgen secretion, progression to metaphase II, and completion of luteinization phase [94,143–145]. All these processes result in ovulation, which is an indicator of the termination of folliculogenesis, and the end product is an oocyte capable of fertilization.
In the early stages of folliculogenesis, there is evidence of the involvement of autophagy-related mechanisms in the regulation of luteinization and ovulation. Recently, it is suggested that the luteinization of GCs is mediated by hyperactivation of autophagy but inhibition of apoptosis [125]. In addition to contribution in luteal formation, autophagy is reported in the corpus luteum during luteal regression, a process that occurs in the absence of pregnancy and is characterized by dropped levels of P4 and structural luteolysis (Figure 4). The initiation of autophagy during luteolysis is mediated through the PGF2A-Ca2+-AMPK signaling pathway, whereas LH-PRKA-MTOR luteotrophic signaling exerts inhibiting effects on autophagy [126]. The presence of BECN1, an autophagy-related protein that links autophagy and apoptosis, and the activity of autophagy in the corpus luteum during the luteal phase lead to an increase in life span rather than cell death, as well as in androgen-secreting cells [127].
Figure 4.
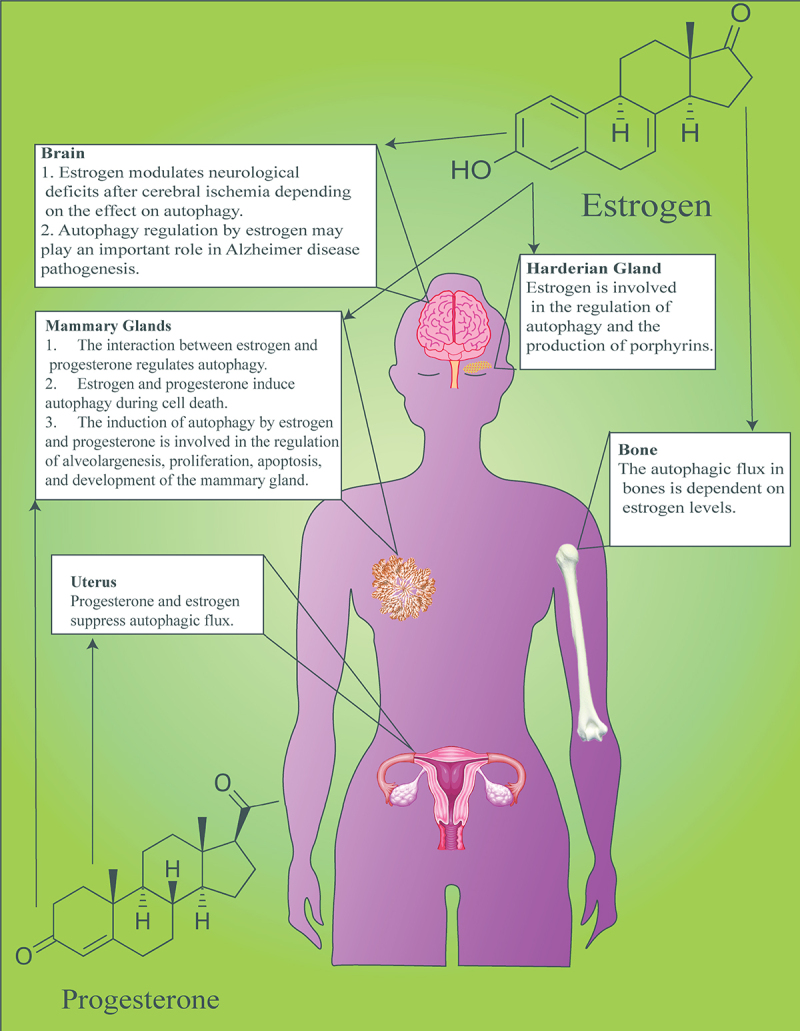
Effects of E2 and P4 on autophagy in various organs. The interaction between sex steroids and autophagy ensures the physiological function of organs associated with the reproductive system such as the central nervous system, harderian gland, mammary gland, uterus, and skeletal system.
Changes in the hydrolytic activity of lysosomes during the preovulatory phase and after ovulation can be considered the first evidence of the role of autophagy in ovulation [146]. Subsequently, increased acid phosphatase activity, autophagy, and lipophilic materials were suggested during corpus luteum formation and its intensification until ovulation in the butterfly Calpodes [147]. Although the two studies primarily suggested a possible role for autophagy in ovulation, they led to further research in this context. The crosstalk between autophagy and apoptosis and the involvement of the Bcl2A/Bcl2 family, Becn1, and Ctsd play a role in regulating ovarian remodeling after fish spawning [148]. The preceding apoptosis by autophagy in characid fish ovaries confirms the crosstalk of these two mechanisms in the involution of follicular atresia and post-ovulatory complex [149]. In Nile tilapia, autophagy is responsible for homeostasis and clearance of ovarian follicles through apoptosis mediated by Ctsd during follicular atresia [121]. In addition, an inflammatory marker such as TNF suppresses ovulation, which is associated with an increase in both apoptosis and autophagy [150]. The time-dependent deterioration of post-ovulatory metaphase II oocytes during the aging process is accompanied by the unregulated expression of ATGs and early apoptosis [151]. Consequently, treatment with rapamycin, an autophagy stimulant by suppressing MTOR expression, in aged oocytes causes cells to survive by improving quality factors such as the increase in the level of MAPK, upregulation of cytoplasmic maturation factors, anti-apoptosis, and development genes, decrease in ROS activity and DNA fragmentation. These alterations were accompanied by the overexpression of autophagy proteins, including BECN1, ATG7, MAP1LC3B, ATG12, GABARAP, and GABARAPL1 [152]. Autophagy promotes the survival of immature eggs, which leads to apoptosis due to the inactivation of Mapk1-Mapk3 [129]. Activation of autophagy in concomitance with the destruction of stress-response, cell-cycle, and calcium signaling pathways leads to post-ovulatory aging of oocytes [153]. The high level of autophagic proteins in zebrafish eggs is considered a low-quality biomarker [154]. In fact, the high level of autophagy contributes to the promotion of the survival of immature eggs [155]. Hence, in low-quality eggs of zebrafish, it has been suggested that autophagy is activated, which is probably due to countering cell death and promoting cell survival. Moreover, low quality could be raised from the failure of oocytes to maturate normally and supply energy [154,156]. Therefore, the increase in the level of autophagy can be assumed as an energy compensatory mechanism. However, this double-edged sword has been associated with unfavored effects such as impairing the normal maturational and disrupting the normal composition and functions of the cytoskeleton. Therefore autophagy could be assumed as a marker of egg low quality.
In oocytes exposed to obesity, damaged mitochondria accumulate due to the inability the activation of mitophagy [157]. A recent study suggested that during ovarian aging mitophagy, regulated by RAB7, contributes to oocyte meiosis and oocyte quality control [123]. In fact, mitochondrial function is regulated by LRRK2 (leucine rich repeat kinase 2), a large multidomain protein that belongs to the Roco GTPase family harboring both GTPase and kinase activities, along with LRRK2 regulation of actin assembly for spindle migration are pivotal for oocyte meiosis [158]. Importantly, the association between mitochondrial membrane potential and autophagy with two other GTPases, ARL2 and ARF5, and the effect of this direct association on oocyte meiosis and quality is reported [159].
Autophagy and female sex hormones
According to the previous section, the ovarian follicular cycle is regulated with both autophagy and apoptosis, however, the question that arises is what can control the balance between these two pathways. Certainly, the crucial role of the endocrine system and gonadotropins must be considered as a fundamental maintainer of GC survival and modulator of autophagy and apoptosis activity.
Higher doses of FSH result in the activation of autophagy via the AKT-MTOR signaling pathway which in turn causes E2 production, elevated expression of FSHR, CYP19, STAR, and BAX along with downregulation of BCL2, and reduced viability of bovine ovarian GCs [160]. Moreover, FSH-induced biosynthesis of P4 in porcine GCs is mediated through the enhancement of autophagy and subsequent acceleration in LDs degradation [161].
In porcine oocytes, the regulation of autophagy by E2 leads to the improvement of developmental ability, reduction of ROS levels, and suppression of apoptosis [162]. Other E2 functions such as modulating neurological deficits after cerebral ischemia depends on the effect on autophagy, specifically the NFE2L2/NRF2-ARE signaling pathway in the brain [163]. Autophagy in female bone tissue is E2-dependent, and postmenopausal osteoporosis may be due to a decrease in autophagic activity and an increase in oxidative stress caused by E2 deficiency, while it is cell-dependent in males [164]. Although the association of autophagy with E2 is involved in the pathogenesis of female Alzheimer disease, it is likely to help postmenopausal women with this disease [165]. Considering the role of steroid hormones, particularly E2, in the regulation of autophagy and the different performances of autophagy in the two genders, perhaps the “gendered-autophagy” term is applicable.
More importantly, the interaction between autophagy and sex steroids controls the fundamental processes in other reproductive tissues as well as non-reproductive organs. In the Syrian hamster Harderian gland, autophagy is regulated by oxidative stress and redox signaling, as higher levels of ROS is associated with more extensive autophagy. The intensity of oxidative stress and consequently autophagy in the female Harderian gland is higher than in the male model, which affects the secretion of hormones in these glands. As in the male Harderian gland autophagy activation leads to survival, while in female glands autophagy peaks in a detachment-derived cell death that leads to a tremendous glandular secretion [166]. Thereby, the “gendered-autophagy” term could be applicated here, too. Porphyrin production by the Harderian gland is closely linked to the estrous cycle and E2 variations affect autophagy [167].
Autophagy during bovine mammary epithelial cell acini formation is regulated by interactions between E2 and P4 [168]. The regulation of autophagy by mitochondrial UCP2 (uncoupling protein 2) in human cumulus cells affects apoptosis, ROS production, and maintenance of gap junction integrity which leads to the management of follicle development, early embryo implantation, and P4 synthesis [169]. Apoptosis and autophagy are activated during mammary epithelial cell death and the dynamic equilibrium between these processes and proliferation contributes to mammary gland growth and involution. Autophagy in mammary epithelial cells is inhibited by epidermal growth factor and insulin-like growth factor-1 via MTOR activation, while E2 and P4 induce autophagy [170]. In the involution of the bovine mammary gland, autophagy is induced by E2, P4, and growth factor TGFB, which is involved in alveologenesis, proliferation and apoptosis regulation, and development of the mammary gland [171]. In human endometrial cancer cells, the crosstalk between E2 and autophagy leads to the overexpression of the E2 receptor by affecting the SRC-EGFR-PI3K-AKT-MTOR signaling pathway and increased release of this receptor from the complex formed with KEAP1 [172]. Ovarian steroids, E2 and P4, suppress autophagic activation in the mouse uterus (Figure 5). This could be explained by the role of uterine autophagy, which is a responsive mechanism to acute inflammation and an energy provider by degrading glycogen under hormone deprivation [173].
Figure 5.
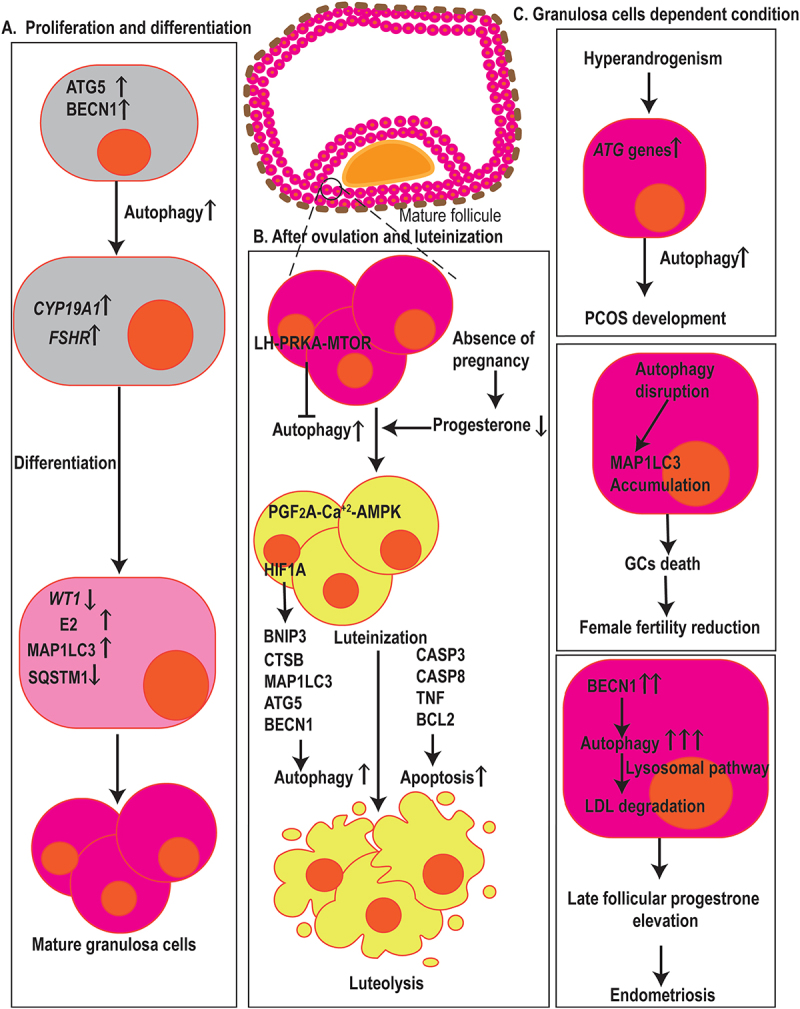
Autophagy in fertilization, menstruation, and infertility. (a) Autophagy has arole in GCs proliferation, differentiation, and death. ATG5 and BECN1 affect the expression of CYP19A1 and FSHR, two genes associated with GCs differentiation, E2 synthesis, and degradation of the WT1 transcription factor, hence contributing to the differentiation of ovarian GCs. Also, elevated levels of MAP1LC3 and decrement in the expression of SQSTM1 resulted in GCs cell differentiation and proliferation. (b) Dropped levels of P4 in the absence of pregnancy result in the induction of autophagy in the corpus luteum during luteal regression. The initiation of autophagy during luteolysis is mediated through the PGF2A-Ca2+-PRKA signaling pathway, whereas LH-PRKA-MTOR luteotrophic signaling exerts inhibiting effects on autophagy. Also, HIF1A regulates GCs through autophagy activation, affecting MAP1LC3 and BECN1, in aBNIP3-dependent manner. Autophagy and apoptosis-related markers such as MAP1LC3, ATG5, CTSB, CASP3, CASP8, TNF, and BCL2 contribute GCs death. (c) in ovarian GCs, ATGsand autophagy markers increase which is positively correlated with hyperandrogenism indicating that autophagy mechanisms are involved in PCOS development. Autophagy-disrupted MAP1LC3 accumulation leads to the death of oocyte-supporting GCs causing areduction in oocyte quality and female fertility. In GCs, higher levels of autophagy, indicated by BECN1, contribut to late follicular P4 elevation by the promotion of LDL degradation via lysosomal pathways which ultimately leads to the aggravation of endometriosis.
