Abstract
Background:
Titanium (Ti) and its alloys are widely used in manufacturing medical devices because of their strength and resistance to corrosion. Although Ti compounds are considered compatible with blood, they appear to support plasma contact activation and may be thrombogenic.
Objectives:
The objective of this study was to compare Ti and titanium nitride (TiN) with known activators of contact activation (kaolin and silica) in plasma-clotting assays and to assess binding and activation of factor XII, (FXII), factor XI (FXI), prekallikrein, and high-molecular-weight kininogen (HK) with Ti/TiN.
Methods:
Ti-based nanospheres and foils were compared with kaolin, silica, and aluminum in plasma-clotting assays. Binding and activation of FXII, prekallikrein, HK, and FXI to surfaces was assessed with western blots and chromogenic assays.
Results:
Using equivalent surface amounts, Ti and TiN were comparable with kaolin and superior to silica, for inducing coagulation and FXII autoactivation. Similar to many inducers of contact activation, Ti and TiN are negatively charged; however, their effects on FXII are not neutralized by the polycation polybrene. Antibodies to FXII, prekallikrein, or FXI or coating Ti with poly-L-arginine blocked Ti-induced coagulation. An antibody to FXII reduced FXII and PK binding to Ti, kallikrein generation, and HK cleavage.
Conclusion:
Titanium compounds induce contact activation with a potency comparable with that of kaolin. Binding of FXII with Ti shares some features with FXII binding to soluble polyanions but may have unique features. Inhibitors targeting FXII or FXI may be useful in mitigating Ti-induced contact activation in patients with titanium-based implants that are exposed to blood.
Keywords: contact activation, factor XII, factor XI, prekallikrein, titanium
1 ∣. INTRODUCTION
The transition metal titanium (Ti) has well-established roles in manufacturing medical devices and implants because of its strength, flexibility, and resistance to corrosion [1,2]. Ti-based materials are generally considered “hemocompatible” because they have relatively limited capacities to trigger blood host-defense responses [3,4]. However, a body of evidence indicates that Ti promotes blood coagulation [5-9]. Clot formation is likely beneficial for osseous integration of implants used in orthopedic, dental, and reconstructive procedures [2-10]. However, it may complicate the use of devices that come into contact with flowing blood. Left ventricular assist devices (LVADs) are an important treatment option for patients with advanced heart failure, either as a bridge to heart transplantation or as destination therapy [11,12]. Components of LVADs that make contact with blood are composed primarily of Ti, Ti alloys, or the ceramic titanium nitride (TiN). Despite the recent changes in LVAD design that reduce thromboembolic events, embolic stroke remains a significant problem [11-14], and most patients with LVADs are treated with warfarin and an antiplatelet agent to reduce the incidence of this complication [15,16]. The inevitable increase in bleeding accompanying anticoagulation and antiplatelet therapy affects up to 50% of patients with LVADs [17-20].
When blood comes into contact with many artificial surfaces, particularly those carrying a negative charge, the plasma zymogens factor XII (FXII) and prekallikrein (PK) bind to the surface and undergo reciprocal conversion to the proteases FXIIa and plasma kallikrein (PKa) [21,22]. The cofactor high-molecular-weight-kininogen (HK) facilitates PK surface binding. PKa cleaves HK, releasing the vasoactive peptide bradykinin, whereas FXIIa promotes blood coagulation by activating factor XI (FXI). Collectively, these reactions are referred to as contact activation. Contact activation contributes to thrombus formation and the need for anticoagulation, in procedures in which blood is exposed to surfaces of medical devices, including cardiopulmonary bypass [23], extracorporeal membrane oxygenation [24,25], and renal dialysis [26]. Several groups reported that Ti induces clotting in a FXII-dependent manner [6,7,27,28]. Here, we compare Ti and TiN with known contact activators for their capacities to induce plasma coagulation and test inhibitors of contact proteases for their abilities to block Ti-initiated clotting.
2 ∣. METHODS
2.1 ∣. Materials
Pooled normal plasma (PNP), Precision BioLogic (Dartmouth, Nova Scotia, Canada). FXII-deficient and PK-deficient plasmas, George King Biomed. Purified human plasma FXII, FXIIa, PK, PKa, and HK, Enzyme Research Laboratory. Plasma-derived FXI, Hematologic Technologies) S-2302 (H-D-prolyl-L-phenylalanyl-L-arginine-p-nitroaniline), DiaPharma. PTT-A micronized silica reagent, Diagnostica Stago. Bivalirudin (Hospira). Hexadimethrine bromide (polybrene) and poly-L-arginine (Sigma-Aldrich). The kallikrein inhibitor KV999272 was previously described [29,30].
2.2 ∣. Nanopowders and foils
Ti (avg. diameter 70 nm, spherical, 20 m2/g), TiN (80 nm, cubic, 30-50 m2/g), and aluminum (Al, 70 nm, spherical, 15-30 m2/g) nanopowders were from US Research Nanomaterials, Inc. Silica (SiO2) nanospheres (NanoXactTM, 100 nm, spherical, 26.8 m2/g) were from nano-Composix Inc. Kaolin was from Acros Organics. Kaolin is a clay with morphology and composition that vary by source. Our calculations, based on study of Pienkoß et al. [31], determined a kaolin surface area of 20 m2/g. To prepare coated Ti, nanopowder (20 mg/mL) was incubated with poly-L-arginine hydrochloride (10 mg/mL, molecular mass 5000-15 000 Daltons) in deionized water for 30 minutes at 37 °C. After incubation, suspensions underwent centrifugation at 14 000 G. Pellets were washed 5 times with 1 mL phosphate buffered saline (PBS), then suspended in PBS at 20 mg/mL and used immediately. Ti foil (0.89 mm thick, 99.7% purity, AlfaAesar) and Al foil (0.018 mm thick, Fisher) were cut into pieces (1 cm2 surface areas) and were polished. Foils were cleaned with 100% ethanol, deionized water, and PBS. Ti foil was incubated with poly-L-arginine in deionized water (1 mg/mL) for 30 minutes at 37 °C, then washed 5 times with PBS and used immediately.
2.3 ∣. Antibodies
Monoclonal IgGs against human FXI (O1A6) or FXII (5C12) [32,33] and biotinylated IgG 14E11 against FXI [34] were reported. IgGs 559C-X181-D06 (D06) and 559A-M202-H03 (H03) against the catalytic active sites of human FXIIa and PKa, respectively, were isolated from a human antibody phage display library, as described [35,36]. Horseradish peroxidase (HRP)-conjugated goat polyclonal antibodies to human FXII and PK were from Affinity Biologicals. Goat anti-human-HK polyclonal IgG was from Nordic-MUbio.
2.4 ∣. Recombinant protein
Complementary DNAs (cDNAs) encoding human FXII with the epidermal growth factor 1 domain (EGF1) replaced with EGF1 from pro-hepatocyte growth factor activator (Pro-HGFA) was expressed in HEK293 cells and purified by IgG chromatography [37]. Purified protein was stored in 4 mM acetate, 150 mM NaCl, pH5.3.
2.5 ∣. Clotting assays—LVAD surfaces
An explanted third-generation centrifugal flow ventricular assist device (HVAD, HeartWare, Framingham, MA) from a patient who had undergone heart transplantation was sequentially incubated with proteinase K and 1 M NaOH to remove surface-bound proteins, followed by thorough rinsing with PBS. PNP (35 μL) was gently distributed over ~2cm2 of Ti or TiN surfaces of the LVAD and incubated for 10 minutes at RT. Control plasma was incubated in a PEG-20 000-coated polypropylene tube. After incubation, plasma was transferred to a cuvette with 70 μL of CaCl2 (9.4 mM), and time to clot formation was measured on an ST-4 Analyzer (Diagnostica Stago).
2.6 ∣. Clotting assays—nanopowders
Ti, TiN, Si, or Al nanopowders or kaolin was suspended in PBS containing 100 μg/mL phosphatidylcholine:phosphatidylserine vesicles (7:3 ratio, Avanti) to achieve a surface area of 20 m2·L−1. Plasma (35 μL) was mixed with an equal volume of nanopowder suspension or PTT-A reagent and incubated for 5 minutes at 37 °C. In total, 25 mM CaCl2 (35 μL) was added, and time to clot formation was measured on the ST-4 Analyzer. The results are presented as means ± 1 SD. Pairs of groups were compared with a Student t-test. Comparisons of more than 2 groups were performed with ANOVA. p < .05 is considered significant.
2.7 ∣. Clotting assays—foils
Blood from healthy donors was collected into bivalirudin (3 μM final concentration), and plasma was prepared by centrifugation at 2000 g for 15 minutes. Plasma (300 μL) with or without 800 nM IgG 5C12 was placed in PEG-coated polypropylene tubes and incubated at 37 °C with one cm2 Ti foil or Ti foil coated with poly-L-arginine. Samples were inspected every minute, and time to clot formation recorded.
2.8 ∣. PKa generation in plasma
Ti and Al foils were incubated in PBS for 2 hours to allow natural oxidation to occur. PNP (5 μL) was mixed with an equal volume of PBS containing antibodies, applied to ~2 cm2 of foil surfaces, and incubated in a 37 °C humidified chamber for 5 minutes. Fifty microliters of 400 μM S2302 was added and incubation continued for 5 minutes. Reactions were stopped with 20% citric acid (final concentration), and the OD at 405 nm was measured.
2.9 ∣. FXII activation—chromogenic assays
FXII autoactivation was studied in 96-well polypropylene plates coated with PEG-20 000.
2.9.1 ∣. Continuous assay
FXII (100 nM) was incubated at 37 °C in PBS containing 200 μM S-2302 and nanopowders or kaolin (surface area 1 m2·L−1). Changes in OD 405 nm were monitored on a spectrophotometer.
