Figure 5.
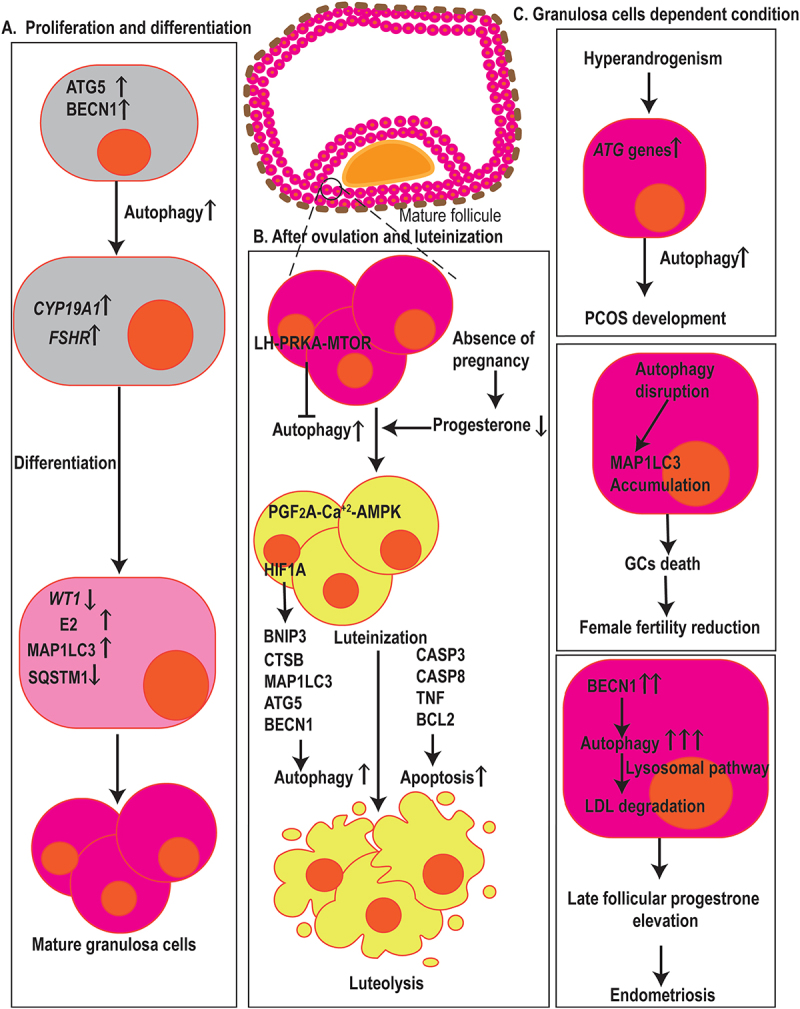
Autophagy in fertilization, menstruation, and infertility. (a) Autophagy has arole in GCs proliferation, differentiation, and death. ATG5 and BECN1 affect the expression of CYP19A1 and FSHR, two genes associated with GCs differentiation, E2 synthesis, and degradation of the WT1 transcription factor, hence contributing to the differentiation of ovarian GCs. Also, elevated levels of MAP1LC3 and decrement in the expression of SQSTM1 resulted in GCs cell differentiation and proliferation. (b) Dropped levels of P4 in the absence of pregnancy result in the induction of autophagy in the corpus luteum during luteal regression. The initiation of autophagy during luteolysis is mediated through the PGF2A-Ca2+-PRKA signaling pathway, whereas LH-PRKA-MTOR luteotrophic signaling exerts inhibiting effects on autophagy. Also, HIF1A regulates GCs through autophagy activation, affecting MAP1LC3 and BECN1, in aBNIP3-dependent manner. Autophagy and apoptosis-related markers such as MAP1LC3, ATG5, CTSB, CASP3, CASP8, TNF, and BCL2 contribute GCs death. (c) in ovarian GCs, ATGsand autophagy markers increase which is positively correlated with hyperandrogenism indicating that autophagy mechanisms are involved in PCOS development. Autophagy-disrupted MAP1LC3 accumulation leads to the death of oocyte-supporting GCs causing areduction in oocyte quality and female fertility. In GCs, higher levels of autophagy, indicated by BECN1, contribut to late follicular P4 elevation by the promotion of LDL degradation via lysosomal pathways which ultimately leads to the aggravation of endometriosis.