Autophagy and endometrium receptivity
In addition to the maintenance of the ovarian reserve, the physiological development of folliculogenesis, and the regulation of the interplay between molecular mechanisms and endocrine activity, the preparation of the uterus and especially the endometrium is necessary for the reception of the formed egg and subsequent processes. Preparation for endometrium receptivity requires massive cell proliferation, paracrine and endocrine secretions, and is accompanied by cell death, which requires intense regulation and energy supply. Therefore, the degradative and recyclate properties of autophagy along with its ability to supply energy and regulate cellular processes involve it as a controlling process.
Importantly, a recent study revealed that endometrial receptivity is regulated by CRIM1 (cysteine rich transmembrane BMP regulator 1), a downstream target for MIR143, by affecting ATG7-dependent autophagy and controlling cell proliferation, cell adhesion, and prostaglandins secretion [174]. Besides, low and high expression of BSCL2 (Berardinelli-Seip congenital lipodystrophy 2 (seipin)), an integral ER membrane protein, in myometrium and uterine luminal epithelium respectively, and its association with MAP1LC3 expression demonstrates regulation of autophagy in the uterine luminal epithelium but not myometrium [175]. In goats, the physiological level of ER stress contributes to early pregnancy development, and autophagy cooperates with the ATF6 (activating transcription factor 6) signaling pathway for the regulation of endometrial function by modulating the MTOR pathway [176]. Additionally, diet-induced obesity impairs autophagy which leads to the impairment of endometrial stromal cell decidualization [177].
Furthermore, the deficiency of BSCL2 causes pathological conditions such as lipodystrophy, diabetes, muscle hypertrophy, and male infertility. Interestingly, the lack of expression of this protein revealed normal embryo implantation and body weight gain during pregnancy, however, a reduction in delivery rates and an increase in gestation period and parturition problems were achieved [175]. During early pregnancy, COPS5 (COP9 signalosome subunit 5) has a negative regulating role on goat endometrial function through ERN1 and MTOR‐autophagy pathways [178]. Interestingly, in oocytes exposed to obesity, the pre-attachment development of bovine embryos depends on an autophagy-ER stress balance, as autophagy promotes this process by reducing ER stress [179]. In the disruption of mitochondrial function, mitophagy is activated by the PINK1-PRKN (parkin RBR E3 ubiquitin protein ligase) pathway leading to the loss of embryonic developmental potential [180,181].
Autophagy, implantation, and fertilization
Embryonic development begins with the fertilization of the egg by sperm. During this process, some intracellular components derived from germ cells must be eliminated. Removal of germ cell organelles includes not only the paternal organelles but the maternal germ cell organelles. In many species, the elimination of paternal mitochondria, but not maternal mitochondria, after fertilization occurs using the process of selective autophagy (Figure 6). During fertilization, the lysosomal pathway has a vital role in the degradation of paternal mitochondria in C. elegans [130]. After fertilization autophagy is triggered localized around sperm mitochondria via sperm-derived components resulting in the engulfment of paternal mitochondria by autophagosomes in early embryogenesis in C. elegans [136]. This process, known as allophagy, is mediated by an autophagy receptor called ALLO-1 in C. elegans embryos. This receptor is vital for the formation of autophagosomes around paternal organelles and binds to the MAP1LC3 homolog LGG-1 directly [131]. FUNDC1 (FUN14 domain containing 1), a mammalian mitophagy receptor expressed on the mitochondrial outer membrane, contributes to mitochondrial quality control. The C. elegans ortholog of FUNDC1, FNDC-1, is strongly expressed in sperm but not in oocytes attribute to paternal mitochondria elimination [182]. Taking together, two different mechanisms are considered for the elimination of paternal mitochondria in C. elegans, one involves ubiquitination and the other one involves the mitochondrial-associated autophagy receptor, FNDC-1 [183]. Additionally, the clearance of other sperm-inherited organelles in C. elegans depends on MAP1LC3-controlled autophagosome targeting a specific pericentral area [184]. In Drosophila, an almost similar mechanism that depends on the autophagic flux is involved in the elimination of paternal mitochondria [132]. Incidentally, in mice, although ATGs such as SQSTM1 and MAP1LC3 were assembled around sperm mitochondria immediately post-fertilization, the engulfment and degradation of sperm mitochondria in lysosomes occurred when ATGs were disengaged from mitochondria. Furthermore, most motile sperms that had arrived at the oviduct had eliminated their mitochondria [133], which is similar to nematodes [183]. Interestingly, if sperm mitochondria entered the zygote, paternal mtDNA could remain and be detected in newborn animals. These findings revealed that the inheritance of mitochondria is not an active process, but is a passive process that depends on pre-fertilization elimination of paternal mtDNA or uneven distribution of mitochondria in embryos [133]. Furthermore, strict maternal transmission of mitochondria in mice depends on mitophagy which is collaborated by E3 ubiquitin ligases, PRKN and MUL1 (mitochondrial E3 ubiquitin protein ligase 1), and is related to depolarization of paternal mitochondria requiring the autophagy receptor SQSTM1, mitochondrial outer membrane protein FIS1, and PINK1 kinase [134]. In higher mammals such as boar, both autophagy and ubiquitin-proteasome systems, including SQSTM1, VCP, and 26S proteasome, contribute to sperm mitophagy in mammalian post-fertilization zygotes [80].
Figure 6.
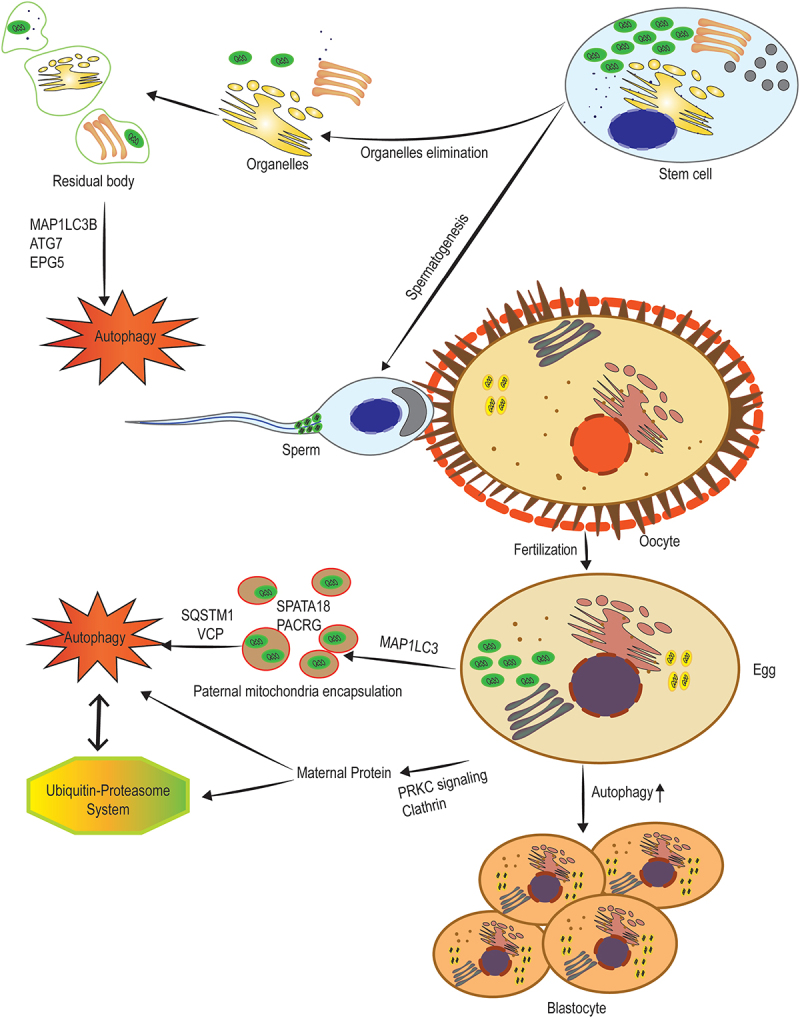
The elimination of paternal and maternal organelles and proteins pre- and post-fertilization. Before spermiogenesis, the segregation of the dominant part of the cytoplasm into residual bodies is involved in the generation of spermatozoa, which are detached and removed from the spermatids. High levels of ATG7, MAP1LC3B, and EPG5 indicate active autophagy in spermiogenesis that is involved in the removal of extra cytoplasm. Post-fertilization degradation of sperm mitochondria is mediated by the interaction of the autophagic pathway with the ubiquitin-proteasome system. SQSTM1, along with VCP are considered key factors during post-fertilization sperm mitophagy. After binding these proteins to sperm mitochondria, two sperm-borne pro-mitophagy proteins including SPATA18 and PACRG underwent alterations in the localization and finally degradation. Moreover, maternal membrane proteins are selectively internalized from the membrane to endosomes, mediated by clathrin and PRKC (protein kinase C) signaling, transported to lysosomes, and finally degraded by both lysosomal and ubiquitination processes.
Maternal proteins in oocytes are degraded after fertilization and the zygotic genome encodes new ones. This process requires autophagy, as fertilization upregulates this catabolic process in early mouse embryos. Furthermore, autophagy-null embryos have a reduced rate of protein synthesis. Together, these findings demonstrate the necessity of oocyte protein degradation by autophagy within early embryos for pre-implantation development [128]. Perhaps autophagy, by degradation of maternal proteins, provides the raw materials necessary for the synthesis of new macromolecules. Therefore, in the absence of autophagy, the cell loses the ability to break down old maternal proteins and suffers subsequent consequences. A recent study, in addition to emphasizing the essential role of autophagy and the ubiquitin-proteasome system in the removal of maternal organelles, determined that in mouse embryos maternal membrane proteins are selectively internalized from the membrane to endosomes, mediated by clathrin and PRKC (protein kinase C) signaling, transported to lysosomes, and finally degraded by both lysosomal and ubiquitination processes [135]. Confirmingly, BECN1−/− mouse embryos are incapable of completing the embryogenesis process. Altogether the data propose that autophagy is a protective growth mechanism for the early embryogenic process. In the blastocyst stage, embryonic cells are differentiated into trophoblast cells and inner cell mass. Autophagy is reported to play a vital role in trophoblast functions, including invasion and vascular remodeling in extravillous trophoblasts, for normal placental development [201]. Furthermore, in the middle of the trophoblast, a cavity called blastocoel is formed when the early dividing cells of the recently fertilized zygote begin to differentiate, which seems to be associated with autophagic cell death. This cavity is vital in the later cell migration during gastrulation. Embryoid bodies derived from cells lacking the autophagy genes, ATG5 or BECN1, fail to cavitate [77].
The process of autophagy from pre-implantation to post-implantation seems to be different in different animals. Although some studies have shown that during pre-implantation development in pig embryos’ and cloned mouse embryos’ the induction of autophagic flux improves embryo viability via the management of the cellular redox state and affects somatic cell nuclear transfer, other studies have associated the increase in the expression of Mir291 clusters in mouse preimplantation embryos with the inhibition of autophagy, as the level of BECN1, ATG5, and autophagosomes gradual decrease from 1-cell stage to the 4–8-cell [137–139]. Contradictory, autophagic flux is documented as an indicator of mice embryo viability [140]. Such conflicts complicate the clarification of the autophagy process in pre-implantation, therefore, conducting further studies considering the types and strains of the studied animals, the length of the study, as well as the application of biochemical-immunological confirmatory methods including western blot, transmission electron microscopy, and flow cytometry are encouraged.
The first studies on the role of autophagy in implantation are related to ultrastructural examinations. In the 1970s, Abraham suggested that autophagy is involved in the destruction of the lysosomal membrane and the release of lysosomal enzymes in the adjacent cytoplasm before and during implantation, indicating the lysosome-dependent role of autophagy in the removal of the barrier so that trophoblast penetrates the uterine epithelium [141]. ACP (acid phosphatase)-dependent autophagy was subsequently indicated by the cytochemical establishment of the enzyme in the bovine endometrial gland epithelium and trophoblast giant cells during implantation [142]. Embryo implantation, which involves breaking the zona pellucida by a mature blastocyst and its penetration into the endometrium, is another cellular process dependent on autophagy. In delayed implantation conditions, blastocysts remain dormitophant in utero via activation of autophagy, called embryo diapause. This can be assumed as an adaptive phenomenon that guarantees embryonic survival during the lack of nutrients in some mammals [202]. Additionally, autophagic activation by FGFR signaling is a pre-condition for multivesicular body formation and accumulation in the trophectoderm of dormant blastocysts upon implantation required activation [203].
In mouse embryos, the induction of autophagy by fertilization is not dependent on MTORC1 [122]. However, MTOR and associated signaling molecules such as PI3K-AKT-MTOR and MAPK/ERK-p-MAPK/ERK participate in implantation [204]. Stimulation of microgravity inhibits decidualization, the differentiation of endometrial stromal cells, by a reduction in proliferation and migration via affecting AKT-MMP2 and FOXO3 (forkhead box O3) transcription factor-autophagic flux [205]. Upon deactivating the Mapk1/Erk2-Mapk3/Erk1 pathway, unfertilized sea urchin eggs are targeted for apoptosis, which autophagy counteracts as a survival program [129].
Angiogenesis and vascularization are critical processes for placenta development, and endangering them could lead to pregnancy complications such as preeclampsia and intrauterine growth restriction. CXCL12 (C-X-C chemokine ligand 12) and its receptor, known as CXCR4, provide a signaling pathway that in collaboration with autophagy regulate placental homeostasis by being a crucial upstream mediator of angiogenesis, vascularization, and cell viability [206]. The CXCL12-CXCR4 axis induces AKT and MTOR phosphorylation in various cell types, and it probably helps to increase autophagy in this way [206]. In addition, one of the most important processes attributed to autophagy is embryonic organogenesis. The basic role of autophagy in neurogenesis, and the development of blood tissue, bone, and heart have been reported. Loss of ATG5 and a positive regulator of the BECN1 known as AMBRA1 (autophagy and beclin 1 regulator 1) at earliest organogenesis led to severe neural tube defect and Spina bifida. These conditions confirm the important role of ATG5 in neurogenesis [207]. ATG5 regulates astrocyte differentiation through the SOCS2-JAK2-STAT3 signaling pathway [208], and promotes the differentiation and proliferation of cortical neural progenitor cells (NPCs) by controlling the CTNNB1 (catenin beta 1) signaling pathway [209]. Altogether, successful pregnancy depends on the sequential happening of biological processes such as embryo implantation, and this process by itself includes blastocyte migration and embryo adhesion as two major parts. Appropriate implantation is an essential factor for the proper interaction between the maternal uterus and embryo, and according to the axial role of autophagy in this process, it might be used as a therapeutic target to improve embryonic transplantation.