2.9.2 ∣. Discontinuous assays
FXII (375 nM) was incubated in PBS at 37 °C in PEG-20 000-coated polypropylene tubes. Varying amounts of nanopowders in PBS were added. At various times, 20 μL samples were removed into 384 well plates containing S-2302 (final concentration 200 μM). Rates of change at OD 405 nm were measured, and FXIIa generated was derived from a standard curve constructed with pure FXIIa.
2.10 ∣. FXII activation—SDS-PAGE
Reactions were conducted in PEG-20 000-coated polypropylene tubes. FXII or FXII-EGF1 (30 ng/μL) in PBS were incubated at 37 °C with nanopowders, kaolin, or Ti nanopowder treated with polyarginine (20 m2·L−1). At various times, aliquots were removed into SDS-sample buffer, size fractionated by reducing SDS-PAGE and stained with Coomassie Blue.
2.11 ∣. Binding studies
PNP (10 μL) was mixed with Ti, TiN, or Al nanopowders (total surface area ~20 cm2) in PEG-20 000-coated polypropylene tubes. Mixtures were incubated on ice for 1 minute or at 37 °C for various times. After incubation, samples underwent centrifugation for 1 minute, 14 000g. Supernatants were mixed with SDS-sample buffer. Pellets were washed 3 times with PBS and then mixed with SDS-sample buffer. Samples were size fractionated by non-reducing SDS-PAGE and transferred to nitrocellulose. Blots were probed with HRP-conjugated polyclonal IgG to human FXII or PK, with detection by chemiluminescence, goat anti-human-HK polyclonal IgG and a fluorescently labeled donkey anti-goat secondary IgG, or biotinylated anti-FXI IgG 14E11 and HRP-conjugated streptavidin.
3 ∣. RESULTS
3.1 ∣. Plasma clotting on LVAD surfaces
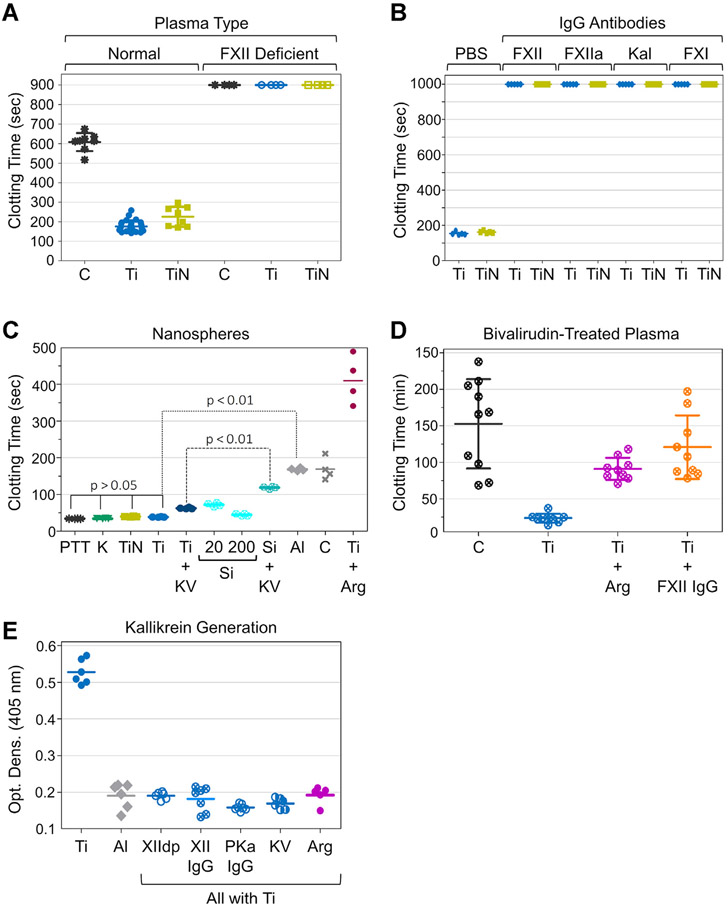
Metallic titanium (Ti) rapidly oxidizes when exposed to air, forming titanium oxide (TiO2). When Ti is referred to in this manuscript, it is reasonable to assume that it is coated with TiO2. The interior of the HeartWare LVAD exposes blood to ~ 60 cm2 of Ti and ~ 30 cm2 of TiN. PNP anticoagulated with sodium citrate was exposed to ~ 2 cm2 of Ti or TiN on the interior surfaces of an LVAD for 10 minutes. After recalcification, clotting times were substantially shorter in samples exposed to Ti (207 ± 28 seconds) or TiN (226 ± 51 seconds) than that of those exposed to polypropylene controls (607 ± 47 seconds, Figure 1A). Exposure to Ti or TiN did not shorten clotting times of FXII-deficient plasma (Figure 1A). IgGs that inhibit FXII (5C12), FXIIa (D06), PKa (H03), or FXI (O1A6) delayed clotting in plasma exposed to Ti or TiN (Figure 1B), indicating that these materials promote clotting by inducing contact activation.
FIGURE 1.
Plasma-clotting studies and kallikrein generation. (A) Recalcification clotting time of pooled normal plasma or FXII-deficient plasma incubated for 10 minutes on surfaces of a ventricular assist device (VAD). Abbreviations are Ti, titanium VAD surface; TiN, titanium nitride VAD surface; and C, polypropylene control. (B) Recalcification clotting times of normal plasma incubated for 5 minutes with Ti or TiN nanopowder (surface area 20 m2·L−1) in PBS with or without monoclonal antibodies to FXII (5C12), FXIIa (D06), PKa (H03), or FXI (O1A6). (C) Clotting times obtained with PTT-reagents (PBS suspension of nanoparticles and phospholipids) containing 20 m2·L−1 kaolin (K), titanium nanoparticles (Ti), titanium nitride nanoparticles (TiN), aluminum nanoparticles (Al), poly-L-arginine-coated titanium nanoparticles (Ti+Arg) or 20 m2·L−1 or 200 m2·L−1 silica nanospheres (Si) compared with micronized silica PTT-A reagent (PTT, ~500 m2·L−1) with or without the small-molecular PKa inhibitor KV999272 (KV). Control (C) is PBS with phospholipids. Experiments in panels A through C where run a minimum of 4 times. (D) Spontaneous or surface-induced clotting of bivalirudin-stabilized (3 μM) normal donor plasma (3 different donors, each run at least 3 times). Pieces of Ti foil (Ti) or poly-L-arginine-coated Ti foil (Ti+Arg) were placed into 300 μL of plasma or plasma containing 800 nM anti-FXIIa IgG 5C12 (Ti+FXII IgG). (E) PKa generation. Normal plasma (5 μL) was incubated on Ti foil (Ti), aluminum foil (Al), or poly-L-arginine-coated Ti foil (Ti+Arg), and PKa generation was determined by chromogenic substrate assay. In some reactions with titanium foil, anti-FXII IgG5C12(XII IgG),PKa IgG H03(PKa IgG), or PKa inhibitor KV999272 (KV) were included. XIIdp indicates a sample containing FXII-deficient plasma rather than normal plasma. Each reaction was run at least 5 times. For all panels, long horizontal bars represent means, and short horizontal bars ± 1 SD.
3.2 ∣. Plasma clotting with Ti nanopowders
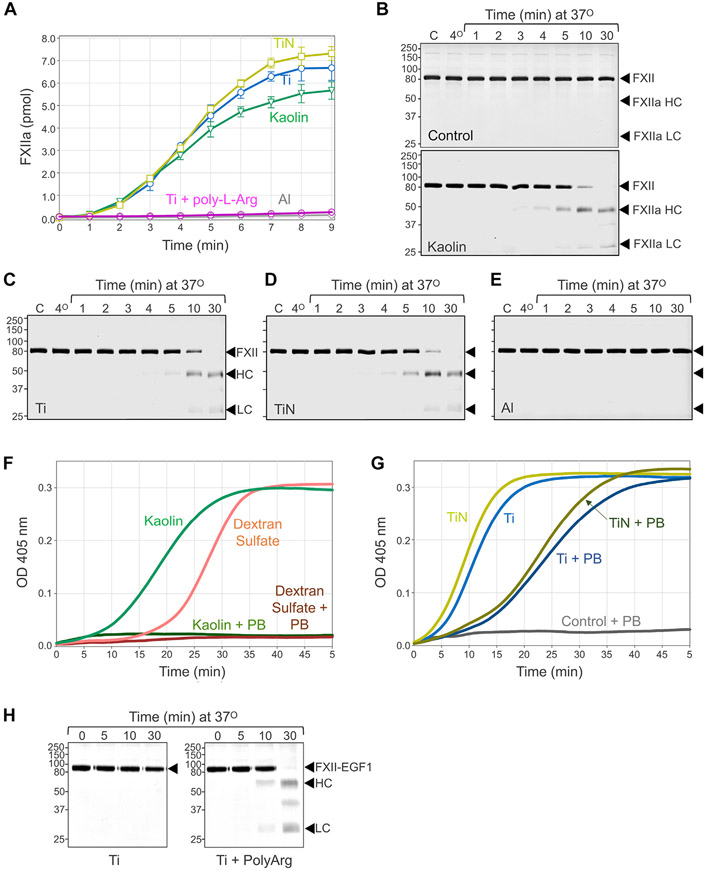
As with many substances that induce contact activation, Ti (TiO2) and TiN have negative surface charges at physiologic pH [38,39]. To investigate their pro-coagulant properties, we used metal nanopowders that allowed us to control the surface area exposed to plasma. This facilitated comparisons with known initiators of contact activation such as kaolin and silica, and with Al (actually Al2O3), which has a positive charge at pH7.4 [40]. Materials were suspended in buffer containing phospholipid vesicles to create mixtures similar to commercial micronized silica reagents such as PTT-A used in activated partial thromboplastin time (aPTT) assays in clinical laboratories. aPTTs generated with 20 m2·L−1 Ti (36.6 ± 2.3 seconds) or TiN (38.3 ± 1.8 seconds) were not significantly different (p > .1) from those obtained with 20 m2·L−1 kaolin (37.7 ± 3.4 seconds) or PTT-A (37.2 ± 4.1 seconds). The surface area of silica in the PTT-A reagent is ~500 m2·L−1. SiO2 nanospheres of comparable size with the Ti and TiN nanospheres were less potent than micronized silica, with clotting times of 72.1 ± 5.1 seconds and 44.7 ± 3.3 seconds at surface areas of 20 m2·L−1 and 200 m2·L−1 (p < .05, Figure 1C), respectively. aPTTs with 20 m2·L−1 Al nanopowder (175.2 ± 9.5 seconds) were similar to those observed in the absence of a surface (179.6 ± 29.9 seconds, Figure 1C) and did not change with increasing surface area (data not shown). The differences between clotting times measured with Ti or TiN were significantly different from those with Al or control (p < .05).
During contact activation, FXII and PK reciprocally convert to FXIIa and PKa. However, the importance of PK activation varies with surface type. Supplementing PNP with a PKa inhibitor (KV999272) prolonged the aPTT to a greater extent with SiO2 nanospheres (119 ± 3 seconds) than Ti nanospheres (62 ± 2 seconds, Figure 1C), suggesting that Ti is a more potent inducer of FXII autoactivation than silica. Alternatively, PKa may form more rapidly on silica and make a greater contribution to clot formation than with Ti.
Poly-L-arginine is a synthetic peptide containing 5 or more arginine residues. It forms strong interactions with negatively charged oxygen groups on cell membranes and metal oxide film coatings through positively charged guanidinium groups on the arginine side chains [41]. When Ti nanoparticles are coated with poly-L-arginine, the capacity to induce contact activation is lost (Figure 1C). Indeed, clotting times with poly-L-Arg-coated Ti were significantly longer (412 ± 65 seconds, p < .05) than those in the absence of a surface, suggesting that poly-L-arginine interferes with coagulation reactions distinct from contact activation. Adding poly-L-arginine directly to plasma inhibits clotting induced by micronized silica (Supplementary Figure S1A). However, poly-L-arginine-coated Ti does not interfere with silica-induced clotting, indicating that surface-bound poly-L-arginine only affects reactions on the Ti surface (Supplementary Figure S1B).
3.3 ∣. Plasma clotting on Ti foil
Collecting plasma into sodium citrate produces an anticoagulant effect by reducing free Ca2+. However, it also alters concentrations of divalent cations such as Mg2+ and Zn2+ that may affect the performance of the contact system [42,43]. Plasma was prepared from blood collected into the reversible thrombin inhibitor bivalirudin to avoid the chelating effect of citrate. Bivalirudin-treated plasma incubated at 37 °C in polypropylene tubes took 152.7 ± 61.2 minutes to clot (Figure 1D). Adding Ti foil shortened clotting times to 23.7 ± 6.2 minutes (p < .01). Coating the Ti foil with poly-L-arginine (91.1 ± 15.2 minutes) or adding the anti-FXII IgG 5C12 to plasma (120.8 ± 43.5 minutes) substantially prolonged clotting times relative to Ti alone (p < .01), consistent with a role for contact activation in clotting on Ti foil.
3.4 ∣. PKa generation on Ti foil
Incubating PNP with Ti foil generates activity that cleaves the chromogenic substrate S-2302 (Figure 1E). This does not occur with Al foil. The specific PKa inhibitor KV999272 blocks amidolytic activity in plasma incubated with Ti foil, indicating that PKa is the main activity source. Activity does not form in PNP containing anti-FXII IgG 5C12 or anti-PKa IgG H03 or if PNP is replaced with FXII-deficient plasma. Coating Ti with poly-L-arginine also prevented development of activity.
3.5 ∣. Contact factor binding to Ti and TiN
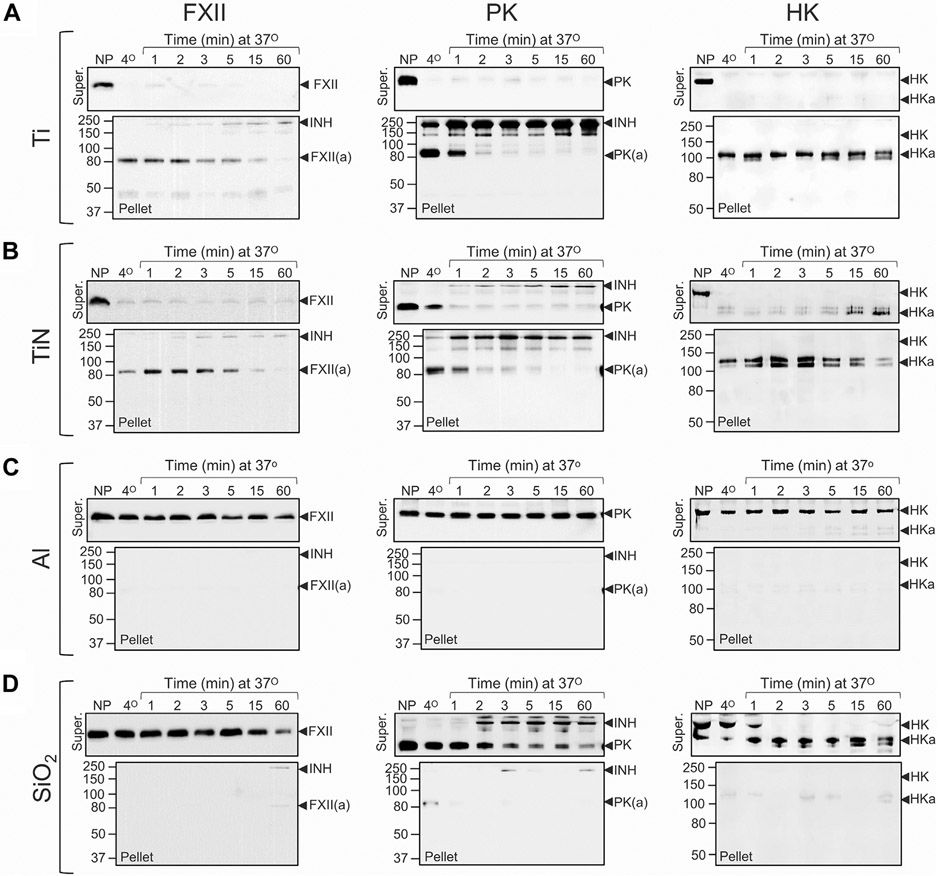
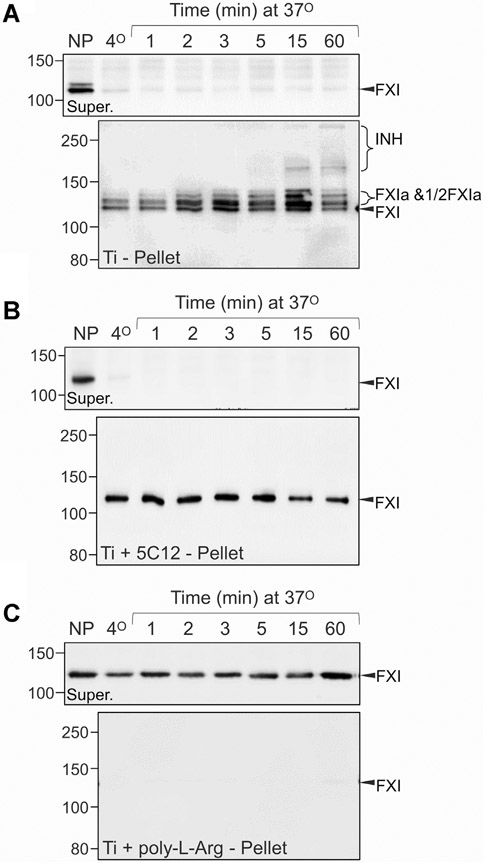
FXII, PK, and HK in plasma bind rapidly to Ti or TiN nanopowder (20 cm2 per reaction) at 37 °C, with evidence of rapid PK activation and HK cleavage (Figure 2A, B) on Western blots. Bands at ~ 250 kDa on PK blots (Figure 2A, B) likely represent PKa in covalent complexes with plasma protease inhibitors. On HK blots, the shift in migration is characteristic of HK cleavage by PKa [30]. Consistent with published data [44], adsorption also occurs rapidly (within 1 minute) at 4°C. There is little evidence of adsorption, PK activation, or HK cleavage on Al nanopowder (Figure 2C). SiO2 nanospheres of particle size comparable with Ti and TiN nanopowders bound contact factors relatively poorly, even at surface areas 10-fold higher (200 cm2) than that for Ti and TiN (Figure 2D). PKa generation and HK cleavage does occur; however, products are in the supernatant rather than attached to silica, indicating lower affinities for silica than Ti. Figure 3A illustrates reactions likely to be occurring on the Ti surface.
FIGURE 2.