Autophagy and infertility
Autophagy and male infertility
Approximately half of the infertility cases are attributed to males. Several factors are involved in male infertility, including spermatogenesis disorder, obstruction of the seminal tract, the disorder of sperm motility and quality, and reduced sperm count [37,210]. Autophagy as a double-edged sword appears to play a dual role in cellular life, whether somatic cells or germ cells. In ejaculated stallion spermatozoa, for example, autophagy promotes cell survival on the first day, although, after 5 days, autophagy enhancement indicates decreased sperm motility and cooperation with apoptosis due to increased membrane permeability [152]. Interestingly, the performance of the various extenders used to increase sperm survival during incubation at 5°C depends on how they interact with autophagy [129]. The cryopreservation of testicular tissue to maintain male fertility in patients with cancer is unsatisfactory due to impairment of human testicular grafting, contamination by malignant cells, and deregulation of angiogenesis-related signaling. However, IVM is more effective as modifies the expression of proteins related to autophagy and apoptosis in mouse testicular tissue, in which the impact of cryopreservation is minimal [153].
Autophagy and oligo/azoospermia
The conditions where there is no sperm to ejaculate or the number of sperm is insufficiently low are known as azoospermia and oligospermia, respectively [211]. Considering the basic role of autophagy in advancing the processes involved in spermatogenesis it appears logical to expect that any disruption in autophagic flux will cause reproductive complications and ultimately infertility. In this regard, a recent study demonstrated that in patients with non-obstructive azoospermia, the expression of BECN1 in testicular tissue was significantly up-regulated. It also was positively related to LH serum levels, and negatively associated with serum TST levels and testicular volume suggesting that autophagy could be assumed as a marker with a predictive value in sperm retrieval rate [212]. The levels of BECN1, MAP1LC3, and ATG7 are highly expressed in non-obstructive azoospermia with a negative correlation with MIR188-3p, a regulator of ATG7 expression [185]. The downregulation of MIR188-3p leads to abnormal sperm formation. In fact, there is a mutual binding site between MIR188-3p and ATG7, and the expression of ATG7 is impeded by MIR188-3p, revealing that MIR188-3p contributes to autophagy regulation by modifying ATG7 expression, leading to spermatogenesis disorder and non-obstructive azoospermia [185]. Moreover, a cross-sectional study on patients with azoospermia reported that the reduction in BECN1, MAP1LC3B, and ATG5 expression is significant in infertile patients [213]. In addition, there is a disease-causing missense variant in an autophagy gene located on human chromosome Xp11.23, known as TBC1D25 (TBC1 domain family member 25), which leads to aberrant interaction with ATG8 homologs, oligospermia, and male infertility [214]. Finally, aberrant autophagy activation germline Foxj2 overexpression-induced LAMP2A upregulation results in the failure of spermatogenesis at the initiation of meiosis leading to infertility revealing the importance of autophagy regulation in spermatogenesis [215].
Autophagy and globozospermia
Round-headed sperm syndrome or globozoospermia is one of the most common types of monomorphic severe teratozoospermia [216]. Dysmorphic spermatozoa lose the ability to normal function and motility [217]. The globozoospermia phenotype usually occurs after a disturbance in acrosome biogenesis [218]. As mentioned earlier, the process of acrosome biogenesis is closely associated with autophagic flux, particularly ATG7. A case-control study demonstrated a significant elevation in ATG7 compared to the control group in patients with globozoospermia [187]. However, MAP1LC3 expression between the two groups was the same. Previously, it was suggested that deacetylation of MAP1LC3 by SIRT1 is a necessity for the localization of MAP1LC3 from the nucleus to the cytoplasm, initiation of autophagy, and acrosome biogenesis [219]. Altogether the results proposed that in globozoospermia autophagy initiates, however, a disorder of other genes involved in acrosome biogenesis inhibits the progression of this pathway. This could be the reason that MAP1LC3 levels remained unchanged. Although, further studies with larger sample sizes and investigation of the levels of sex steroids, gonadotropins, and other genes involved in acrosome biogenesis are encouraged.
Autophagy, metabolic homeostasis, and male infertility
The processes involved in cell death, including apoptosis, necrosis, and autophagy in spermatocytes may be influenced by the metabolic energy source. The lack of an energy source leads to necrosis, whereas the absence of lactate, the main source of ATP in spermatocytes and spermatids, is associated with increased apoptosis. However, autophagy increases either in the presence of lactate or its deficiency in cultured cells [220]. Highly disrupted autophagy in testicular LCs is a feature of non-breeding males described at the reduced levels of ATG7, ATG5, MAP1LC3B, and MAP1LC3A, and the number of autophagosomes. With disrupted autophagy TST production suppression, testicular atrophy, and decreased levels of androgens occur [221]. Furthermore, testicular dysfunction due to diabetes is caused by autophagy abnormalities [188]. Hyperlipidemia is accompanied by higher levels of fatty acids which are able to interfere with steroidogenesis via the downregulation of CYP11A1 and inhibition of late-stage autophagy [189]. Autophagy inhibition, due to the lack of degradation of cholesteryl esters to free cholesterol, is involved in increasing the number and size of LDs and total cholesterol, lowering free cholesterol, and reducing TST synthesis in rat primary LCs. Interestingly, the initial inhibition of autophagy due to the induction of hypoxia is associated with increased TST secretion, although over time this effect becomes abolished [88]. Starvation promotes autophagy-dependent maturation in Macrobrachium rosenbergii, a giant freshwater prawn [222]. Surgically-induced cryptorchidism induces both apoptosis and autophagy in mice spermatogenic cells [223].
Varicocele-induced infertility
Varicocele (VC) is an abnormal venous dilatation and/or tortuosity of the pampiniform plexus in the scrotum which is one of the most prevalent causes of male infertility. VC can alter the testis normal physiological function by increasing of local temperature, inducing hypoxia and/or oxidative stress. Chronic VC changes the seminiferous tubule’s structure and causes disorganization in seminiferous epithelial cells. All these factors eventually disturb the process of spermatogenesis [224,225]. Autophagy plays an important role in the VC-induced infertility of male rats. The HIF1A-BNIP3-BECN1 signaling pathway in VC rat testes leads to the initiation of autophagy [226]. But the question that arises here is whether the activation of autophagy in these conditions is a VC accelerator factor or a protective testicular response against VC. Pathological states such as hypoxia and impaired testicular blood flow at the beginning of VC could impair the energy supply system necessary for the activity of testicular cells. It seems that in this situation autophagy activation is a protective mechanism that can be a way to recycle damaged organelles and a way to supply the required energy from alternative sources. However, apoptosis inhibits autophagy in later stages [227,228].
Autophagy, endocrine-disrupting chemicals, and male infertility
Endocrine disruptors (EDs) are described as exogenous substances that shift the endocrine function and consequently cause harmful effects in an organism. Exposure to EDs may exacerbate the process of development and differentiation of male germ cells by increasing or decreasing the autophagy flux, leading to infertility, which may be consistent with or against apoptosis [119,229]. For example, mitochondrial dysfunction along with alterations in autophagic flux and metabolomic homeostasis in spermatogonia as well as spermatogenic failure, germ cell loss, and testicular structural abnormalities accompanied by the induction of apoptosis occur in animals and offspring upon exposure to arsenic trioxide or fluoride [230,231]. In toxicity induced by co-exposure to bisphenol a and nonylphenol, which are associated with spermatogenic epithelial atrophy and loss of germ cells, both autophagy and apoptosis have increased, possibly reinforcing each other’s effects [48].
4-Nonylphenol toxicity, a xenoestrogen, was reported to induce apoptosis in various cell types through oxidative stress and ROS production. 4-Nonylphenol eventually could phosphorylate and activate AMPK and simultaneously suppresses MTOR activity in the testis. It seems that autophagy is involved in SCs’ survival against apoptosis and necrosis caused by 4-nonylphenol [191]. However, exposure to this xenoestrogen by oxidative stress-mediated activation of autophagic and apoptotic mechanisms, and involving the PI3K-AKT-MTOR pathway, leads to morphological abnormalities in epididymal sperm, TST depletion, and decreased semen quality [232]. The difference in the effect of 4-nonylphenol on prepubertal and adult rats can be a result of the dependence of autophagic flux on the spermatogenesis stages. In acute exposure to ethanol, for example, the increase in autophagy in SCs during androgen-dependent stages, spermatogenesis stages, is more significant than in other stages. However, the reason for and role of stage-depended altered activity have not been determined [233]. Microcystin-leucine-arginine (MLR) as a hazardous material induces apical ectoplasmic specialization disassembly. It seems that it can function through the disruptions of F-actin arrangement. In addition, its accumulation is able to induce testicular atrophy, spermatogenic cell apoptosis, and endocrine disruption and affects the motility and morphology of sperm, contributing to spermatogenesis impairment [234–236]. Interestingly, in the di-2-ethylhexyl phthalate-induced toxicity, autophagy triggers apoptosis activation, which has side effects such as decreased TST output, the insult to LCs, the disintegration of the germinal epithelium, inhibition of cell viability, and decreased sperm density in the epididymis [237]. Conversely, developmental impairment induced by flutamide and di-2-ethylhexyl phthalate in the Cryptorchid rats is mediated by excessive apoptosis and autophagy disruption which results in sperm abnormality, testicular damage, and decreased TST [231]. By the mentioned findings, it is understood that contrary to traditional belief, autophagy does not act just like a cell protector. Interestingly, autophagy appears to play a dual role in cellular life, whether in somatic cells or germ cells. Therefore, in the interaction between autophagy and apoptosis after exposure to EDs, synergistic or confrontational approaches could be expected that depends on the type of exposed ED and probably the concentration and route of exposure. Hence, predicting the outputs and how autophagy behaves, whether as a cell protector or as a facilitator of cell death, requires further specialized studies.
Autophagy and female infertility
Autophagy and endometriosis
Endometriosis is a chronic, E2-dependent disease characterized by the implantation of endometrial glands and stroma randomly into the outside uterine cavity. Endometriosis could be considered one of the most prevalent causes of pelvic pain, amenorrhea, dysmenorrhea, and infertility [238,239]. Previous studies indicated that the BCL1 level is significantly low in stroma cells of endometriosis tissues. Currently, it is known that disrupted autophagy occurs in endometriosis, which can be caused by inflammation or under the influence of E2 levels. Autophagy-disrupted MAP1LC3 accumulation leads to the death of oocyte-supporting GCs causing a reduction in oocyte quality and female fertility [193]. The autophagy dysregulation accompanied by endometrial inflammation causes recurrent implantation failure with chronic endometritis in women [240]. Recently, it is reported that reduced Indian hedgehog signaling could activate autophagy markers such as MAP1LC3B in endometrial tissues of women with endometriosis or adenomyosis leading to improper survival of ectopic sites endometrial cells of these two gynecological disorders [241]. Higher levels of autophagy, indicated by BECN1, in GCs, contribute to late follicular P4 elevation by the promotion of LDL degradation via lysosomal pathways which ultimately leads to the aggravation of endometriosis [242]. On the contrary, elevated p-AKT and MTOR expressions accompanied by reduced BECN1 and MAP1LC3B levels in endometriosis lesions suggest that autophagy inhibition could be a causative agent in the development of endometriosis. Surprisingly, the activation of autophagy and mitophagy leads to increased apoptosis and reduced angiogenesis in the lesions revealing that autophagy activation may be a therapeutic target against endometriosis [194]. Similarly, we demonstrated that reduced autophagy, along with decreased levels of NQO1 (NAD(P)H quinone dehydrogenase 1) enzyme activity and gene expression levels of NFE2L2 and MTOR overexpression and ROS production are involved in ectopic endometrial tissues which could alleviate by activation of autophagy [243]. On the one hand, these contradictions could be considered appropriate examples of the dual role of autophagy as a protector and destroyer of female fecundity, and on the other hand, it highlights the importance of autophagy in maintaining female reproductive health, as any alteration in autophagic flux, whether suppression or enhancement, leads to female reproductive abnormalities (Table 3).
Table 3.
Autophagy and infertility.
| Autophagy Contribution | Refs | ||
|---|---|---|---|
| Male Infertility | Oligo/Azoospermia |
|
[185,186] |
| Globozospermia |
|
[187] | |
| Metabolic Homeostasis |
|
[88,188,189] | |
| Varicocele |
|
[190] | |
| Chemicals |
|
[48,97,191,192] | |
| Female Infertility | Endometriosis |
|
[193,194] |
| PCOS |
|
[195,196] | |
| POI |
|
[160,197,198] | |
| Chemicals |
|
[120,199,200] | |
Note: Considering the significant role of autophagy in the vital mechanisms of maintaining reproductive ability in both genders, it appears logical to expect that any dysregulation in autophagy can lead to fertility disorders. Dysregulation of autophagic flux could be caused by environmental factors such as exposure to toxins and chemotherapeutics or genetic deviations. More importantly, whether the increase or decrease of autophagy has been reported in reproductive disorders.
Autophagy and PCOS
Polycystic ovary syndrome (PCOS) is a common endocrine disorder prevalent among women of reproductive age. PCOS is frequently caused by hyperandrogenism [244]. One of the most clinical consequences of PCOS is infertility and the pathophysiology of it is still unclear. Considering the relationship between sex steroids, especially TST, and autophagy, PCOS might be associated with autophagy [245]. Impaired autophagy during different stages of folliculogenesis is reported in the PCOS ovary [246]. Immunohistochemistry study of ATGs demonstrated an accumulation of SQSTM1 and ubiquitin in the theca cells layer in the PCOS ovary [195]. The suppression of autophagy is followed by higher levels of ROS. Conversely, elevated levels of palmitic acid are also found in sera of patients with PCOS. It is reported that palmitic acid inhibits the final stages of the autophagy pathway and could induce androgen production via ROS-MAPK/p38 and MAPK8 signaling [195]. Although in ovarian GC ATG7 and ATG5 increase which is positively correlated with hyperandrogenism indicating that autophagy mechanisms are involved in PCOS development [196]. The comparison of a healthy follicle and PCOS one reveals impaired folliculogenesis in the early stages suggesting the vital role of MTOR-autophagy in the development of PCOS or normal folliculogenesis [195]. Moreover, the analysis of the MTOR expression in the DHEA-induced PCOS ovary showed an elevated level of this regulator of autophagy [247].