Binding and activation of contact proteins on nanopowders. Binding and activation of factor XII (FXII), prekallikrein (PK), and high-molecular-weight kininogen (HK) in normal plasma (10 μL) on nanopowders of (A) titanium (Ti, ~20 cm2), (B) titanium nitride (TiN, ~20 cm2), (C) aluminum (Al, ~20 cm2), or on (D) silica (SiO2) nanospheres (~200 cm2). Samples of normal plasma (10 μL) were incubated at 4 °C for 1 min or at 37 °C for 1, 2, 3, 5, 15, or 60 minutes. Untreated normal plasma was used as a control. After incubation, each sample underwent centrifugation for 1 minute (14 000 g, 4 °C). Supernatant plasmas were mixed with non-reducing SDS-sample buffer. Pellets were washed 3 times with ice-cold PBS, then mixed with SDS-sample buffer. Samples were size fractionated by non-reducing SDS-PAGE (10% acrylamide) and transferred to nitrocellulose membranes. Each protein and condition is represented by a pair of blots. The top blot of each pair shows results for plasma supernatants (Super), and the lower blot shows results for eluates from nanopowder pellets (Pellet). FXII or PK blots were probed with horseradish peroxidase–conjugated polyclonal IgG to FXII or PK and detected with chemiluminescence. HK blots were probed with goat anti-human-HK polyclonal antibody, using a fluorescent donkey anti-goat secondary antibodies. Positions of molecular mass standards are shown to the left of each figure. Positions of migration for FXII and FXIIa (FXII(a)), PK and PKa (PK(a)), HK and cleaved HK (HKa), and active protease in complex with plasma inhibitors (INH) are shown to the right of each panel. In all panels, representative blots are shown. For each conditions, experiments were run at least 3 times, except for experiments with aluminum (Panel C), which were run twice.
FIGURE 3.
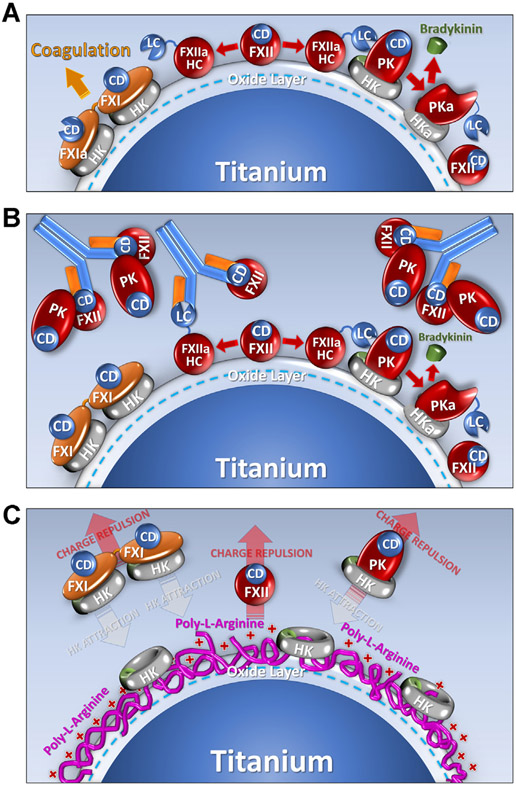
Contact activation on titanium. Shown are diagrams of proposed mechanisms by which a titanium (Ti) surface supports contact activation, and the effects of the anti-FXII antibody 5C12, or poly-L-arginine surface coating on the process. The Ti surface is assumed to be covered with a layer of TiO2 that carries a negative surface charge at physiologic pH. (A). Contact Activation. When plasma is exposed to Ti the protease zymogens factor XII (FXII), factor XI (FXI), and prekallikrein (PK) bind to the Ti. For PK and FXII, surface binding is enhanced by the cofactor high-molecular-weight-kininogen (HK). The abbreviation CD indicates the catalytic domain of the zymogens, and HC (heavy chain) and LC (light chain) are the 2 parts of the activated proteases FXIIa, FXIa, and plasma kallikrein (PKa). During contact activation, FXII undergoes autoactivation to FXIIa, which converts PK to PKa. PKa, in turn, reciprocally activates FXII to FXIIa. FXIIa converts FXI to FXIa to promote thrombin generation and blood coagulation. PKa cleaves HK to release the vasoactive peptide bradykinin. (B). Effect of IgG 5C12. 5C12 binds to the catalytic domain of FXII and FXIIa blocking catalytic activity, which limits downstream activation of FXI and PK, blocking clotting and bradykinin formation. 5C12 also seems to displace FXII from the Ti surface. Interestingly, PK also is displaced from the surface by 5C12, suggesting that some PK binds to the Ti surface through an interaction with FXII. (C). Effect of poly-L-arginine coating of Ti. Poly-L-arginine appears to avidly bind to the Ti surface and prevents access to binding sites for FXII, PK, and FXI. It may also repel FXII, FXI, and PK from the surface. Interestingly, HK can bind to poly-L-arginine coated Ti, but it seems to not be associated with PK or FXI in this situation. Nearly, all FXI and most PK circulates in plasma in a complex with HK. These complexes apparently do not bind well to poly-L-arginine (perhaps due to repulsion of FXI and PK).
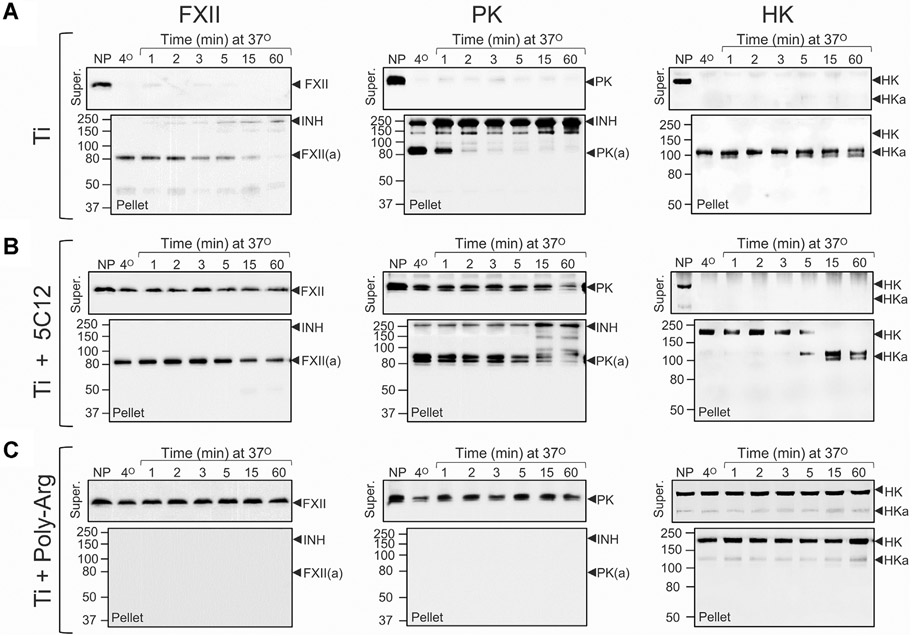
Contact activation is initiated by autocatalytic conversion of FXII to FXIIa [21,22]. IgG 5C12 binds to the FXII and FXIIa catalytic domains, inhibiting protease activity [33]. 5C12 not only inhibited PK activation and HK cleavage in plasma exposed to Ti but seemed to reduce FXII and PK surface binding (compare Figure 4A, B, illustrated in Figure 3B). PK and FXII likely interact in solution [36,37]. Disrupting FXII surface binding with 5C12 may result in some PK dissociating from the surface because it is bound to FXII-5C12 complexes (Figure 3B). With time, some PK activation and HK cleavage occur even with saturating 5C12, perhaps due to traces of free FXII in the system or a FXII-independent mechanism for PK activation. Coating Ti with poly-L-arginine prevented FXII and PK binding to Ti (Figure 4C, illustrated in Figure 3C). HK binding was reduced, but not completely blocked. Interestingly, in the absence of PK, HK binds avidly to poly-L-arginine coated Ti (Supplementary Figure S2). Addition of PK results in some HK dissociating from the surface. At physiologic pH, a charge-based repulsion between PK and poly-L-arginine may occur. As HK is associated with PK, it may be dissociated from the poly-L-arginine-coated surface along with PK (Figure 3C). Binding aside, there is little evidence for PK activation or HK cleavage on poly-L-arginine-coated Ti.
FIGURE 4.
Binding and activation of contact proteins on titanium. Binding and activation of factor XII (FXII), prekallikrein (PK), and high-molecular-weight kininogen (HK) in normal plasma (10 μL) on titanium nanopowder (20 cm2). (A) Untreated normal plasma with titanium nanopowder (This panel contains the same images as in Figure 2A), (B) Normal plasma containing 800 nM anti-FXII IgG 5C12 with titanium nanopowder, or (C) untreated normal plasma with poly-L-arginine-coated titanium nanopowder. Samples were processed as described in the legend for Figure 2. Each protein and condition is represented by a pair of blots. The top blot of each pair shows results for plasma supernatants (Super) and the lower blot shows results for eluates from nanopowder pellets (Pellet). Positions of molecular mass standards are shown to the left of each figure. Positions of migration for FXII and FXIIa (FXII(a), PK and PKa (PK(a), HK and cleaved HK (HKa), and active protease in complex with plasma inhibitors (INH) are shown to the right of each panel. In all panels, representative blots are shown. For each conditions, experiments were run at least 3 times.
FXIIa drives plasma coagulation primarily through surface-dependent conversion of FXI to FXIa [45]. FXI is a homodimeric protein that can have either one or 2 activated subunits when converted to FXIa [45,46]. The activated species migrate slightly differently from FXI, and from each other, on non-reducing SDS-PAGE [46]. When PNP is exposed to Ti nanopowder, a progression from FXI to FXIa with one active subunit, and then FXIa with 2 active subunits, is observed (Figure 5A). Even larger species accumulate with time, likely representing FXIa in complex with plasma protease inhibitors. FXI in PNP treated with 5C12 adsorbs onto Ti, but is not activated, consistent with the importance of surface-bound FXIIa to FXI activation (Figure 5B). Ti coated with poly-L-arginine prevents FXI binding and activation (Figure 5C).
FIGURE 5.