Autophagy and POI
POI is another cause of female infertility characterized by premature depletion of ovarian follicles and high levels of FSH [248]. POI etiology is highly heterogeneous but disrupted autophagy-induced follicular atresia might be one of the main causative factors. Premature ovarian failure (POF) is thus considered the end stage of POI with FSH > 40 IU/L [249].
High doses of FSH result in the activation of autophagy via the inhibiting the AKT-MTOR signaling pathway which in turn enhances the E2 production, FSHR expression, CYP19, STAR, and BAX along with downregulation of BCL2, and reduced viability of bovine ovarian GCs [160]. In addition, the administration of FSH provoked autophagy in mouse GC via upregulation of HIF1A [197]. Conversely, due to the pivotal role of autophagy in follicular atresia a group of studies claimed that overactivated autophagy results in follicular atresia and POI [250,251]. Studies conducted in this regard have shown that using POI ameliorative compounds that can activate the PI3K-AKT-MTOR or AMPK-MTOR pathways could result in the attenuation of POI GCs [252,253]. Since FSH is an activator of autophagy, it is logical to expect that alteration in FSH levels could be followed by the activation of autophagy hence contributing to the pathogenesis of POI.
However, as autophagy represented a dual function in other pathological states, this property of autophagy may be involved in the development of POI. In silico analysis of two variants affecting ATGs, which were identified in women with POI, showed the decreased expression of ATG7 and ATG9A representing haploinsufficiency and ATG7 p. Phe403Leu and ATG9A p. Arg758Cys variants were deleterious for autophagy [198]. It is proposed that the induction of autophagy in patients with ATG variants at birth was low in ovaries which might lead to an alteration of the ovarian reserve [198]. Additionally, atg7 knockout in murine germ cells led to POI, as the number of follicles and oocytes was markedly diminished in the adult mutant animals [254].
Autophagy and chemicals-caused infertility
Environmental contaminants are considered the main causative factors for female reproductive disorders [119,120]. Exposure to chemicals can affect the ovaries in both direct and indirect ways. For instance, exposure to BPA causes alterations in apoptotic factor, autophagy, and oxidative stress gene expression in the ovaries, and eventually, germ cell nest breakdown is even passed on to future generations In the mealworm Tenebrio molitor, after Neb-colloostatin injection, both apoptosis and autophagy processes are involved in follicle cell death which is accompanied by ovarian atresia, delayed ovulation, slowed vitellogenesis, reduced number of eggs laid, F-actin cytoskeleton disorganization, and changes in chromatin organization [255].
Chemotherapeutic agents especially alkylating agents are able to exert their cellular toxicity via transferring the alkyl group. One of the vulnerable parts of the cell is the genomic DNA that if get damaged, could trigger programmed cell death. Adjuvants are reported to protect ovarian tissue from reproductive toxicity induced by doxorubicin, a well-known antineoplastic agent, through suppression of both autophagy and apoptosis [120,199]. Investigation of the possible reason for ovarian cancer resistance to cisplatin, a chemotropic agent, showed that MAPK-mediated autophagy can lead to cisplatin resistance [200]. In cisplatin resistance cells autophagy is significantly activated. It seems that effect of this chemotherapeutic agent is mediated through the ERK pathway and affecting on ATG5 levels. In addition, the inhibition of autophagic activity or knockdown of ATG5 results in cisplatin-induced apoptosis in ovarian cancer cells. It is proposed that the sensitivity to cisplatin is regulated via the interaction between autophagy and apoptosis [200].
Conclusions and perspectives
The indispensable importance of autophagic flux, by preserving its dynamic characteristics, which is accompanied by a highly regulated and conserved increase and decrease in different stages, coordination with intracellular signaling mechanisms, and a highly processed crosstalk with regulated cell death programs at each relevant reproductive-associated stage is completely evident for both sexes fertility. Considerably, any disruption and inconsistency will be accompanied by the lack of formation of essential organelles, the lack of removal of extra organelles, cell malformation, destructive irregular somatic and germ cells’ death, defects in cell development, impairment of the production of intracellular or secretory macromolecules, and finally reproductive disorders and infertility. More importantly, autophagy can be considered a promising favored molecular target for the prevention or treatment of reproductive disorders, although further studies are required.
Acknowledgements
The authors would like to appreciate all those who contributed to the provision of this manuscript.
Funding Statement
The author(s) reported there is no funding associated with the work featured in this article.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- [1].Ashford TP, Porter KR.. Cytoplasmic components in hepatic cell lysosomes. J Cell Bio. 1962;12(1):198. doi: 10.1083/jcb.12.1.198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kirkin V. History of the selective autophagy research: how did it begin and where does it stand today? J Mol Biol. 2020;432(1):3–27. doi: 10.1016/j.jmb.2019.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Tsukada M, Ohsumi Y. Isolation and characterization of autophagy-defective mutants of Saccharomyces cerevisiae. FEBS Lett. 1993;333(1–2):169–174. doi: 10.1016/0014-5793(93)80398-E [DOI] [PubMed] [Google Scholar]
- [4].Hansen M, Rubinsztein DC, Walker DW. Autophagy as a promoter of longevity: insights from model organisms. Nat Rev Mol Cell Biol. 2018;19(9):579–593. doi: 10.1038/s41580-018-0033-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kaushik S, Cuervo AM. The coming of age of chaperone-mediated autophagy. Nat Rev Mol Cell Biol. 2018;19(6):365–381. doi: 10.1038/s41580-018-0001-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Aman Y, Schmauck-Medina T, Hansen M, et al. Autophagy in healthy aging and disease. Nature Aging. 2021;1(8):634–650. doi: 10.1038/s43587-021-00098-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bjørkøy G, Lamark T, Brech A, et al. P62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J Cell Bio. 2005;171(4):603–614. doi: 10.1083/jcb.200507002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Clarke AJ, Simon AK. Autophagy in the renewal, differentiation and homeostasis of immune cells. Nat Rev Immunol. 2019;19(3):170–183. doi: 10.1038/s41577-018-0095-2 [DOI] [PubMed] [Google Scholar]
- [9].Denton D, Kumar S. Autophagy-dependent cell death. Cell Death Diff. 2019;26(4):605–616. doi: 10.1038/s41418-018-0252-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Mizushima N, Levine B, Cuervo AM, et al. Autophagy fights disease through cellular self-digestion. nature. Nature. 2008;451(7182):1069–1075. doi: 10.1038/nature06639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Napoletano F, Baron O, Vandenabeele P, et al. Intersections between regulated cell death and autophagy. Trends in cell biology. Trends Cell Biol. 2019;29(4):323–338. doi: 10.1016/j.tcb.2018.12.007 [DOI] [PubMed] [Google Scholar]
- [12].Saha S, Panigrahi DP, Patil S, et al. Autophagy in health and disease: A comprehensive review. Biomed Pharmacother. 2018;104:485–495. DOI: 10.1016/j.biopha.2018.05.007 [DOI] [PubMed] [Google Scholar]
- [13].Guerri G, Maniscalchi T, Barati S, et al. Non-syndromic monogenic female infertility. Acta Bio Medica. Atenei Parmensis. 2019;90(Suppl 10):68–74. doi: 10.23750/abm.v90i10-S.8763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Laganà AS, Uccella S, Chiantera V, et al. Molecular biology of human fertility: stepping towards a tailored approach. MDPI. 2022;23(14):7517. doi: 10.3390/ijms23147517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gao H, Khawar MB, Li W. Essential role of autophagy in resource allocation during sexual reproduction. Autophagy. 2020;16(1):18–27. doi: 10.1080/15548627.2019.1628543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Oestreich AK, Chadchan SB, Popli P, et al. The autophagy gene Atg16L1 is necessary for endometrial decidualization. Endocrinology. 2020;161(1):bqz039. doi: 10.1210/endocr/bqz039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Al‐Bari MAA, Xu P. Molecular regulation of autophagy machinery by Mtor‐dependent and‐independent pathways. Ann N Y Acad Sci. 2020;1467(1):3–20. doi: 10.1111/nyas.14305 [DOI] [PubMed] [Google Scholar]
- [18].Chung CY-S, Shin HR, Berdan CA, et al. Covalent targeting of the vacuolar H±ATPase activates autophagy via mTORC1 inhibition. Nat Chem Biol. 2019;15(8):776–785. doi: 10.1038/s41589-019-0308-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Li Y, Chen Y. AMPK and Autophagy. Vol. 1206, Autophagy: Biology And Diseases. Singapore: Springer; 2019. p. 85–108. doi: 10.1007/978-981-15-0602-4_4 [DOI] [PubMed] [Google Scholar]
- [20].Mao K, Klionsky DJ. AMPK activates autophagy by phosphorylating ULK1. Circ Res. 2011;108(7):787–788. doi: 10.1161/RES.0b013e3182194c29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kim S, Choi KJ, Cho S-J, et al. Fisetin stimulates autophagic degradation of phosphorylated tau via the activation of TFEB and Nrf2 transcription factors. Sci Rep. 2016;6(1):1–13. doi: 10.1038/srep24933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Settembre C, Zoncu R, Medina DL, et al. A lysosome‐to‐nucleus signalling mechanism senses and regulates the lysosome via mTOR and TFEB. Embo J. 2012;31(5):1095–1108. doi: 10.1038/emboj.2012.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Boya P, Reggiori F, Codogno P. Emerging regulation and functions of autophagy. Nat Cell Biol. 2013;15(7):713–720. doi: 10.1038/ncb2788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zalckvar E, Berissi H, Mizrachy L, et al. DAP‐kinase‐mediated phosphorylation on the BH3 domain of beclin 1 promotes dissociation of beclin 1 from Bcl‐XL and induction of autophagy. EMBO Rep. 2009;10(3):285–292. doi: 10.1038/embor.2008.246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Dikic I, Elazar Z. Mechanism and medical implications of mammalian autophagy. Nat Rev Mol Cell Biol. 2018;19(6):349–364. doi: 10.1038/s41580-018-0003-4 [DOI] [PubMed] [Google Scholar]
- [26].Yu L, Chen Y, Tooze SA. Autophagy pathway: cellular and molecular mechanisms. Autophagy. 2018;14(2):207–215. doi: 10.1080/15548627.2017.1378838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Levine B, Kroemer G. Biological functions of autophagy genes: a disease perspective. Cell. 2019;176(1–2):11–42. doi: 10.1016/j.cell.2018.09.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Torii S, Yoshida T, Arakawa S, et al. Identification of PPM1D as an essential Ulk1 phosphatase for genotoxic stress‐induced autophagy. EMBO Rep. 2016;17(11):1552–1564. doi: 10.15252/embr.201642565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Carlsson SR, Simonsen A. Membrane dynamics in autophagosome biogenesis. J Cell Sci. 2015;128(2):193–205. doi: 10.1242/jcs.141036 [DOI] [PubMed] [Google Scholar]
- [30].Stolz A, Ernst A, Dikic I. Cargo recognition and trafficking in selective autophagy. Nat Cell Biol. 2014;16(6):495–501. doi: 10.1038/ncb2979 [DOI] [PubMed] [Google Scholar]
- [31].Yun CW, Lee SH. The roles of autophagy in cancer. Int J Mol Sci. 2018;19(11):3466. doi: 10.3390/ijms19113466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Wild P, McEwan DG, Dikic I. The LC3 interactome at a glance. J Cell Sci. 2014;127(1):3–9. doi: 10.1242/jcs.140426 [DOI] [PubMed] [Google Scholar]
- [33].Thurston TL, Boyle KB, Allen M, et al. Recruitment of TBK 1 to cytosol‐invading Salmonella induces WIPI 2‐dependent antibacterial autophagy. Embo J. 2016;35(16):1779–1792. doi: 10.15252/embj.201694491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Qian X, Mruk DD, Cheng Y-H, et al. Actin binding proteins, spermatid transport and spermiation . Seminars in cell & developmental biology. 2014;30:75–85. doi: 10.1016/j.semcdb.2014.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ge R-S, Li X, Wang Y. Leydig Cell and Spermatogenesis. Vol. 1381, Molecular Mechanisms in Spermatogenesis. Cham: Springer; 2021. p. 111–129. doi: 10.1007/978-3-030-77779-1_6 [DOI] [PubMed] [Google Scholar]
- [36].Hai Y, Hou J, Liu Y, et al. The roles and regulation of Sertoli cells in fate determinations of spermatogonial stem cells and spermatogenesis . Seminars in cell & developmental biology. 2014;29:66–75. doi: 10.1016/j.semcdb.2014.04.007 [DOI] [PubMed] [Google Scholar]
- [37].Krausz C. Male infertility: pathogenesis and clinical diagnosis. Best practice & research clinical endocrinology & metabolism. Best Pract Res Clin Endocrinol Metab. 2011;25(2):271–285. doi: 10.1016/j.beem.2010.08.006 [DOI] [PubMed] [Google Scholar]
- [38].Wang M, Zeng L, Su P, et al. Autophagy: a multifaceted player in the fate of sperm. Human Reproduction Update. 2022;28(2):200–231. doi: 10.1093/humupd/dmab043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Schlatt S, Ehmcke J. Regulation of spermatogenesis: An evolutionary biologist's perspective. Seminars in cell & developmental biology. 2014;29:2–16. doi: 10.1016/j.semcdb.2014.03.007 [DOI] [PubMed] [Google Scholar]
- [40].Liu C, Wang H, Shang Y, et al. Autophagy is required for ectoplasmic specialization assembly in sertoli cells. Autophagy. 2016;12(5):814–832. doi: 10.1080/15548627.2016.1159377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Cheng Y, Lai F, Wang X, et al. Srag regulates autophagy via integrating into a preexisting autophagy pathway in testis. Mol Biol Evol. 2021;38(1):128–141. doi: 10.1093/molbev/msaa195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Herpin A, Englberger E, Zehner M, et al. Defective autophagy through epg5 mutation results in failure to reduce germ plasm and mitochondria. Faseb J. 2015;29(10):4145–4161. doi: 10.1096/fj.14-265462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Liu Y, Hu Y, Wang L, et al. Expression of transcriptional factor EB (TFEB) in differentiating spermatogonia potentially promotes cell migration in mouse seminiferous epithelium. Reprod Biol Endocrinol. 2018;16(1):105. doi: 10.1186/s12958-018-0427-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Ferder IC, Fung L, Ohguchi Y, et al. Meiotic gatekeeper STRA8 suppresses autophagy by repressing Nr1d1 expression during spermatogenesis in mice. PLoS Genet. 2019;15(5):e1008084. doi: 10.1371/journal.pgen.1008084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Huang Y, Yang P, Liu T, et al. Subcellular evidence for biogenesis of autophagosomal membrane during spermiogenesis in vivo. Frontiers in physiology. Front Physiol. 2016;7:470. DOI: 10.3389/fphys.2016.00470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Yang P, Ahmed N, Wang L, et al. In vivo autophagy and biogenesis of autophagosomes within male haploid cells during spermiogenesis. Oncotarget. 2017;8(34):56791. doi: 10.18632/oncotarget.18221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Qu W, Tarique I, Deng B, et al. Cellular evidence of autophagy in Sertoli cells during spermatogenesis in goats. Theriogenology. 2020;154:237–245. DOI: 10.1016/j.theriogenology.2020.05.024 [DOI] [PubMed] [Google Scholar]
- [48].Asgari R, Bakhtiari M, Rezazadeh D, et al. TSGA10 as a potential key factor in the process of spermatid differentiation/maturation: deciphering its association with autophagy pathway. Reproductive Sciences. 2021;28(11):3228–3240. doi: 10.1007/s43032-021-00648-6 [DOI] [PubMed] [Google Scholar]
- [49].Vives-Bauza C, Zhou C, Huang Y, et al. PINK1-dependent recruitment of Parkin to mitochondria in mitophagy. Proc Nat Acad Sci. 2010;107(1):378–383. doi: 10.1073/pnas.0911187107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Varuzhanyan G, Rojansky R, Sweredoski MJ, et al. Mitochondrial fusion is required for spermatogonial differentiation and meiosis. Elife. 2019;8:e51601. DOI: 10.7554/eLife.51601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Rajender S, Rahul P, Mahdi AA. Mitochondria, spermatogenesis and male infertility. Mitochondrion. 2010;10(5):419–428. doi: 10.1016/j.mito.2010.05.015 [DOI] [PubMed] [Google Scholar]
- [52].Varuzhanyan G, Chan DC. Mitochondrial dynamics during spermatogenesis. Journal Of Cell Science. 2020;133(14). doi: 10.1242/jcs.235937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Ho HC, Wey S. Three dimensional rendering of the mitochondrial sheath morphogenesis during mouse spermiogenesis. Microsc Res Tech. 2007;70(8):719–723. doi: 10.1002/jemt.20457 [DOI] [PubMed] [Google Scholar]
- [54].Xu X, Zhang Y, Cheng H, et al. SPATA33 functions as a mitophagy receptor in mammalian germline. Autophagy. 2021;17(5):1284–1286. doi: 10.1080/15548627.2021.1909836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].CHEMES H. The phagocytic function of Sertoli cells: a morphological, biochemical, and endocrinological study of lysosomes and acid phosphatase localization in the rat testis. Endocrinology. 1986;119(4):1673–1681. doi: 10.1210/endo-119-4-1673 [DOI] [PubMed] [Google Scholar]
- [56].Huang J, Wang H, Chen Y, et al. Residual body removal during spermatogenesis in C. elegans requires genes that mediate cell corpse clearance. Development. Development. 2012;139(24):4613–4622. doi: 10.1242/dev.086769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Blanco-Rodríguez J, Martínez-García C. Apoptosis is physiologically restricted to a specialized cytoplasmic compartment in rat spermatids. Biol Reprod. 1999;61(6):1541–1547. doi: 10.1095/biolreprod61.6.1541 [DOI] [PubMed] [Google Scholar]
- [58].Breucker H, Schäfer E, Holstein A-F. Morphogenesis and fate of the residual body in human spermiogenesis. Cell Tissue Res. 1985;240(2):303–309. doi: 10.1007/BF00222339 [DOI] [PubMed] [Google Scholar]
- [59].Bose R, Manku G, Culty M, et al. Ubiquitin–Proteasome System in Spermatogenesis. Posttranslational Protein Modifications in the Reproductive System. NY: Springer; . 2014;181–213. doi: 10.1007/978-1-4939-0817-2_9 [DOI] [PubMed] [Google Scholar]
- [60].Hermo L, Pelletier RM, Cyr DG, et al. Surfing the wave, cycle, life history, and genes/proteins expressed by testicular germ cells. Part 4: intercellular bridges, mitochondria, nuclear envelope, apoptosis, ubiquitination, membrane/voltage-gated channels, methylation/acetylation, and transcription factors. Microsc Res Tech. 2010;73(4):364–408. doi: 10.1002/jemt.20785 [DOI] [PubMed] [Google Scholar]
- [61].Shang Y, Wang H, Jia P, et al. Autophagy regulates spermatid differentiation via degradation of PDLIM1. Autophagy. 2016;12(9):1575–1592. doi: 10.1080/15548627.2016.1192750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Song Z, Yu H-Y, Wang P, et al. Germ cell-specific Atg7 knockout results in primary ovarian insufficiency in female mice. Cell death & disease. Cell Death Dis. 2015;6(1):e1589–e1589. doi: 10.1038/cddis.2014.559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Huang Q, Liu Y, Zhang S, et al. Autophagy core protein ATG5 is required for elongating spermatid development, sperm individualization and normal fertility in male mice. Autophagy. 2021;17(7):1753–1767. doi: 10.1080/15548627.2020.1783822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Wang H, Wan H, Li X, et al. Atg7 is required for acrosome biogenesis during spermatogenesis in mice. Cell Res. 2014;24(7):852–869. doi: 10.1038/cr.2014.70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Umer N, Phadke S, Shakeri F, et al. PFN4 is required for manchette development and acrosome biogenesis during mouse spermiogenesis. Development. 2022;149(16):dev200499. doi: 10.1242/dev.200499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Ahmed N, Liu Y, Chen H, et al. Novel cellular evidence of lipophagy within the Sertoli cells during spermatogenesis in the turtle. Aging (Albany NY. Aging. 2017;9(1):41. doi: 10.18632/aging.101070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Tarique I, Vistro WA, Bai X, et al. LIPOPHAGY: a novel form of steroidogenic activity within the LEYDIG cell during the reproductive cycle of turtle. Reprod Biol Endocrinol. 2019;17(1):19. doi: 10.1186/s12958-019-0462-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Mancilla H, Maldonado R, Cereceda K, et al. Glutathione depletion induces spermatogonial cell autophagy. J Cell Biochem. 2015;116(10):2283–2292. doi: 10.1002/jcb.25178 [DOI] [PubMed] [Google Scholar]
- [69].Mruk DD, Cheng CY. Cell–cell interactions at the ectoplasmic specialization in the testis. Trends Endocrinol Metab. 2004;15(9):439–447. doi: 10.1016/j.tem.2004.09.009 [DOI] [PubMed] [Google Scholar]
- [70].Rossi M, Colecchia D, Ilardi G, et al. MAPK15 upregulation promotes cell proliferation and prevents DNA damage in male germ cell tumors. Oncotarget. 2016;7(15):20981. doi: 10.18632/oncotarget.8044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Aslani F, Sebastian T, Keidel M, et al. Resistance to apoptosis and autophagy leads to enhanced survival in Sertoli cells. MHR: Basic science of reproductive medicine. Mol Hum Reprod. 2017;23(6):370–380. doi: 10.1093/molehr/gax022 [DOI] [PubMed] [Google Scholar]
- [72].Pereira R, Sá R, Barros A, et al. Major regulatory mechanisms involved in sperm motility. Asian J Androl. 2017;19(1):5. doi: 10.4103/1008-682X.167716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Hargreaves CA, Rogers S, Hills F, et al. Effects of co-trimoxazole, erythromycin, amoxycillin, tetracycline and chloroquine on sperm function in vitro. Hum Reprod. 1998;13(7):1878–1886. doi: 10.1093/humrep/13.7.1878 [DOI] [PubMed] [Google Scholar]
- [74].Hori YS, Hosoda R, Akiyama Y, et al. Chloroquine potentiates temozolomide cytotoxicity by inhibiting mitochondrial autophagy in glioma cells. J Neurooncol. 2015;122(1):11–20. doi: 10.1007/s11060-014-1686-9 [DOI] [PubMed] [Google Scholar]
- [75].Long L, Yang X, Southwood M, et al. Chloroquine prevents progression of experimental pulmonary hypertension via inhibition of autophagy and lysosomal bone morphogenetic protein type II receptor degradation. Circ Res. 2013;112(8):1159–1170. doi: 10.1161/CIRCRESAHA.111.300483 [DOI] [PubMed] [Google Scholar]
- [76].Zhang Z, Li W, Zhang Y, et al. Intraflagellar transport protein IFT20 is essential for male fertility and spermiogenesis in mice. Mol Biol Cell. 2016;27(23):3705–3716. doi: 10.1091/mbc.e16-05-0318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Aparicio IM, Espino J, Bejarano I, et al. Autophagy-related proteins are functionally active in human spermatozoa and may be involved in the regulation of cell survival and motility. Sci Rep. 2016;6(1):33647. doi: 10.1038/srep33647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Zhang J, Zhang X, Liu Y, et al. Leucine mediates autophagosome-lysosome fusion and improves sperm motility by activating the PI3K/Akt pathway. Oncotarget. 2017;8(67):111807–111818. doi: 10.18632/oncotarget.22910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Song W-H, Yi Y-J, Sutovsky M, et al. The ART and science of sperm mitophagy. Autophagy. 2016;12(12):2510–2511. doi: 10.1080/15548627.2016.1239004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Song W-H, Yi Y-J, Sutovsky M, et al. Autophagy and ubiquitin–proteasome system contribute to sperm mitophagy after mammalian fertilization. Proc Nat Acad Sci. 2016;113(36):E5261–E5270. doi: 10.1073/pnas.1605844113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Song W-H, Zuidema D, Yi Y-J, et al. Mammalian cell-free system recapitulates the early events of post-fertilization sperm mitophagy. Cells. 2021;10(9):2450. doi: 10.3390/cells10092450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Lei Y, Zhang X, Xu Q, et al. Autophagic elimination of ribosomes during spermiogenesis provides energy for flagellar motility. Dev Cell. 2021;56(16):2313–2328. e7. doi: 10.1016/j.devcel.2021.07.015 [DOI] [PubMed] [Google Scholar]
- [83].Guo Y, Ma Y, Zhang J, et al. Alteration in autophagy gene expression profile correlates with low sperm quality. Reprod Biol. 2021;21(4):100546. doi: 10.1016/j.repbio.2021.100546 [DOI] [PubMed] [Google Scholar]
- [84].Yi J, Tang XM. Functional implication of autophagy in steroid-secreting cells of the rat. Anat Rec. 1995;242(2):137–146. doi: 10.1002/ar.1092420202 [DOI] [PubMed] [Google Scholar]
- [85].Li WR, Chen L, Chang Z-J, et al. Autophagic deficiency is related to steroidogenic decline in aged rat Leydig cells. Asian J Androl. 2011;13(6):881–888. doi: 10.1038/aja.2011.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Khawar MB, Liu C, Gao F, et al. Sirt1 regulates testosterone biosynthesis in Leydig cells via modulating autophagy. Protein Cell. 2021;12(1):67–75. doi: 10.1007/s13238-020-00771-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Gao F, Li G, Liu C, et al. Autophagy regulates testosterone synthesis by facilitating cholesterol uptake in Leydig cells. J Cell Bio. 2018;217(6):2103–2119. doi: 10.1083/jcb.201710078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Ma Y, Zhou Y, Zhu Y-C, et al. Lipophagy contributes to Testosterone biosynthesis in male Rat Leydig Cells. Endocrinology. 2018;159(2):1119–1129. doi: 10.1210/en.2017-03020 [DOI] [PubMed] [Google Scholar]
- [89].Sokanovic SJ, Baburski AZ, Kojic Z, et al. Aging-related increase of cGMP disrupts mitochondrial homeostasis in leydig cells. J Gerontol A. 2021;76(2):177–186. doi: 10.1093/gerona/glaa132 [DOI] [PubMed] [Google Scholar]
- [90].Zhou L, Lv M-Q, Ge P, et al. The expression of Beclin-1 in testicular tissues of non-obstructive azoospermia patients and its predictive value in sperm retrieval rate. Transl Androl Urol. 2021;10(8):3267–3274. doi: 10.21037/tau-21-320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Ma Y, Yang H-Z, Xu L-M, et al. Testosterone regulates the autophagic clearance of androgen binding protein in rat Sertoli cells. Sci Rep. 2015;5(1):8894. doi: 10.1038/srep08894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Kumar R, Mendonca J, Owoyemi O, et al. Supraphysiologic testosterone induces ferroptosis and activates immune pathways through nucleophagy in prostate cancer. Cancer Res. 2021;81(23):5948–5962. doi: 10.1158/0008-5472.CAN-20-3607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Tal R, Seifer DB. Ovarian reserve testing: a user’s guide. Am J Obstet Gynecol. 2017;217(2):129–140. doi: 10.1016/j.ajog.2017.02.027 [DOI] [PubMed] [Google Scholar]
- [94].Esencan E, Beroukhim G, Seifer DB. Age-related changes in Folliculogenesis and potential modifiers to improve fertility outcomes-A narrative review. Reprod Biol Endocrinol. 2022;20(1):1–18. doi: 10.1186/s12958-022-01033-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Matzuk MM, Burns KH, Viveiros MM, et al. Intercellular communication in the mammalian ovary: oocytes carry the conversation. Science. 2002;296(5576):2178–2180. doi: 10.1126/science.1071965 [DOI] [PubMed] [Google Scholar]