Titanium-induced activation of factor XI in plasma. Binding and activation of factor XI (FXI) in normal plasma (10 μL) to titanium nanopowder. FXI is a homodimeric protein. During activation, zymogen FXI is converted first to a species with one activated subunit which runs slightly above FXI on non-reducing gels (1/2FXIa), and then to a species with both subunits activated that runs above FXI and 1/2FXIa [46]. (A) Binding and activation of FXI in normal plasma (10 μL) on titanium nanopowder (~ 20 cm2). (B) Binding and activation of FXI in normal plasma (10 μL) supplemented with 800 nM anti-FXII IgG 5C12 on titanium nanopowder (~ 20 cm2). (C) Binding and activation of FXI in normal plasma (10 μL) on poly-L-arginine-coated titanium nanopowder (~ 20 cm2). Samples were processed as described in the legend for Figure 2. Each protein and condition is represented by a pair of blots. The top blot of each pair shows results for plasma supernatants (Super.) and the lower blot shows results for eluates from nanopowder pellets (Pellet). For panels B, C, and D, positions of molecular mass standards are shown to the left of each figure. Positions of migration for FXI, FXIa (FXIa and 1/2FXIa), and FXIa in complex with plasma protease inhibitors (INH) are indicated on the right of each panel. In all panels, representative blots are shown. For each conditions, experiments were run twice.
3.6 ∣. FXII autoactivation on Ti compounds
Surface binding exposes the FXII activation cleavage site, facilitating autoactivation [37,47,48]. Ti and TiN nanopowders are comparable with kaolin in their capacity to induce FXII autoactivation (Figure 6A-D), whereas Al nanopowder does not support the reaction (Figure 6A, E). Coating Ti with poly-L-arginine reduces FXII autoactivation (Figure 6A). Although Ti and TiN are negatively charged, FXII binding may involve more than simple charge interactions. The polycation hexadimethrine bromide (polybrene) disrupts charge-based protein-surface interactions. Polybrene prevents FXII autoactivation on kaolin and the polyanion dextran sulfate probably by displacing FXII from the polyanion (Figure 6F). However, polybrene only has a modest effect on FXII autoactivation with Ti or TiN nanopowders (Figure 6G), raising the possibility that the FXII interactions with Ti and TiN is not entirely charge based.
FIGURE 6.
Factor XII autoactivation. (A) FXII (30 μg/mL) was incubated in PBS with ~ 5 m2·L−1 kaolin, or with nanopowders of titanium (Ti), titanium nitride (TiN), titanium coated with poly-L-arginine (Ti +poly-L-Arg), or aluminum (Al). At the indicated times, samples were removed, and FXIIa generation determine with a chromogenic substrate assay. Shown are means for duplicate runs± 1 SD (B-E) Reducing Coomassie Blue-stained SDS-polyacrylamide gels showing time courses of human FXII (20 μg/mL) incubated in the (B) absence (control) or presence of (~ 20 m2·L−1) kaolin, (C) titanium (Ti), (D) titanium nitride (TiN), or (E) aluminum (Al). In panels B through E, representative stained gels are shown. For each conditions, experiments were run twice. At indicated times, samples were removed into reducing SDS-sample buffer and size fractionated by SDS-PAGE. For all panels, positions of molecular mass standards in kiloDaltons are shown on the left, and positions of standards for FXII, and the heavy chain (HC) and light chain (LC) or FXIIa are on the right. (F) FXII (100 nM) was incubated in PBS containing 200 μM S-2302 in the presence of kaolin (~ 1 m2·L−1) or dextran sulfate (10 μg/mL) with or without 150 μg/mL Polybrene (PB). Change in optical density at 405 nm was continuously monitored on a spectrophotometer. (G) As in panel F, except that FXII was incubated with ~ 1 m2·L−1 titanium (Ti) or titanium nitride (TiN) with or without 150 μg/mL Polybrene (PB). For panels F and G, representative tracings are shown. Each experiment was run at least 3 times. (H) FXII-EGF1 (30 μg/mL) in PBS was incubated with titanium nanopowder or poly-L-arginine-coated titanium nanopowder (~20 m2·L−1). At indicated times, samples were removed into reducing SDS-sample buffer and size fractionated by SDS-PAGE. Positions of molecular mass standards in kiloDaltons are shown on the left, and positions of standards for FXII-EGF1, and the heavy chain (HC) and light chain (LC) or FXIIa-EGF1 are on the right.
FXII is a homolog of Pro-HGFA [37,49]. Pro-HGFA is structurally similar to FXII but does not autoactivate on anionic surfaces. We showed that FXII in which the EGF1 domain is replaced with the Pro-HGFA EGF1 domain (FXII-EGF1) autoactivates poorly on polyphosphate and dextran sulfate [37]. The FXII EGF1 domain carries a positive charge, consistent with a role in polyanion-binding. Pro-HGFA EGF1 is negatively charged and would not be expected to bind polyanions. However, FXII-EGF1 autoactivates on a cationic polymer, polyethyleneimine, probably because the negatively charged Pro-HGFA EGF1 domain binds to it [37]. Consistent with the hypothesis that FXII binds Ti at least partly through charge-based interactions requiring EGF1, FXII-EGF1 did not autoactivate on Ti (Figure 6H, left panel). Consistent with our previous experiment with polyethyleneimine, poly-L-arginine-coated Ti (which also has a positive surface charge) supported FXII-EGF1 autoactivation (Figure 6H, right panel).
3.7 ∣. Evidence for a template mechanism
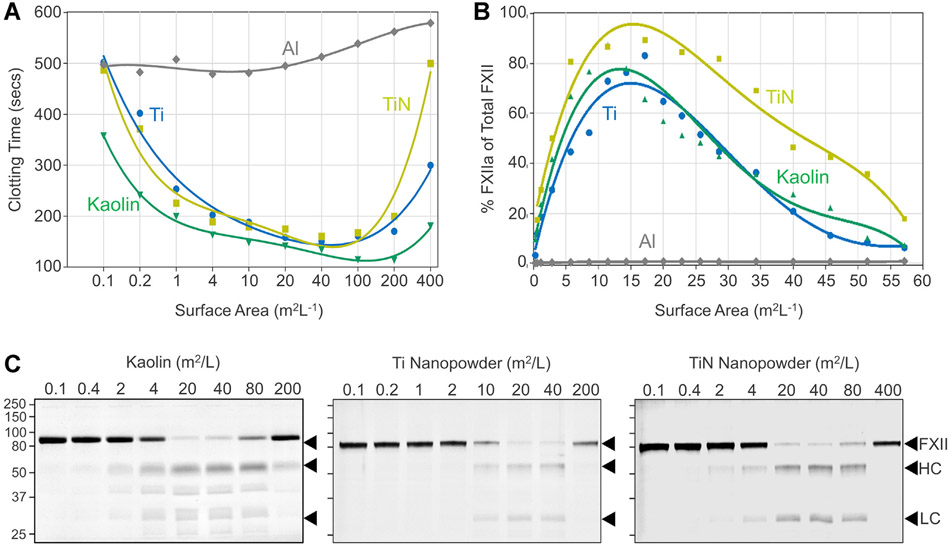
Surfaces enhance FXII autoactivation and reciprocal activation of FXII and PK by 2 mechanisms. First, the surface-bound protein adopts an “open” conformation that facilitates cleavage at the activation site [37,47,48]. Second, the proteins are brought into proximity with each other when surface bound, a “template” mechanism [47]. Reactions involving template mechanisms display a bell-shaped dependence on surface concentration. Induction of blood coagulation (Figure 7A) and FXII activation (Figure 7B, C) by kaolin, Ti, or TiN, all show this type of dependence.
FIGURE 7.
FXII autoactivation—surface-dependency. (A) Recalcification clotting times of pooled normal plasma incubated for 5 minutes with different amounts of kaolin or titanium (Ti), titanium nitride (TiN), or aluminum (Al) nanopowders. Each point represents a single measurement. (B) FXII (30 μg/mL) was incubated in PBS different amounts of the insoluble materials used in panel A. After 10 minutes incubation, percent of total FXII converted to FXIIa was determined by chromogenic assay. For panels A and B, each point represents a single measurement. (C) FXII (30 μg/mL) in PBS was incubated with increasing amounts of kaolin, Ti, or TiN nanopowders. After 60 minutes incubation samples were removed into reducing SDS-sample buffer and size fractionated by SDS-PAGE. Positions of molecular mass standards in kiloDaltons are shown on the left, and positions of standards for FXII, and the heavy chain (HC) and light chain (LC) or FXIIa are on the right. Shown are representative blots from 2 separate experiments.
DISCUSSION
In the 1960s, Leo Vroman and his colleagues described a competitive process for blood plasma protein adsorption onto surfaces [44,50,51]. In the Vroman Effect, the abundant proteins albumin and immunoglobulin initially coat the surface. With time, they are replaced by proteins that bind with higher affinity, including fibrinogen, FXII, and HK, that support platelet adhesion and thrombin generation [50,51]. It is now apparent that contact protein assembly on many surfaces is more rapid than initially described by the Vroman Effect [51], with FXII, PK, and HK binding in significant amounts within seconds to minutes of surface exposure [50,51]. This binding has generally been considered non-specific, but the efficiency of assembly of the contact phase is consistent with a coordinated response to a foreign substance.
Ti (TiO2 after exposure to air) [1], Ti alloys such as nitinol (titanium-nickel) [28] and titanium-aluminum-vanadium [52], and ceramics such as TiN [13,53] are used in manufacturing medical implants, including LVADs and heart valves that are exposed to flowing blood for prolonged periods. Internal surfaces of the widely used HeartMate 3 continuous flow device (Abbott Inc, Abbott Parkway, IL) are made of bare Ti or Ti microspheres sintered onto Ti. A predecessor, the HVAD (HeartWare, Framingham, MA), has Ti and TiN components. Although most studies of blood compatibility focus on albumin and fibrinogen absorption, platelet retention, and hemolysis [20-23], there are reports indicating that Ti induces contact activation [5,27]. The work shown here supports the conclusion that FXII, PK, HK, and FXI rapidly adsorb onto Ti and TiN, promoting clot formation, kallikrein generation, and HK cleavage. The capacities of Ti and TiN to support contact activation are comparable with the classic contact activator kaolin and greater than that of silica. Interestingly, kaolin (an aluminum silicate clay) often contains transition metals, including Ti [54,55], that may contribute to its pro-coagulant properties.