- [96].Liu C, Peng J, Matzuk MM, et al. Lineage specification of ovarian theca cells requires multicellular interactions via oocyte and granulosa cells. Nat Commun. 2015;6(1):6934. doi: 10.1038/ncomms7934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Sun Y-C, Wang Y-Y, Sun X-F, et al. The role of autophagy during murine primordial follicle assembly. Aging (Albany NY. Aging. 2018;10(2):197. doi: 10.18632/aging.101376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Chen Z, Zuo X, Li H, et al. Effects of melatonin on maturation, histone acetylation, autophagy of porcine oocytes and subsequent embryonic development. Anim Sci J. 2017;88(9):1298–1310. doi: 10.1111/asj.12779 [DOI] [PubMed] [Google Scholar]
- [99].Nezis IP, Stravopodis DJ, Margaritis LH, et al. Programmed cell death of follicular epithelium during the late developmental stages of oogenesis in the fruit flies Bactrocera oleae and Ceratitis capitata (Diptera, Tephritidae) is mediated by autophagy. Deve Growth Differ. 2006;48(3):189–198. doi: 10.1111/j.1440-169X.2006.00856.x [DOI] [PubMed] [Google Scholar]
- [100].Santos A, Ramos I. ATG3 is important for the chorion ultrastructure during oogenesis in the insect vector Rhodnius prolixus. Front Physiol. 2021;12:638026. doi: 10.3389/fphys.2021.638026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Rodrigues P, Limback D, McGinnis LK, et al. Multiple mechanisms of germ cell loss in the perinatal mouse ovary. Reproduction. 2009;137(4):709–720. doi: 10.1530/REP-08-0203 [DOI] [PubMed] [Google Scholar]
- [102].Barth J, Szabad J, Hafen E, et al. Autophagy in Drosophila ovaries is induced by starvation and is required for oogenesis. Cell Death Diff. 2011;18(6):915–924. doi: 10.1038/cdd.2010.157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Watanabe R, Kimura N. Non-suckling starvation of neonatal mice promotes primordial follicle formation with activation of ovarian autophagy. J Reprod Dev. 2018;64(1):89–94. doi: 10.1262/jrd.2017-126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Hou Y-C-C, Chittaranjan S, Barbosa SG, et al. Effector caspase Dcp-1 and IAP protein Bruce regulate starvation-induced autophagy during Drosophila melanogaster oogenesis. J Cell Bio. 2008;182(6):1127–1139. doi: 10.1083/jcb.200712091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Tworzydlo W, Kisiel E, Jankowska W, et al. Exclusion of dysfunctional mitochondria from Balbiani body during early oogenesis of Thermobia. Cell and tissue research. Cell Tissue Res. 2016;366(1):191–201. doi: 10.1007/s00441-016-2414-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Rojas-Ríos P, Chartier A, Pierson S, et al. Translational control of autophagy by Orb in the Drosophila germline. Dev Cell. 2015;35(5):622–631. doi: 10.1016/j.devcel.2015.11.003 [DOI] [PubMed] [Google Scholar]
- [107].Zhihan T, Xinyi M, Qingying L, et al. Autophagy participates in cyst breakdown and primordial folliculogenesis by reducing reactive oxygen species levels in perinatal mouse ovaries. J Cell Physiol. 2019;234(5):6125–6135. doi: 10.1002/jcp.27367 [DOI] [PubMed] [Google Scholar]
- [108].Wang Y-Y, Sun Y-C, Sun X-F, et al. Starvation at birth impairs germ cell cyst breakdown and increases autophagy and apoptosis in mouse oocytes. Cell Death Dis. 2017;8(2):e2613–e2613. doi: 10.1038/cddis.2017.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Shao T, Ke H, Liu R, et al. Autophagy regulates differentiation of ovarian granulosa cells through degradation of WT1. Autophagy. 2022;18(8):1–15. doi: 10.1080/15548627.2021.2005415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].He H, Li D, Tian Y, et al. miRNA sequencing analysis of healthy and atretic follicles of chickens revealed that miR-30a-5p inhibits granulosa cell death via targeting Beclin1. J Animal Sci Biotechnol. 2022;13(1):1–22. doi: 10.1186/s40104-022-00697-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Chen C, Ahmad MJ, Ye T, et al. Cathepsin B regulates Mice Granulosa cells’ apoptosis and proliferation in vitro. Int J Mol Sci. 2021;22(21):11827. doi: 10.3390/ijms222111827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].D’Ignazio L, Michel M, Beyer M, et al. Lhx8 ablation leads to massive autophagy of mouse oocytes associated with DNA damage†. Biol Reprod. 2018;98(4):532–542. doi: 10.1093/biolre/iox184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Gawriluk TR, Hale AN, Flaws JA, et al. Autophagy is a cell survival program for female germ cells in the murine ovary. Reproduction. 2011;141(6):759. doi: 10.1530/REP-10-0489 [DOI] [PubMed] [Google Scholar]
- [114].Omari S, Waters M, Naranian T, et al. Mcl-1 is a key regulator of the ovarian reserve. Cell death & disease. Cell Death Dis. 2015;6(5):e1755–e1755. doi: 10.1038/cddis.2015.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Nezis IP, Lamark T, Velentzas AD, et al. Cell death during Drosophila melanogaster early oogenesis is mediated through autophagy. Autophagy. 2009;5(3):298–302. doi: 10.4161/auto.5.3.7454 [DOI] [PubMed] [Google Scholar]
- [116].Tanner EA, Blute TA, Brachmann CB, et al. Bcl-2 proteins and autophagy regulate mitochondrial dynamics during programmed cell death in the Drosophila ovary. Development. 2011;138(2):327–338. doi: 10.1242/dev.057943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Choutka C, DeVorkin L, Go NE, et al. Hsp83 loss suppresses proteasomal activity resulting in an upregulation of caspase-dependent compensatory autophagy. Autophagy. 2017;13(9):1573–1589. doi: 10.1080/15548627.2017.1339004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Chon SJ, Umair Z, Yoon M-S. Premature ovarian insufficiency: past, present, and future. Frontiers in cell and developmental biology, 2021. Front Cell Dev Biol. 2021;9:672890. doi: 10.3389/fcell.2021.672890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Samare-Najaf M, Samareh A, Namavar Jahromi B, et al. Female infertility caused by organophosphates: an insight into the latest biochemical and histomorphological findings. Toxin Reviews. 2022;42(1):1–28. doi: 10.1080/15569543.2022.2120897 [DOI] [Google Scholar]
- [120].Samare-Najaf M, Zal F, Safari S. Primary and secondary markers of doxorubicin-induced female infertility and the alleviative properties of quercetin and vitamin E in a rat model. Reprod Toxicol. 2020;96:316–326. doi: 10.1016/j.reprotox.2020.07.015 [DOI] [PubMed] [Google Scholar]
- [121].Sales CF, Melo RMC, Pinheiro APB, et al. Autophagy and Cathepsin D mediated apoptosis contributing to ovarian follicular atresia in the Nile tilapia. Mol Reprod Dev. 2019;86(11):1592–1602. doi: 10.1002/mrd.23245 [DOI] [PubMed] [Google Scholar]
- [122].Yamamoto A, Mizushima N, Tsukamoto S. Fertilization-induced autophagy in mouse embryos is independent of mTORC1. Biol Reprod. 2014;91(1):7, 1–7. doi: 10.1095/biolreprod.113.115816 [DOI] [PubMed] [Google Scholar]
- [123].Jin X, Wang K, Wang L, et al. RAB7 activity is required for the regulation of mitophagy in oocyte meiosis and oocyte quality control during ovarian aging. Autophagy. 2022;18(3):643–660. doi: 10.1080/15548627.2021.1946739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Lee G-K, Shin H, Lim HJ. Rapamycin influences the efficiency of in vitro fertilization and development in the mouse: a role for autophagic activation. Asian-Australas J Anim Sci. 2016;29(8):1102. doi: 10.5713/ajas.15.0762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Tang Z, Zhang Z, Lin Q, et al. HIF-1α/BNIP3-mediated autophagy contributes to the luteinization of granulosa cells during the formation of corpus luteum. Frontiers in cell and developmental biology, 2021. Front Cell Dev Biol. 2021;8:619924. DOI: 10.3389/fcell.2020.619924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Przygrodzka E, Monaco CF, Plewes MR, et al. Protein kinase a and 5′ AMP-Activated protein kinase signaling pathways exert opposite effects on induction of autophagy in luteal cells. Frontiers in cell and developmental biology, 2021. Front Cell Dev Biol. 2021;9. doi: 10.3389/fcell.2021.723563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Gaytán M, Morales C, Sánchez-Criado JE, et al. Immunolocalization of beclin 1, a bcl-2-binding, autophagy-related protein, in the human ovary: possible relation to life span of corpus luteum. Cell Tissue Res. 2008;331(2):509–517. doi: 10.1007/s00441-007-0531-2 [DOI] [PubMed] [Google Scholar]
- [128].Tsukamoto S, Kuma A, Murakami M, et al. Autophagy is essential for preimplantation development of mouse embryos. Science. 2008;321(5885):117–120. doi: 10.1126/science.1154822 [DOI] [PubMed] [Google Scholar]
- [129].Houel-Renault L, Philippe L, Piquemal M, et al. Autophagy is used as a survival program in unfertilized sea urchin eggs that are destined to die by apoptosis after inactivation of MAPK1/3 (ERK2/1). Autophagy. 2013;9(10):1527–1539. doi: 10.4161/auto.25712 [DOI] [PubMed] [Google Scholar]
- [130].Zhou Q, Li H, Xue D. Elimination of paternal mitochondria through the lysosomal degradation pathway in C. elegans. Cell Res. 2011;21(12):1662–1669. doi: 10.1038/cr.2011.182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Sato M, Sato K, Tomura K, et al. The autophagy receptor ALLO-1 and the IKKE-1 kinase control clearance of paternal mitochondria in Caenorhabditis elegans. Nat Cell Biol. 2018;20(1):81–91. doi: 10.1038/s41556-017-0008-9 [DOI] [PubMed] [Google Scholar]
- [132].Politi Y, Gal L, Kalifa Y, et al. Paternal mitochondrial destruction after fertilization is mediated by a common endocytic and autophagic pathway in Drosophila. Dev Cell. 2014;29(3):305–320. doi: 10.1016/j.devcel.2014.04.005 [DOI] [PubMed] [Google Scholar]
- [133].Luo S-M, Ge Z-J, Wang Z-W, et al. Unique insights into maternal mitochondrial inheritance in mice. Proc Nat Acad Sci. 2013;110(32):13038–13043. doi: 10.1073/pnas.1303231110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Rojansky R, Cha M-Y, Chan DC. Elimination of paternal mitochondria in mouse embryos occurs through autophagic degradation dependent on PARKIN and MUL1. Elife. 2016;5:e17896. doi: 10.7554/eLife.17896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Morita A, Satouh Y, Kosako H, et al. Clathrin-mediated endocytosis is essential for the selective degradation of maternal membrane proteins and preimplantation development. Development. 2021;148(14):dev199461. doi: 10.1242/dev.199461 [DOI] [PubMed] [Google Scholar]
- [136].Sato M, Sato K. Degradation of paternal mitochondria by fertilization-triggered autophagy in C. elegans embryos. Science. 2011;334(6059):1141–1144. doi: 10.1126/science.1210333 [DOI] [PubMed] [Google Scholar]
- [137].Elahi F, Lee H, Lee J, et al. Effect of rapamycin treatment during post-activation and/or in vitro culture on embryonic development after parthenogenesis and in vitro fertilization in pigs. Reprod Domest Anim. 2017;52(5):741–748. doi: 10.1111/rda.12974 [DOI] [PubMed] [Google Scholar]
- [138].Shen X, Zhang N, Wang Z, et al. Induction of autophagy improves embryo viability in cloned mouse embryos. Sci Rep. 2015;5(1):17829. doi: 10.1038/srep17829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Lu L, Wang X, Zhao H, et al. MiR-291a/b-5p inhibits autophagy by targeting Atg5 and Becn1 during mouse preimplantation embryo development. RSC Adv. 2019;9(16):9331–9341. doi: 10.1039/C9RA00017H [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Tsukamoto S, Hara T, Yamamoto A, et al. Fluorescence-based visualization of autophagic activity predicts mouse embryo viability. Sci Rep. 2014;4(1):4533. doi: 10.1038/srep04533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Abraham R, Hendy R, Dougherty WJ, et al. Participation of lysosomes in early implantation in the rabbit. Exp Mol Pathol. 1970;13(3):329–345. doi: 10.1016/0014-4800(70)90095-X [DOI] [PubMed] [Google Scholar]
- [142].Leiser R, Wille KH. Cytochemical establishment of acid phosphatase in the bovine endometrium and trophoblast during implantation. Anat Embryol (Berl). 1975;148(2):159–173. doi: 10.1007/BF00315267 [DOI] [PubMed] [Google Scholar]
- [143].Eppig J, Chesnel F, Hirao Y, et al. Oocyte control of granulosa cell development: how and why. Hum Reprod. 1997;12(11 Suppl):127–132. [PubMed] [Google Scholar]
- [144].Sorensen RA, Wassarman PM. Relationship between growth and meiotic maturation of the mouse oocyte. Dev Biology. 1976;50(2):531–536. doi: 10.1016/0012-1606(76)90172-X [DOI] [PubMed] [Google Scholar]
- [145].Zeleznik AJ. Follicle selection in primates: “many are called but few are chosen”. Biol Reprod. 2001;65(3):655–659. doi: 10.1095/biolreprod65.3.655 [DOI] [PubMed] [Google Scholar]
- [146].Richart RM, Ferenczy A. Endometrial morphologic response to hormonal environment. Gynecol Oncol. 1974;2(2–3):180–197. doi: 10.1016/0090-8258(74)90008-0 [DOI] [PubMed] [Google Scholar]
- [147].Griffith C, Lai-Fook J. Corpus luteum formation and ovulation in the butterfly Calpodes. Tissue Cell. 1986;18(5):783–792. doi: 10.1016/0040-8166(86)90077-7 [DOI] [PubMed] [Google Scholar]
- [148].Morais RDVS, Thomé RG, Santos HB, et al. Relationship between bcl-2, bax, beclin-1, and cathepsin-D proteins during postovulatory follicular regression in fish ovary. Theriogenology. 2016;85(6):1118–1131. doi: 10.1016/j.theriogenology.2015.11.024 [DOI] [PubMed] [Google Scholar]
- [149].Cassel M, de Paiva Camargo M, Oliveira de Jesus LW, et al. Involution processes of follicular atresia and post-ovulatory complex in a characid fish ovary: a study of apoptosis and autophagy pathways. J Mol Hist. 2017;48(3):243–257. doi: 10.1007/s10735-017-9723-6 [DOI] [PubMed] [Google Scholar]
- [150].Yamamoto Y, Kuwahara A, Taniguchi Y, et al. Tumor necrosis factor alpha inhibits ovulation and induces granulosa cell death in rat ovaries. Reprod Med Biol. 2015;14(3):107–115. doi: 10.1007/s12522-014-0201-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Yang Q, Dai S, Luo X, et al. Melatonin attenuates postovulatory oocyte dysfunction by regulating SIRT1 expression. Reproduction. 2018;156(1):81–92. doi: 10.1530/REP-18-0211 [DOI] [PubMed] [Google Scholar]
- [152].Lee SE, Kim EY, Choi HY, et al. Rapamycin rescues the poor developmental capacity of aged porcine oocytes. Asian-Australas J Anim Sci. 2014;27(5):635–647. doi: 10.5713/ajas.2013.13816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].McGinnis LK, Pelech S, Kinsey WH. Post-ovulatory aging of oocytes disrupts kinase signaling pathways and lysosome biogenesis. Mol Reprod Dev. 2014;81(10):928–945. doi: 10.1002/mrd.22413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Yilmaz O, Patinote A, Nguyen TV, et al. Scrambled eggs: Proteomic portraits and novel biomarkers of egg quality in zebrafish (Danio rerio). Plos One. 2017;12(11):e0188084. doi: 10.1371/journal.pone.0188084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Leopardo NP, Velazquez ME, Cortasa S, et al. A dual death/survival role of autophagy in the adult ovary of Lagostomus maximus (Mammalia-Rodentia). Plos one. Plos One. 2020;15(5):e0232819. doi: 10.1371/journal.pone.0232819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Yilmaz O, Patinote A, Com E, et al. Knock out of specific maternal vitellogenins in zebrafish (Danio rerio) evokes vital changes in egg proteomic profiles that resemble the phenotype of poor quality eggs. BMC Genomics. 2021;22(1):308. doi: 10.1186/s12864-021-07606-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Boudoures AL, Saben J, Drury A, et al. Obesity-exposed oocytes accumulate and transmit damaged mitochondria due to an inability to activate mitophagy. Dev Biology. 2017;426(1):126–138. doi: 10.1016/j.ydbio.2017.04.005 [DOI] [PubMed] [Google Scholar]
- [158].Pan Z-N, Liu J-C, Ju J-Q, et al. LRRK2 regulates actin assembly for spindle migration and mitochondrial function in mouse oocyte meiosis. J Mol Cell Biol. 2022;14(1):mjab079. doi: 10.1093/jmcb/mjab079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Zhou CX, Wang Y, Shi L-Y, et al. Gtpases Arf5 and Arl2 function partially distinctly during oocyte meiosis. J Cell Biochem. 2021;122(2):198–208. doi: 10.1002/jcb.29839 [DOI] [PubMed] [Google Scholar]
- [160].Tang X, Ma L, Guo S, et al. High doses of FSH induce autophagy in bovine ovarian granulosa cells via the AKT/mTOR pathway. Reproduct Domestic Animals. 2021;56(2):324–332. doi: 10.1111/rda.13869 [DOI] [PubMed] [Google Scholar]
- [161].Liu Q, Gao H, Yang F, et al. FSH promotes progesterone synthesis by enhancing autophagy to accelerate lipid droplet degradation in porcine granulosa cells. Frontiers in cell and developmental biology, 2021. Front Cell Dev Biol. 2021;9:626927. DOI: 10.3389/fcell.2021.626927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Duan J, Chen H, Xu D, et al. 17β-estradiol improves the developmental ability, inhibits reactive oxygen species levels and apoptosis of porcine oocytes by regulating autophagy events. J Steroid Biochem Mol Biol. 2021;209:105826. DOI: 10.1016/j.jsbmb.2021.105826 [DOI] [PubMed] [Google Scholar]
- [163].Li L, Chen J, Sun S, et al. Effects of estradiol on autophagy and Nrf-2/ARE signals after cerebral ischemia. Cell Physiol Biochem. 2017;41(5):2027–2036. doi: 10.1159/000475433 [DOI] [PubMed] [Google Scholar]
- [164].Camuzard O, Santucci-Darmanin S, Breuil V, et al. Sex-specific autophagy modulation in osteoblastic lineage: a critical function to counteract bone loss in female. Oncotarget. 2016;7(41):66416. doi: 10.18632/oncotarget.12013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Yao Q, Feng M, Yang B, et al. Effects of ovarian hormone loss on neuritic plaques and autophagic flux in the brains of adult female APP/PS1 double-transgenic mice. Acta Biochim Biophys Sin (Shanghai). 2018;50(5):447–455. doi: 10.1093/abbs/gmy032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Vega-Naredo I, Caballero B, Sierra V, et al. Sexual dimorphism of autophagy in Syrian hamster Harderian gland culminates in a holocrine secretion in female glands. Autophagy. 2009;5(7):1004–1017. doi: 10.4161/auto.5.7.9610 [DOI] [PubMed] [Google Scholar]
- [167].García-Macia M, Rubio-Gonzalez A, de Luxán-Delgado B, et al. Autophagic and proteolytic processes in the Harderian gland are modulated during the estrous cycle. Histochemistry and cell biology. Histochem Cell Biol. 2014;141(5):519–529. doi: 10.1007/s00418-013-1170-1 [DOI] [PubMed] [Google Scholar]
- [168].Zielniok K, Motyl T, Gajewska M. Functional interactions between 17β-estradiol and progesterone regulate autophagy during acini formation by bovine mammary epithelial cells in 3D cultures. Bio Med Res Int. 2014;2014:1–16. doi: 10.1155/2014/382653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Ge H, Zhang F, Duan P, et al. Mitochondrial Uncoupling Protein 2 in human cumulus cells is associated with regulating autophagy and apoptosis, maintaining gap junction integrity and progesterone synthesis. Mol Cell Endocrinol. 2017;443:128–137. DOI: 10.1016/j.mce.2017.01.020 [DOI] [PubMed] [Google Scholar]
- [170].Sobolewska A, Gajewska M, Zarzyńska J, et al. IGF-I, EGF, and sex steroids regulate autophagy in bovine mammary epithelial cells via the mTOR pathway. Eur J Cell Biol. 2009;88(2):117–130. doi: 10.1016/j.ejcb.2008.09.004 [DOI] [PubMed] [Google Scholar]
- [171].Sobolewska A, Motyl T, Gajewska M. Role and regulation of autophagy in the development of acinar structures formed by bovine BME-UV1 mammary epithelial cells. Eur J Cell Biol. 2011;90(10):854–864. doi: 10.1016/j.ejcb.2011.06.007 [DOI] [PubMed] [Google Scholar]
- [172].Tsai C-L, Lin C-Y, Chao A, et al. GPR30 activation by 17β-estradiol promotes p62 phosphorylation and increases estrogen receptor α protein expression by inducing its release from a complex formed with KEAP1. J Pers Med. 2021;11(9):906. doi: 10.3390/jpm11090906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Choi S, Shin H, Song H, et al. Suppression of autophagic activation in the mouse uterus by estrogen and progesterone. J Endocrinol. 2014;221(1):39–50. doi: 10.1530/JOE-13-0449 [DOI] [PubMed] [Google Scholar]
- [174].Yang D, Liu A, Zhang Y, et al. Essential role of crim1 on endometrial receptivity in goat. Int J Mol Sci. 2021;22(10):5323. doi: 10.3390/ijms22105323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Zowalaty AEE, Ye X. Seipin deficiency leads to defective parturition in mice. Biol Reprod. 2017;97(3):378–386. doi: 10.1093/biolre/iox088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Yang D, Jiang T, Liu J, et al. Hormone regulates endometrial function via cooperation of endoplasmic reticulum stress and Mtor‐autophagy. J Cell Physiol. 2018;233(9):6644–6659. doi: 10.1002/jcp.26315 [DOI] [PubMed] [Google Scholar]
- [177].Rhee JS, Saben JL, Mayer AL, et al. Diet-induced obesity impairs endometrial stromal cell decidualization: a potential role for impaired autophagy. Hum Reprod. 2016;31(6):1315–1326. doi: 10.1093/humrep/dew048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Yang D, Zhang B, Wang Z, et al. COPS5 negatively regulates goat endometrial function via the ERN1 and Mtor‐autophagy pathways during early pregnancy. J Cell Physiol. 2019;234(10):18666–18678. doi: 10.1002/jcp.28505 [DOI] [PubMed] [Google Scholar]
- [179].Song B-S, Yoon S-B, Kim J-S, et al. Induction of autophagy promotes preattachment development of bovine embryos by reducing endoplasmic reticulum stress. Biol Reprod. 2012;87(1):8, 1–11. doi: 10.1095/biolreprod.111.097949 [DOI] [PubMed] [Google Scholar]
- [180].Xu J, Sun L, Wu C, et al. Involvement of PINK1/Parkin-mediated mitophagy in mitochondrial functional disruption under oxidative stress in vitrified porcine oocytes. Theriogenology. 2021;174:160–168. DOI: 10.1016/j.theriogenology.2021.08.028 [DOI] [PubMed] [Google Scholar]
- [181].Xu J, Zhang D, Ju S, et al. Mitophagy is involved in the mitochondrial dysfunction of vitrified porcine oocytes. Mol Reprod Dev. 2021;88(6):427–436. doi: 10.1002/mrd.23472 [DOI] [PubMed] [Google Scholar]
- [182].Lim Y, Rubio-Peña K, Sobraske PJ, et al. Fndc-1 contributes to paternal mitochondria elimination in C. elegans. Dev Biology. 2019;454(1):15–20. doi: 10.1016/j.ydbio.2019.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Molina P, Lim Y, Boyd L. Ubiquitination is required for the initial removal of paternal organelles in C. elegans. Dev Biology. 2019;453(2):168–179. doi: 10.1016/j.ydbio.2019.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Djeddi A, Al Rawi S, Deuve JL, et al. Sperm-inherited organelle clearance in C. elegans relies on LC3-dependent autophagosome targeting to the pericentrosomal area. Development. 2015;142(9):1705–1716. doi: 10.1242/dev.117879 [DOI] [PubMed] [Google Scholar]
- [185].Wang Y, Tian C-C, Jiao Y-Y, et al. MiR-188-3p-targeted regulation of ATG7 affects cell autophagy in patients with nonobstructive azoospermia. Reprod Biol Endocrinol. 2022;20(1):1–14. doi: 10.1186/s12958-022-00951-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Bai F-R, Wu Q-Q, Wu Y-J, et al. Germline FOXJ2 overexpression causes male infertility via aberrant autophagy activation by LAMP2A upregulation. Cell Death Dis. 2022;13(7):1–13. doi: 10.1038/s41419-022-05116-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Foroozan-Boroojeni S, Tavalaee M, Zakeri Z, et al. Assessment of Atg7 and LC3II/LC3, as the mArkers of autophagy, in sperm of infertile men with globozoospermia: a case-control study. Cell J (Yakhteh). 2021;23(1):70. doi: 10.22074/cellj.2021.7023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Shi G-J, Zheng J, Han X-X, et al. Lycium barbarum polysaccharide attenuates diabetic testicular dysfunction via inhibition of the PI3K/Akt pathway-mediated abnormal autophagy in male mice. Cell Tissue Res. 2018;374(3):653–666. doi: 10.1007/s00441-018-2891-1 [DOI] [PubMed] [Google Scholar]
- [189].Huang C, Hsu H-J, Wang M-E, et al. Fatty acids suppress the steroidogenesis of the MA-10 mouse Leydig cell line by downregulating CYP11A1 and inhibiting late-stage autophagy. Sci Rep. 2021;11(1):1–12. doi: 10.1038/s41598-021-92008-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Li Z, Li Y, Zhou X, et al. Autophagy involved in the activation of the Nrf2-antioxidant system in testes of heat-exposed mice. J Therm Biol. 2018;71:142–152. DOI: 10.1016/j.jtherbio.2017.11.006 [DOI] [PubMed] [Google Scholar]
- [191].Duan P, Hu C, Quan C, et al. 4-Nonylphenol induces apoptosis, autophagy and necrosis in Sertoli cells: involvement of ROS-mediated AMPK/AKT-Mtor and JNK pathways. Toxicology. 2016;341:28–40. DOI: 10.1016/j.tox.2016.01.004 [DOI] [PubMed] [Google Scholar]
- [192].Li W, Li D, Kuang H, et al. Berberine increases glucose uptake and intracellular ROS levels by promoting Sirtuin 3 ubiquitination. Biomed Pharmacother. 2020;121:109563. DOI: 10.1016/j.biopha.2019.109563 [DOI] [PubMed] [Google Scholar]
- [193].Kang W, Ishida E, Yamatoya K, et al. Autophagy-disrupted LC3 abundance leads to death of supporting cells of human oocytes. Biochem Biophys Rep. 2018;15:107–114. DOI: 10.1016/j.bbrep.2018.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Siracusa R, D’Amico R, Impellizzeri D, et al. Autophagy and mitophagy promotion in a rat model of endometriosis. Int J Mol Sci. 2021;22(10):5074. doi: 10.3390/ijms22105074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Kumariya S, Ubba V, Jha RK, et al. Autophagy in ovary and polycystic ovary syndrome: role, dispute and future perspective. Autophagy. 2021;17(10):2706–2733. doi: 10.1080/15548627.2021.1938914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Li X, Qi J, Zhu Q, et al. The role of androgen in autophagy of granulosa cells from PCOS. Gynecological Endocrinol. 2019;35(8):669–672. doi: 10.1080/09513590.2018.1540567 [DOI] [PubMed] [Google Scholar]
- [197].Zhou J, Yao W, Li C, et al. Administration of follicle-stimulating hormone induces autophagy via upregulation of HIF-1α in mouse granulosa cells. Cell Death Dis. 2017;8(8):e3001–e3001. doi: 10.1038/cddis.2017.371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Delcour C, Amazit L, Patino LC, et al. ATG7 and ATG9A loss-of-function variants trigger autophagy impairment and ovarian failure. Genet Med. 2019;21(4):930–938. doi: 10.1038/s41436-018-0287-y [DOI] [PubMed] [Google Scholar]
- [199].Boussada M, Dias TR, Crisóstomo L, et al. A new thiocyanoacetamide (2-cyano-2-p-nitrophenyl-N-benzylthioamide) reduces doxorubicin-induced in vitro toxicity in Sertoli cells by decreasing apoptosis and autophagy. Theriogenology. 2019;140:188–200. DOI: 10.1016/j.theriogenology.2019.08.030 [DOI] [PubMed] [Google Scholar]
- [200].Wang J, Wu GS. Role of autophagy in cisplatin resistance in ovarian cancer cells. J Biol Chem. 2014;289(24):17163–17173. doi: 10.1074/jbc.M114.558288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Nakashima A, Yamanaka-Tatematsu M, Fujita N, et al. Impaired autophagy by soluble endoglin, under physiological hypoxia in early pregnant period, is involved in poor placentation in preeclampsia. Autophagy. 2013;9(3):303–316. doi: 10.4161/auto.22927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Lee J-E, Oh H-A, Song H, et al. Autophagy regulates embryonic survival during delayed implantation. Endocrinology. 2011;152(5):2067–2075. doi: 10.1210/en.2010-1456 [DOI] [PubMed] [Google Scholar]