FXII triggers contact activation and surface-initiated coagulation [47,56]. Previously, we showed that the FXII EGF1 domain is required for interactions with soluble polyanions such as polyphosphate, dextran sulfate, and heparin [37]. EGF1 is also involved in FXII autoactivation on Ti, consistent with a negative charge on the metal oxide surface. However, the interaction of FXII with Ti is only slightly affected by the polycation polybrene, raising the possibility that FXII binding to Ti is much tighter than to soluble polycations or perhaps involves a complex mechanism that is not entirely charge based. In support of the notion that negative surface charge alone may not be the sole determinant of FXII binding to Ti, Mutch et al. [57] showed that several transition metals in their divalent cationic forms (Ni2+, Cu2+, Co2+, and Zn2+) support FXII activation. Precoating Ti with poly-L-arginine to prevent FXII binding to Ti may, therefore, be partly due to blockade of noncharge-based binding interactions.
Patients with LVADs are at substantial risk for thromboembolism, probably as a direct consequence of turbulent flow and blood exposure to foreign surfaces [58]. The HeartMate 3, which utilizes a levitated rotor to generate centrifugal flow, is the most recent generation of LVAD. Its larger flow channels (to reduce sheer-stress) [59] and pulsatile flow (to reduce stasis) [59,60] may lower the risk of LVAD thrombosis [61]. However, despite claims of improved hemocompatibility, the incidence of thromboembolic stroke remains high with the HeartMate 3 [15], and non-surgical bleeding (NSB), particularly within the gastrointestinal tract, is a significant problem [61]. To reduce thrombotic complications, current standard of care includes chronic systemic anticoagulation (usually with warfarin) in combination with an antiplatelet agent (aspirin or a P2Y12 inhibitor), which undoubtedly contributes to NSB. LVAD patients also frequently develop an acquired von Willebrand syndrome [62] and arteriovenous malformations (AVMs) [63] that compound bleeding problems. Tabit et al. [63] observed that LVAD patients have relatively high plasma levels of angiopoietin-2, an angiogenesis factor that contributes to AVM formation. Patients with higher angiopoietin-2 levels were at higher risk of NSB. They also observed increased levels of thrombin (despite warfarin therapy), FXIIa, and FXIa in plasma from LVAD patients [63]. As thrombin releases angiopoietin-2 from endothelial cell Weibel-Palade bodies [64,65], this work raises the possibility that LVAD-initiated contact activation contributes to AVM formation and thromboembolism.
Although the contributions of Ti to LVAD-associated thromboembolism and AVMs are unknown, it seems likely that Ti-based device components support contact activation and thrombin generation. As suggested by experiments with poly-L-arginine, altering Ti surfaces to prevent direct exposure of metal to flowing blood, or to repel contact proteins, could reduce contact activation. This approach is being actively pursued [11,66]. In the past, some LVAD components were coated with TiN, but our results indicate that this is unlikely to reduce contact activation. Any coating for an LVAD component would need to be sufficiently durable to stand up to months or years of use. Pharmacologic blockade of FXIIa and/or PKa shows great promise for limiting the reciprocal activation of FXII and PK that triggers bouts of bradykinin-induced soft tissue swelling in patients with the genetic disorder hereditary angioedema [67,68]. As reciprocal activation is central to contact activation, such strategies may have a role in limiting contact-induced thromboembolism with Ti. Inhibiting or reducing FXII(a) or PK(a) provides protection from thrombosis in mouse arterial-injury models in which clot formation is probably triggered by contact activation [69,70]. As FXIIa and PKa are not required for hemostasis, inhibitors to these proteases (alone or in combination) might safely be added to current therapeutic regimens without increasing bleeding risk. This, in turn, may reduce the intensity of conventional anticoagulation required to prevent thromboembolism.
Pre-clinical and early clinical data suggest that FXIIa drives thrombus formation by activating FXI [34,71,72]. FXI or FXIa inhibitors may, therefore, be useful for limiting thromboembolism in LVAD patients. Agents targeting FXI(a) have been effective in preventing venous thrombosis after knee replacement surgery with minimal compromise of hemostasis [73-77], consistent with observations that congenital FXI deficiency reduces the incidence of venous thrombosis [78]. FXI-deficient individuals also have a relatively low incidence of ischemic stroke [79], and FXIa inhibitors may be particularly useful for reducing this complication in LVAD patients. Two recent phase 2 secondary stroke prevention trials (The AXIOMATIC-SSP and PACIFIC-SSP trials) examined the effects of FXIa inhibition superimposed on dual antiplatelet therapy in patients with recent ischemic strokes or transient ischemic attacks [80,81]. FXIa inhibition had relatively small effects on bleeding in these trials, suggesting that it may be safely administered to patients on antiplatelet agents. In both studies, secondary analyses suggested that FXIa inhibition may reduce the incidence of recurrent symptomatic stroke.
In summary, Ti is a potent inducer of contact activation. Therapies targeting contact proteases that are currently being developed for other purposes may prove useful in blocking this effect in patients with Ti-based devices, such as LVADs, that are exposed to flowing blood.
Supplementary Material
Essentials.
Intravascular devices made of titanium and its alloys require anticoagulation to prevent thromboembolic complications.
We investigated binding and activation of plasma contact proteins on titanium surfaces and compared them with the well-known contact activators silica and kaolin.
Contact activation on titanium surfaces may contribute significantly to the thrombogenicity of intravascular devices.
Therapies targeting contact proteases and surface coating with positively charged peptides may prove useful in blocking contact activation in patients with titanium-based intravascular devices.
ACKNOWLEDGMENTS
The authors wish to acknowledge generous support from the National Heart, Lung, and Blood Institute of the National Institutes of Health: Awards R35 HL140025 (to D.G.), HL101972 and HL144113 (to O.J.T.M.), and HL126235 (to E.I.T.); and the Ernest W. Goodpasture Chair in Experimental Pathology for Translational Research, Vanderbilt University Medical Center.
Footnotes
DECLARATION OF COMPETING INTEREST STATEMENT
D.G. is a consultant for pharmaceutical companies (Anthos Therapeutics; Aronora, Inc.; Bayer Pharma; Bristol-Myers Squibb; Ionis Pharmaceuticals; Janssen, Pharmaceuticals) with interests in targeting factor XI, factor XII, and prekallikrein for therapeutic purposes. M.W. and E.I.T are employees of Aronora, and they, as well as Oregon Health and Sciences University, may have financial interest in the results of this study. EPF is an employee of KalVista Pharmaceuticals Inc. The other authors have no conflicts to report.
SUPPLEMENTARY MATERIAL
The online version contains supplementary material available at https://doi.org/10.1016/j.jtha.2022.12.014
REFERENCES
- [1].Rack HJ, Qazi JI. Titanium alloys for biomedical applications. Mater Sci Eng C. 2006;26:1269–77. [Google Scholar]
- [2].Sarraf M, Rezvani Ghomi E, Alipour S, Ramakrishna S, Liana Sukiman N. A state-of-the-art review of the fabrication and characteristics of titanium and its alloys for biomedical applications. Biodes Manuf. 2021;5:371–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Prinzing A, Herold U, Berkefeld A, Krane M, Lange R, Voss B. Left ventricular assist devices—current state and perspectives. J Thorac Dis. 2016;8:E660–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kuchinka J, Willems C, Telyshev DV, Groth T. control of blood coagulation by hemocompatible material surfaces—a review. Bioengineering (Basel). 2021;8:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hong J, Andersson J, Nilsson Ekdahl K, Elgue G, Axén N, Larsson R, Nilsson B. Titanium is a highly thrombogenic biomaterial: possible implications for osteogenesis. Thromb Haemost. 1999;82:58–64. [PubMed] [Google Scholar]
- [6].Hong J, Azens A, Ekdahl KN, Granqvist CG, Nilsson B. Material-specific thrombin generation following contact between metal surfaces and whole blood. Biomaterials. 2005;26:1397–14403. [DOI] [PubMed] [Google Scholar]
- [7].Thor A, Rasmusson L, Wennerberg A, Thomsen P, Hirsch JM, Nilsson B, Hong J. The role of whole blood in thrombin generation in contact with various titanium surfaces. Biomaterials. 2007;28:966–74. [DOI] [PubMed] [Google Scholar]
- [8].Walkowiak-Przybyło M, Klimek L, Okrõj W, Jakubowski W, Chwiłka M, Czajka A, Walkowiak B. Adhesion, activation, and aggregation of blood platelets and biofilm formation on the surfaces of titanium alloys Ti6Al4V and Ti6Al7Nb. J Biomed Mater Res A. 2012;100:768–75. [DOI] [PubMed] [Google Scholar]
- [9].Ekstrand-Hammarström B, Hong J, Davoodpour P, Sandholm K, Ekdahl KN, Bucht A, Nilsson B. TiO2 nanoparticles tested in a novel screening whole human blood model of toxicity trigger adverse activation of the kallikrein system at low concentrations. Biomaterials. 2015;51:58–68. [DOI] [PubMed] [Google Scholar]
- [10].Hanawa T. Titanium-tissue interface reaction and its control with surface treatment. Front Bioeng Biotechnol. 2019;7:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Malone G, Abdelsayed G, Bligh F, al Qattan F, Syed S, Varatharajullu P, Msellati A, Mwipatayi D, Azhar M, Malone A, Fatimi SH, Conway C, Hameed A. Advancements in left ventricular assist devices to prevent pump thrombosis and blood coagulopathy. J Anat (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Jefferson HL, Kent WDT, Kelsey Macqueen TM, Miller RJH, Holloway DD, Hassanabad AF. Left ventricular assist devices: A comprehensive review of major clinical trials, devices, and future directions. J Card Surg. 2021;36:1480–91. [DOI] [PubMed] [Google Scholar]
- [13].Dion I, Baquey C, Candelon B, Monties JR. Hemocompatibility of titanium nitride. Int J Artif Organs. 1992;15:617–21. [PubMed] [Google Scholar]
- [14].Ishihara K. Revolutionary advances in 2-methacryloyloxyethyl phosphorylcholine polymers as biomaterials. J Biomed Mater Res A. 2019;107:933–43. [DOI] [PubMed] [Google Scholar]
- [15].Plecash AR, Byrne D, Flexman A, Toma M, Field TS. Stroke in patients with left ventricular assist devices. Cerebrovasc Dis. 2022;51:3–13. [DOI] [PubMed] [Google Scholar]
- [16].Yuan N, Arnaoutakis GJ, George TJ, Allen JG, Ju DG, Schaffer JM, Russell SD, Shah AS, Conte JV. The spectrum of complications following left ventricular assist device placement. J Card Surg. 2012;27:630–8. [DOI] [PubMed] [Google Scholar]
- [17].Eckman PM, John R. Bleeding and thrombosis in patients with continuous-flow ventricular assist devices. Circulation. 2012;125:3038–47. [DOI] [PubMed] [Google Scholar]
- [18].Long B, Robertson J, Koyfman A, Brady W. Left ventricular assist devices and their complications: A review for emergency clinicians. Am J Emerg Med. 2019;37:1562–70. [DOI] [PubMed] [Google Scholar]
- [19].Sage W, Gottiparthy A, Lincoln P, Tsui SSL, Pettit SJ. Improving anticoagulation of patients with an implantable left ventricular assist device. BMJ Open Qual. 2018;7:e000250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].den Exter PL, Beeres SLMA, Eikenboom J, Klok FA, Huisman M. Anticoagulant treatment and bleeding complications in patients with left ventricular assist devices. Expert Rev Cardiovasc Ther. 2020;18:363–72. [DOI] [PubMed] [Google Scholar]
- [21].Schmaier AH. The contact activation and kallikrein/kinin systems: pathophysiologic and physiologic activities. J Thromb Haemost. 2016;14:28–39. [DOI] [PubMed] [Google Scholar]
- [22].Long AT, Kenne E, Jung R, Fuchs TA, Renné T. Contact system revisited: an interface between inflammation, coagulation, and innate immunity. J Thromb Haemost. 2016;14:427–37. [DOI] [PubMed] [Google Scholar]
- [23].Sniecinski RM, Chandler WL. Activation of the hemostatic system during cardiopulmonary bypass. Anesth Analg. 2011;113:1319–33. [DOI] [PubMed] [Google Scholar]
- [24].Larsson M, Rayzman V, Nolte MW, Nickel KF, Björkqvist J, Jämsä A, Hardy MP, Fries M, Schmidbauer S, Hedenqvist P, Broomé M, Pragst I, Dickneite G, Wilson MJ, Nash AD, Panousis C, Renné T. A factor XIIa inhibitory antibody provides thromboprotection in extracorporeal circulation without increasing bleeding risk. Sci Transl Med. 2014;6:222ra17. [DOI] [PubMed] [Google Scholar]
- [25].Wallisch M, Lorentz CU, Lakshmanan HHS, Johnson J, Carris MR, Puy C, Gailani D, Hinds MT, McCarty OJT, Gruber A, Tucker EI. Antibody inhibition of contact factor XII reduces platelet deposition in a model of extracorporeal membrane oxygenator perfusion in nonhuman primates. Res Pract Thromb Haemost. 2020;4:205–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ekdahl KN, Soveri I, Hilborn J, Fellström B, Nilsson B. Cardiovascular disease in haemodialysis: role of the intravascular innate immune system. undefined Nat Rev Nephrol. 2017;13:285–96. [DOI] [PubMed] [Google Scholar]
- [27].Arvidsson S, Askendal A, Tengvall P. Blood plasma contact activation on silicon, titanium and aluminum. Biomaterials. 2007;28:1346–54. [DOI] [PubMed] [Google Scholar]
- [28].Gegenschatz-Schmid K, Buzzi S, Grossmann J, Roschitzki B, Urbanet R, Heuberger R, Glück D, Zucker A, Ehrbar M. Reduced thrombogenicity of surface-treated nitinol implants steered by altered protein adsorption. Acta Biomater. 2022;137:331–45. [DOI] [PubMed] [Google Scholar]
- [29].Clermont A, Murugesan N, Zhou Q, Kita T, Robson PA, Rushbrooke LJ, Evans DM, Aiello LP, Feener EP. Plasma kallikrein mediates vascular endothelial growth factor–induced retinal dysfunction and thickening. Invest Ophthalmol Vis Sci. 2016;57:2390–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Dickeson SK, Kumar S, Sun MF, Mohammed BM, Phillips DR, Whisstock JC, Quek AJ, Feener EP, Law RHP, Gailani D. A mechanism for hereditary angioedema caused by a lysine 311-to-glutamic acid substitution in plasminogen. Blood. 2022;139:2816–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Pienkoß F, Ochoa-Hernández C, Theyssen N, Leitner W. Kaolin: A natural low-cost material as catalyst for isomerization of glucose to fructose. ACS Sustain Chem Eng. 2018;6:8782–9. [Google Scholar]
- [32].Tucker EI, Marzec UM, White TC, Hurst S, Rugonyi S, McCarty OJT, Gailani D, Gruber A, Hanson SR. Prevention of vascular graft occlusion and thrombus-associated thrombin generation by inhibition of factor XI. Blood. 2009;113:936–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kohs TCL, Lorentz CU, Johnson J, Puy C, Olson SR, Shatzel JJ, Gailani D, Hinds MT, Tucker EI, Gruber A, McCarty OJT, Wallisch M. Development of coagulation factor XII antibodies for inhibiting vascular device-related thrombosis. Cell Mol Bioeng. 2021;14:161–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Cheng Q, Tucker EI, Pine MS, Sisler I, Matafonov A, Sun MF, White-Adams TC, Smith SA, Hanson SR, McCarty OJT, Renné T, Gruber A, Gailani D. A role for factor XIIa-mediated factor XI activation in thrombus formation in vivo. Blood. 2010;116:3981–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kenniston JA, Faucette RR, Martik D, Comeau SR, Lindberg AP, Kopacz KJ, Conley GP, Chen J, Viswanathan M, Kastrapeli N, Cosic J, Mason S, DiLeo M, Abendroth J, Kuzmic P, Ladner RC, Edwards TE, TenHoor C, Adelman BA, Nixon AE, et al. Inhibition of plasma kallikrein by a highly specific active site blocking antibody. J Biol Chem. 2014;289:23596–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ivanov I, Matafonov A, Sun MF, Cheng Q, Dickeson SK, Verhamme IM, Emsley J, Gailani D. Proteolytic properties of single-chain factor XII: A mechanism for triggering contact activation. Blood. 2017;129:1527–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Shamanaev A, Ivanov I, Sun M-F, Litvak M, Srivastava P, Mohammed BM, Shaban R, Maddur A, Verhamme IM, McCarty OJT, Law RHP, Gailani D. Model for surface-dependent factor XII activation: the roles of factor XII heavy chain domains. Blood Adv. 2022;6:3142–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Tengvall P, Lundström I. Physico-chemical considerations of titanium as a biomaterial. Clin Mater. 1992;9:115–34. [DOI] [PubMed] [Google Scholar]
- [39].Parks GA. The isoelectric points of solid oxides, solid hydroxides, and aqueous hydroxo complex systems. Chem Rev. 2002;65:177–98. [Google Scholar]
- [40].Larson I, Attard P. Surface charge of silver iodide and several metal oxides. are all surfaces Nernstian? J Colloid Interface Sci. 2000;227:152–63. [DOI] [PubMed] [Google Scholar]
- [41].Lai BH, Yeh CC, Chen DH. Surface modification of iron oxide nanoparticles with polyarginine as a highly positively charged magnetic nano-adsorbent for fast and effective recovery of acid proteins. Proc Biochem. 2012;47:799–805. [Google Scholar]
- [42].Bjorkqvist J, Lecher B, Maas C, Renné T. Zinc-dependent contact system activation induces vascular leakage and hypotension in rodents. Biol Chem. 2013;394:1195–204. [DOI] [PubMed] [Google Scholar]
- [43].Chaudhry SA, Serrata M, Tomczak L, Higgins S, Ryu J, Laprise D, Enjyoji K, Bekendam R, Kaushik V, Flaumenhaft R, Bendapudi PK. Cationic zinc is required for factor XII recruitment and activation by stimulated platelets and for thrombus formation in vivo. J Thromb Haemost. 2020;18:2318–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Vroman L, Adams AL, Fischer GC, Munoz PC. Interaction of high molecular weight kininogen, factor XII, and fibrinogen in plasma at interfaces. Blood. 1980;55:156–9. [PubMed] [Google Scholar]
- [45].Mohammed BM, Matafonov A, Ivanov I, Sun M-f, Cheng Q, Dickeson SK, Li C, Sun D, Verhamme IM, Emsley J, Gailani D. An update on factor XI structure and function. Thromb Res. 2018;161:94–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Smith SB, Verhamme IM, Sun MF, Bock PE, Gailani D. Characterization of novel forms of coagulation factor XIa: independence of factor XIa subunits in factor IX activation. J Biol Chem. 2008;283:66966705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Shamanaev A, Litvak M, Gailani D. Recent advances in factor XII structure and function. Curr Opin Hematol. 2022;29:233–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Clark CC, Hofman ZLM, Sanrattana W, den Braven L, de Maat S, Maas C. The fibronectin type II domain of factor XII ensures zymogen quiescence. Thromb Haemost. 2020;120:400–11. [DOI] [PubMed] [Google Scholar]