- [203].Shin H, Bang S, Kim J, et al. The formation of multivesicular bodies in activated blastocysts is influenced by autophagy and FGF signaling in mice. Sci Rep. 2017;7(1):41986. doi: 10.1038/srep41986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Ekizceli G, Inan S, Oktem G, et al. Assessment of mTOR pathway molecules during implantation in rats. Biotechnic Histochemist. 2017;92(6):450–458. doi: 10.1080/10520295.2017.1350749 [DOI] [PubMed] [Google Scholar]
- [205].Cho H-J, Baek M-O, Khaliq SA, et al. Microgravity inhibits decidualization via decreasing Akt activity and FOXO3a expression in human endometrial stromal cells. Sci Rep. 2019;9(1):1–11. doi: 10.1038/s41598-019-48580-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Runyan CL, McIntosh SZ, Maestas MM, et al. CXCR4 signaling at the ovine fetal–maternal interface regulates vascularization, CD34+ cell presence, and autophagy in the endometrium†. Biol Reprod. 2019;101(1):102–111. doi: 10.1093/biolre/ioz073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Maria Fimia G, Stoykova A, Romagnoli A, et al. Ambra1 regulates autophagy and development of the nervous system. Nature. 2007;447(7148):1121–1125. doi: 10.1038/nature05925 [DOI] [PubMed] [Google Scholar]
- [208].Wang S, Li B, Qiao H, et al. Autophagy‐related gene Atg5 is essential for astrocyte differentiation in the developing mouse cortex. EMBO Rep. 2014;15(10):1053–1061. doi: 10.15252/embr.201338343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Lv X, Jiang H, Li B, et al. The crucial role of Atg5 in cortical neurogenesis during early brain development. Sci Rep. 2014;4(1):1–9. doi: 10.1038/srep06010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Gimenes F, Souza RP, Bento JC, et al. Male infertility: a public health issue caused by sexually transmitted pathogens. Nat Rev Urol. 2014;11(12):672–687. doi: 10.1038/nrurol.2014.285 [DOI] [PubMed] [Google Scholar]
- [211].Mehta RH, Makwana S, Ranga GM, et al. Prevalences of oligozoospermia and azoospermia in male partners of infertile couples from different parts of India. Asian J Andrology. 2006;8(1):89–93. doi: 10.1111/j.1745-7262.2006.00050.x [DOI] [PubMed] [Google Scholar]
- [212].Zhou L, Lv M-Q, Ge P, et al. The expression of Beclin-1 in testicular tissues of non-obstructive azoospermia patients and its predictive value in sperm retrieval rate. Transl Androl Urol. 2021;10(8):3267. doi: 10.21037/tau-21-320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Asgari R, Bakhtiari M, Rezazadeh D, et al. Autophagy related gene expression status in patients diagnosed with azoospermia: A cross-sectional study. J Gene Med. 2020;22(4):e3161. doi: 10.1002/jgm.3161 [DOI] [PubMed] [Google Scholar]
- [214].Nawaz S, Hussain S, Basit S, et al. First evidence of involvement of TBC1D25 in causing human male infertility. Eur J Med Genet. 2021;64(2):104142. doi: 10.1016/j.ejmg.2021.104142 [DOI] [PubMed] [Google Scholar]
- [215].Bai F-R, Wu Q-Q, Wu Y-J, et al. Germline FOXJ2 overexpression causes male infertility via aberrant autophagy activation by LAMP2A upregulation. Cell Death Dis. 2022;13(7):665. doi: 10.1038/s41419-022-05116-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].De Braekeleer M, Nguyen MH, Morel F, et al. Genetic aspects of monomorphic teratozoospermia: a review. J Assist Reprod Genet. 2015;32(4):615–623. doi: 10.1007/s10815-015-0433-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Fesahat F, Henkel R, Agarwal A. Globozoospermia syndrome: an update. Andrologia. 2020;52(2):e13459. doi: 10.1111/and.13459 [DOI] [PubMed] [Google Scholar]
- [218].Alvarez Sedó C, Rawe VY, Chemes HE. Acrosomal biogenesis in human globozoospermia: immunocytochemical, ultrastructural and proteomic studies. Human reproduction. Hum Reprod. 2012;27(7):1912–1921. doi: 10.1093/humrep/des126 [DOI] [PubMed] [Google Scholar]
- [219].Liu C, Song Z, Wang L, et al. Sirt1 regulates acrosome biogenesis by modulating autophagic flux during spermiogenesis in mice. Development. 2017;144(3):441–451. doi: 10.1242/dev.147074 [DOI] [PubMed] [Google Scholar]
- [220].Bustamante-Marín X, Quiroga C, Lavandero S, et al. Apoptosis, necrosis and autophagy are influenced by metabolic energy sources in cultured rat spermatocytes. Apoptosis. 2012;17(6):539–550. doi: 10.1007/s10495-012-0709-2 [DOI] [PubMed] [Google Scholar]
- [221].Yang W, Li L, Huang X, et al. Levels of Leydig cell autophagy regulate the fertility of male naked mole-rats. Oncotarget. 2017;8(58):98677. doi: 10.18632/oncotarget.22088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Kankuan W, Wanichanon C, Morani F, et al. Starvation promotes autophagy-associated maturation of the testis in the giant freshwater prawn, macrobrachium rosenbergii. Front Physiol. 2019;10:1219. DOI: 10.3389/fphys.2019.01219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Zheng Y, Zhang P, Zhang C, et al. Surgery-induced cryptorchidism induces apoptosis and autophagy of spermatogenic cells in mice. Zygote. 2019;27(2):101–110. doi: 10.1017/S096719941900011X [DOI] [PubMed] [Google Scholar]
- [224].Eisenberg ML, Lipshultz LI. Varicocele-induced infertility: newer insights into its pathophysiology. Indian J Urol. 2011;27(1):58. doi: 10.4103/0970-1591.78428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Jensen CFS, Østergren P, Dupree JM, et al. Varicocele and male infertility. Nat Rev Urol. 2017;14(9):523–533. doi: 10.1038/nrurol.2017.98 [DOI] [PubMed] [Google Scholar]
- [226].Zhu SM, Rao T, Yang X, et al. Autophagy may play an important role in varicocele. Molecular medicine reports. Mol Med Rep. 2017;16(4):5471–5479. doi: 10.3892/mmr.2017.7253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Sadeghi N, Erfani-Majd N, Tavalaee M, et al. Signs of ROS-associated autophagy in testis and sperm in a rat model of varicocele. Oxid Med Cell Longevity. 2020;2020:1–11. DOI: 10.1155/2020/5140383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].Uribe P, Meriño J, Matus CE, et al. Autophagy is activated in human spermatozoa subjected to oxidative stress and its inhibition impairs sperm quality and promotes cell death. Hum Reprod. 2022;37(4):680–695. doi: 10.1093/humrep/deac021 [DOI] [PubMed] [Google Scholar]
- [229].Eertmans F, Dhooge W, Stuyvaert S, et al. Endocrine disruptors: effects on male fertility and screening tools for their assessment. Toxicology in vitro. Toxicol In Vitro. 2003;17(5–6):515–524. doi: 10.1016/S0887-2333(03)00121-8 [DOI] [PubMed] [Google Scholar]
- [230].Chen H, Liu G, Qiao N, et al. Toxic effects of arsenic trioxide on spermatogonia are associated with oxidative stress, mitochondrial dysfunction, autophagy and metabolomic alterations. Ecotoxicol Environ Saf. 2020;190:110063. DOI: 10.1016/j.ecoenv.2019.110063 [DOI] [PubMed] [Google Scholar]
- [231].Wei Y, Zhou Y, Tang X-L, et al. Testicular developmental impairment caused by flutamide-induced and DEHP-induced cryptorchid rat models is mediated by excessive apoptosis and deficient autophagy. Toxicol Mech Methods. 2018;28(7):507–519. doi: 10.1080/15376516.2018.1459994 [DOI] [PubMed] [Google Scholar]
- [232].Huang W, Quan C, Duan P, et al. Nonylphenol induced apoptosis and autophagy involving the Akt/mTOR pathway in prepubertal Sprague-Dawley male rats in vivo and in vitro. Toxicology. 2016;373:41–53. DOI: 10.1016/j.tox.2016.11.006 [DOI] [PubMed] [Google Scholar]
- [233].Horibe A, Eid N, Ito Y, et al. Ethanol-induced autophagy in Sertoli cells is specifically marked at androgen-dependent stages of the spermatogenic cycle: Potential mechanisms and implications. Int J Mol Sci. 2019;20(1):184. doi: 10.3390/ijms20010184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [234].Chen Y, Wang J, Zhang Q, et al. Microcystin-leucine arginine exhibits immunomodulatory roles in testicular cells resulting in orchitis. Environ Pollut. 2017;229:964–975. DOI: 10.1016/j.envpol.2017.07.081 [DOI] [PubMed] [Google Scholar]
- [235].Xu D, Wang J, Ma Y, et al. Microcystin-leucine-arginine induces apical ectoplasmic specialization disassembly. Chemosphere. 2021;264:128440. DOI: 10.1016/j.chemosphere.2020.128440 [DOI] [PubMed] [Google Scholar]
- [236].Zhou Y, Chen Y, Yuan M, et al. In vivo study on the effects of microcystin—LR on the apoptosis, proliferation and differentiation of rat testicular spermatogenic cells of male rats injected i.P. with toxins. J Toxicol Sci. 2013;38(5):661–670. doi: 10.2131/jts.38.661 [DOI] [PubMed] [Google Scholar]
- [237].Sun Y, Shen J, Zeng L, et al. Role of autophagy in di-2-ethylhexyl phthalate (DEHP)-induced apoptosis in mouse Leydig cells. Environ Pollut. 2018;243:563–572. DOI: 10.1016/j.envpol.2018.08.089 [DOI] [PubMed] [Google Scholar]
- [238].McLennan MT, Steele A, Leong FC. Obstetrics and Gynecology Clinics of North America. Pelvic pain in women. Preface Obstetrics And Gynecology Clinics Of North America. 2014;41(3):xiii–xiv. doi: 10.1016/j.ogc.2014.06.003 [DOI] [PubMed] [Google Scholar]
- [239].Laux-Biehlmann A, d’Hooghe T, Zollner TM. Menstruation pulls the trigger for inflammation and pain in endometriosis. Trends in pharmacological sciences. Trends Pharmacol Sci. 2015;36(5):270–276. doi: 10.1016/j.tips.2015.03.004 [DOI] [PubMed] [Google Scholar]
- [240].Wang W-J, Zhang H, Chen Z-Q, et al. Endometrial TGF-β, IL-10, IL-17 and autophagy are dysregulated in women with recurrent implantation failure with chronic endometritis. Reprod Biol Endocrinol. 2019;17(1):1–9. doi: 10.1186/s12958-018-0444-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [241].Zhou Y, Peng Y, Xia Q, et al. Decreased Indian hedgehog signaling activates autophagy in endometriosis and adenomyosis. Reproduction. 2021;161(2):99–109. doi: 10.1530/REP-20-0172 [DOI] [PubMed] [Google Scholar]
- [242].Ding Y, Zhu Q, He Y, et al. Induction of autophagy by Beclin-1 in granulosa cells contributes to follicular progesterone elevation in ovarian endometriosis. Transl Res. 2021;227:15–29. DOI: 10.1016/j.trsl.2020.06.013 [DOI] [PubMed] [Google Scholar]
- [243].Jamali N, Zal F, Mostafavi-Pour Z, et al. Ameliorative effects of quercetin and metformin and their combination against experimental endometriosis in rats. Reproductive Sciences. 2021;28(3):683–692. doi: 10.1007/s43032-020-00377-2 [DOI] [PubMed] [Google Scholar]
- [244].Escobar Morreale HF. Polycystic ovary syndrome: treatment strategies and management. Expert Opin Pharmacother. 2008;9(17):2995–3008. doi: 10.1517/14656560802559932 [DOI] [PubMed] [Google Scholar]
- [245].Neisy A, Koohpeyma F, Khorchani M, et al. Quercetin modulates ovarian autophagy–related molecules and stereological parameters in a rat model of PCOS. Asian Pac J Tropical Biomedicine. 2023;13(1):9. doi: 10.4103/2221-1691.367686 [DOI] [Google Scholar]
- [246].Dai G, Lu G. Different protein expression patterns associated with polycystic ovary syndrome in human follicular fluid during controlled ovarian hyperstimulation. Reproduction, fertility and development. Reprod Fertil Dev. 2012;24(7):893–904. doi: 10.1071/RD11201 [DOI] [PubMed] [Google Scholar]
- [247].Song X, Shen Q, Fan L, et al. Dehydroepiandrosterone-induced activation of mTORC1 and inhibition of autophagy contribute to skeletal muscle insulin resistance in a mouse model of polycystic ovary syndrome. Oncotarget. 2018;9(15):11905. doi: 10.18632/oncotarget.24190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [248].Jiao X, Ke H, Qin Y, et al. Molecular genetics of premature ovarian insufficiency. Trends Endocrinol Metab. 2018;29(11):795–807. doi: 10.1016/j.tem.2018.07.002 [DOI] [PubMed] [Google Scholar]
- [249].Welt CK. Primary ovarian insufficiency: a more accurate term for premature ovarian failure. Clinical Endocrinol. 2008;68(4):499–509. doi: 10.1111/j.1365-2265.2007.03073.x [DOI] [PubMed] [Google Scholar]
- [250].Chon SJ, Umair Z, Yoon M-S. Premature ovarian insufficiency: past, present, and future. Frontiers in cell and developmental biology. Front Cell Dev Biol. 2021;9:1119. doi: 10.3389/fcell.2021.672890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [251].Szeliga A, Calik-Ksepka A, Maciejewska-Jeske M, et al. Autoimmune diseases in patients with premature ovarian insufficiency—our current state of knowledge. Int J Mol Sci. 2021;22(5):2594. doi: 10.3390/ijms22052594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [252].Dou X, Jin X, Chen X, et al. Bu-Shen-Ning-Xin decoction alleviates premature ovarian insufficiency (POI) by regulating autophagy of granule cells through activating PI3K/AKT/mTOR pathway. Gynecol Endocrinol. 2022;38(9):754–764. doi: 10.1080/09513590.2022.2112941 [DOI] [PubMed] [Google Scholar]
- [253].Lu X, Bao H, Cui L, et al. hUMSC transplantation restores ovarian function in POI rats by inhibiting autophagy of theca-interstitial cells via the AMPK/mTOR signaling pathway. Stem Cell Res Ther. 2020;11(1):268. doi: 10.1186/s13287-020-01784-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [254].Gawriluk TR, Hale AN, Flaws JA, et al. Autophagy is a cell survival program for female germ cells in the murine ovary. Reproduction. 2011;141(6):759–765. doi: 10.1530/REP-10-0489 [DOI] [PubMed] [Google Scholar]
- [255].Czarniewska E, Rosiński G, Gabała E, et al. The natural insect peptide neb-colloostatin induces ovarian atresia and apoptosis in the mealworm tenebrio molitor. BMC Dev Biol. 2014;14(1):1–10. doi: 10.1186/1471-213X-14-4 [DOI] [PMC free article] [PubMed] [Google Scholar]