- [49].Ponczek MB, Shamanaev A, LaPlace A, Dickeson SK, Srivastava P, Sun MF, Gruber A, Kastrup C, Emsley J, Gailani D. The evolution of factor XI and the kallikrein-kinin system. Blood Adv. 2020;4:6135–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Vroman L, Lukosevicius A. Ellipsometer recordings of changes in optical thickness of adsorbed films associated with surface activation of blood clotting. Nature. 1964;204:701–3. [DOI] [PubMed] [Google Scholar]
- [51].Vroman L. The importance of surfaces in contact phase reactions. Semin Thromb Hemost. 1987;13:79–85. [DOI] [PubMed] [Google Scholar]
- [52].Dion I, Baquey C, Monties JR, Havlik P. Haemocompatibility of TI6A14V alloy. Biomaterials. 1993;14:122–6. [DOI] [PubMed] [Google Scholar]
- [53].Geetha CS, Sabareeswaran A, Mohanan PV. Pre-clinical evaluation of titanium nitride coated titanium material. Toxicol Mech Methods. 2012;22:144–50. [DOI] [PubMed] [Google Scholar]
- [54].Ramaswamy S, Raghavan P. Significance of impurity mineral identification in the value addition of kaolin—a case study with reference to an acidic kaolin from India. JMMCE. 2011;10:1007–25. [Google Scholar]
- [55].Gardner DJ. A study of mineral impurities within the Georgia kaolins. Thesis, Georgia State University; 2016. [Google Scholar]
- [56].Naudin C, Burillo E, Blankenberg S, Butler L, Renné T. Factor XII contact activation. Semin Thromb Hemost. 2017;43:814–26. [DOI] [PubMed] [Google Scholar]
- [57].Mutch NJ, Waters EK, Morrissey JH. Immobilized transition metal ions stimulate contact activation and drive factor XII-mediated coagulation. J Thromb Haemostas. 2012;10:2108–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Fatullayev J, Samak M, Sabashnikov A, Zeriouh M, Rahmanian PB, Choi YH, Schmack B, Kallenbach K, Ruhparwar A, Eghbalzadeh K, Dohmen PM, Karck M, Wippermann J, Wahlers T, Popov AF, Simon AR, Weymann A. Continuous-flow left ventricular assist device thrombosis: a danger foreseen is a danger avoided. Med Sci Monit Basic Res. 2015;21:141–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Bourque K, Cotter C, Dague C, Harjes D, Dur O, Duhamel J, Spink K, Walsh K, Burke E. Design rationale and preclinical evaluation of the HeartMate 3 Left Ventricular Assist System for hemocompatibility. Am Soc Artificial Int Organs. 2016;62:375–83. [DOI] [PubMed] [Google Scholar]
- [60].Bourque K, Dague C, Farrar D, Harms K, Tamez D, Cohn W, Tuzun E, Poirier V, Frazier OH. In vivo assessment of a rotary left ventricular assist device-induced artificial pulse in the proximal and distal aorta. Artificial Organs. 2006;30:638–42. [DOI] [PubMed] [Google Scholar]
- [61].Mehra MR, Uriel N, Naka Y, Cleveland JC Jr, Yuzefpolskaya M, Salerno CT, Walsh MN, Milano CA, Patel CB, Hutchins SW, Ransom J, Ewald GA, Itoh A, Raval NY, Silvestry SC, Cogswell R, John R, Bhimaraj A, Bruckner BA, Lowes BD, et al. A fully magnetically levitated left ventricular assist device-final report. N Engl J Med. 2019;380:1618–27. [DOI] [PubMed] [Google Scholar]
- [62].Nascimbene A, Neelamegham S, Frazier OH, Moake JL, Dong JF. Acquired von Willebrand syndrome associated with left ventricular assist device. Blood. 2016. Jun 23;127:3133–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Tabit CE, Chen P, Kim GH, Fedson SE, Sayer G, Coplan MJ, Jeevanandam V, Uriel N, Liao JK. Elevated angiopoietin-2 level in patients with continuous-flow left ventricular assist devices leads to altered angiogenesis and is associated with higher nonsurgical bleeding. Circulation. 2016;134:141–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Huang YQ, Li JJ, Hu L, Lee M, Karpatkin S. Thrombin induces increased expression and secretion of angiopoietin-2 from human umbilical vein endothelial cells. Blood. 2002;99:1646–50. [DOI] [PubMed] [Google Scholar]
- [65].Bae JS, Rezaie AR. Thrombin upregulates the angiopoietin-Tie2 axis: endothelial protein C receptor occupancy prevents the thrombin mobilization of angiopoietin 2 and P-selectin from Weibel-Palade bodies. J Thromb Haemost. 2010;8:1107–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Zhang M, Tansley TG, Dargusch MS, Fraser JF, Pauls JP. Surface coatings for rotary ventricular assist devices: a systematic review. ASAIO J. 2022;68:623–32. [DOI] [PubMed] [Google Scholar]
- [67].Fijen LM, Levi M. Prophylaxis with anti-activated factor XII for hereditary angioedema. Lancet. 2022;399:889–90. [DOI] [PubMed] [Google Scholar]
- [68].Craig T, Magerl M, Levy DS, Reshef A, Lumry WR, Martinez-Saguer I, Jacobs JS, Yang WH, Ritchie B, Aygören-Pürsün E, Keith PK, Busse P, Feuersenger H, Pawaskar D, Jacobs I, Pragst I, Doyle MK. Prophylactic use of an anti-activated factor XII monoclonal antibody, garadacimab, for patients with C1-esterase inhibitor-deficient hereditary angioedema: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2022;399:945–55. [DOI] [PubMed] [Google Scholar]
- [69].Revenko AS, Gao D, Crosby JR, Bhattacharjee G, Zhao C, May C, Gailani D, Monia BP, MacLeod AR. Selective depletion of plasma prekallikrein or coagulation factor XII inhibits thrombosis in mice without increased risk of bleeding. Blood. 2011;118:5302–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Müller F, Mutch NJ, Schenk WA, Smith SA, Esterl L, Spronk HM, Schmidbauer S, Gahl WA, Morrissey JH, Renné T. Platelet polyphosphates are proinflammatory and procoagulant mediators in vivo. Cell. 2009;139:1143–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Lorentz CU, Verbout NG, Wallisch M, Hagen MW, Shatzel JJ, Olson SR, Puy C, Hinds MT, McCarty OJT, Gailani D, Gruber A, Tucker EI. Contact Activation Inhibitor and Factor XI Antibody, AB023, Produces Safe, Dose-Dependent Anticoagulation in a Phase 1 First-In-Human Trial. Arterioscler Thromb Vasc Biol. 2019;39:799–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Lorentz CU, Tucker EI, Verbout NG, Shatzel JJ, Olson SR, Markway BD, Wallisch M, Ralle M, Hinds MT, McCarty OJT, Gailani D, Weitz JI, Gruber A. The contact activation inhibitor AB023 in heparin-free hemodialysis: results of a randomized phase 2 clinical trial. Blood. 2021;138:2173–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Büller HR, Bethune C, Bhanot S, Gailani D, Monia BP, Raskob GE, Segers A, Verhamme P, Weitz JI. Factor XI antisense oligonucleotide for prevention of venous thrombosis. N Engl J Med. 2015;372:232–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Weitz JI, Bauersachs R, Becker B, Berkowitz SD, Freitas MCS, Lassen MR, Metzig C, Raskob GE. Effect of osocimab in preventing venous thromboembolism among patients undergoing knee arthroplasty: the foxtrot randomized clinical trial. JAMA. 2020;323:130–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Verhamme P, Yi BA, Segers A, Salter J, Bloomfield D, Büller HR, Raskob GE, Weitz JI. Abelacimab for prevention of venous thromboembolism. N Engl J Med. 2021;385:609–17. [DOI] [PubMed] [Google Scholar]
- [76].Weitz JI, Strony J, Ageno W, Gailani D, Hylek EM, Lassen MR, Mahaffey KW, Notani RS, Roberts R, Segers A, Raskob GE. Milvexian for the prevention of venous thromboembolism. N Engl J Med. 2021;385:2161–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Presume J, Ferreira J, Ribeiras R, Mendes M. Achieving higher efficacy without compromising safety with Factor XI inhibitors versus low-molecular-weight heparin for the prevention of venous thromboembolism in major orthopedic surgery - Systematic Review and Meta-Analysis. J Thromb Haemost. 2022;20:2930–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Meijers JCM, Tekelenburg WLH, Bouma BN, Bertina RM, Rosendaal FR. High levels of coagulation factor XI as a risk factor for venous thrombosis. N Engl J Med. 2000;342:696–701. [DOI] [PubMed] [Google Scholar]
- [79].Salomon O, Steinberg DM, Koren-Morag N, Tanne D, Seligsohn U. Reduced incidence of ischemic stroke in patients with severe factor XI deficiency. Blood. 2008;111:4113–7. [DOI] [PubMed] [Google Scholar]
- [80].Shoamanesh A, Mundl H, Smith EE, Masjuan J, Milanov I, Hirano T, Agafina A, Campbell B, Caso V, Mas JL, Dong Q, Turcani P, Christensen H, Ferro JM, Veltkamp R, Mikulik R, De Marchis GM, Robinson T, Lemmens R, Stepien A, et al. Factor XIa inhibition with asundexian after acute non-cardioembolic ischaemic stroke (PACIFIC-Stroke): an international, randomised, double-blind, placebo-controlled, phase 2b trial. Lancet (in press). [DOI] [PubMed] [Google Scholar]
- [81].Sharma M, Molina CA. Hankey G for the AXIOMATIC-SSP Steering Committee and Investigators. Efficacy and safety of the FXIa inhibitor milvexian for secondary stroke prevention: final results of the AXIOMATIC-SSP dose-finding randomization trial. Presented at a Hot Line session at the European Society of Cardiology Congress. 2022. August 28, 2022. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.