ABSTRACT
Lactate is a glycolysis product that is produced from pyruvate by LDH (lactate dehydrogenase) and plays an important role in physiological and pathological processes. However, whether lactate regulates autophagy is still unknown. We recently reported that LDHA is phosphorylated at serine 196 by ULK1 (unc-51 like kinase 1) under nutrient-deprivation conditions, promoting lactate production. Then, lactate mediates PIK3C3/VPS34 lactylation at lysine 356 and lysine 781 via acyltransferase KAT5/TIP60. PIK3C3/VPS34 lactylation enhances the association of PIK3C3/VPS34 with BECN1 (beclin 1, autophagy related), ATG14 and UVRAG, increases PIK3C3/VPS34 lipid kinase activity, promotes macroautophagy/autophagy and facilitates the endolysosomal degradation pathway. PIK3C3/VPS34 hyperlactylation induces autophagy and plays an essential role in skeletal muscle homeostasis and cancer progression. Overall, this study describes an autophagy regulation mechanism and the integration of two highly conserved life processes: glycolysis and autophagy.
Lactate is an intracellular glycolysis product that is produced from pyruvate by LDH. In contrast to its early portrayal as a metabolic waste product, lactate plays a critical role in physiological and pathological processes, as shown by the discovery of lactate shuttling and the Warburg effect in tumor cells. Lactate is a metabolic intermediate that is involved in not only central carbon metabolism (major energy source and gluconeogenic precursor) but also regulating tumor immunity, antiviral processes and endoplasmic reticulum (ER)-mitochondrial Mg2+ dynamics. Recently, emerging evidence has shown that lactate dynamically drives the lysine lactylation of histones and nonhistone proteins to regulate gene expression and protein activity in macrophages, somatic cells, cancer cells and brain cells in a glycolysis-dependent manner. Several posttranslational modifications (PTMs), including phosphorylation, glycosylation, ubiquitination, lipidation and acetylation, regulate the structure and biological function of core autophagy machinery. However, it is unclear whether lactate regulates autophagy by mediating the lactylation of core autophagy proteins.
We found that cytosolic lactate levels are significantly increased under serum-starvation or amino acid-deprivation conditions by the genetically coded fluorescent sensor FiLa. To determine the reason for the increase in lactate during this process, we first found that LDHA, which is one of the key subunits of the LDH enzyme in the glycolytic pathway that converts pyruvate to lactate with concomitant interconversion of NADH and NAD+, is phosphorylated by ULK1 at serine 196. This molecular mechanism was confirmed by specific phosphorylation antibodies, yeast two-hybrid assays, immunoprecipitation, mass spectrometry and in vitro phosphorylation assays. LDHA phosphorylation at serine 196 enhances enzyme activity and promotes the production of lactate through enzymatic activity in vitro and in vivo, as shown by the genetically coded fluorescent sensor FiLa and traditional enzyme biochemistry [1].
To explore the relationship between autophagy and lactate, we hypothesized that lactate-mediated lactylation of the core autophagy proteins regulates autophagy activity. The lactylation of the core autophagy proteins was examined by immunoprecipitation and western blotting with an anti-panlactylation antibody. Intriguingly, the results identified many lactylated core autophagy proteins in different autophagy stages (PIK3C3/VPS34, ULK1, UVRAG, etc.), indicating that lactylation played an important role in autophagy regulation. PIK3C3/VPS34 is a catalytic subunit of the class III phosphatidylinositol 3-kinase/PtdIns3K complex that phosphorylates phosphatidylinositol (PtdIns) to produce phosphatidylinositol-3-phosphate (PtdIns3P), thus promoting autophagy and endolysosomal trafficking. PIK3C3/VPS34 lactylation sites (lysine 356 and lysine 781) were identified by mass spectrometry and are mediated by the acetyltransferase KAT5/TIP60, as shown by in vivo and in vitro lactylation assays. PIK3C3/VPS34 lactylation promotes PIK3C3/VPS34 lipid kinase activity by enhancing the ability of PIK3C3/VPS34 to interact with BECN1, ATG14, and UVRAG. PIK3C3/VPS34 delactylation blocks SQSTM1/p62 degradation and GFP-LC3 cleavage at the cellular level in normal medium and under EBSS-induced starvation conditions [1]. In addition, PIK3C3/VPS34 delactylation impairs endosome-lysosomal degradation through EGFR (epidermal growth factor receptor) degradation and decreased proteolytic maturation of CTSD (cathepsin D). These results demonstrated that PIK3C3/VPS34 lactylation is required for autophagosome formation and maturation and endosome-lysosomal degradation.
Glycolysis and autophagy are two highly conserved processes that play important roles in physiological and pathological processes. We speculated that lactate, which is a signaling molecule, links glycolysis and autophagy. Intense exercise can cause muscle protein denaturation and mitochondrial damage through physiological processes. Autophagy maintains muscle cell homeostasis by eliminating damaged organelles and proteins and restoring metabolic balance. Intense muscle exercise produces a large amount of lactate, promotes the lactylation level of PIK3C3/VPS34, enhances the activity of PIK3C3/VPS34 kinase, increases PtdIns3P levels, and induces autophagy in muscle tissue. In addition to the AMPK pathway, lactate-mediated lactylation, which is a bypass regulatory pathway, regulates the homeostasis of muscle tissue. Lactate not only contributes to central metabolism in tumor cells but also orchestrates immunosuppressive communication in the tumor microenvironment. Lactate levels are increased by the Warburg effect in lung cancer and gastric cancer. High lactate mediates PIK3C3/VPS34 lactylation and induces autophagy. Autophagy can promote cancer progression by removing damaged organelles and proteins and thereby increase the metabolic fitness of malignant cells to help them survive in established tumor lesions. Therefore, lactate acts as a bridge linking glycolysis and autophagy.
In conclusion, our study uncovered a novel mechanism by which the glycolytic metabolite lactate regulates autophagy (Figure 1). Lactate is a signaling molecule that connects glycolysis and autophagy. In addition, we found that other core autophagy proteins are lactylated and can play a key role in physiological and pathological processes, such as sepsis, ischemia, neoplasia, and mitochondrial diseases. This new molecular mechanism needs to be explored further. These results may provide a theoretical basis for clinical diseases associated with glycolysis and autophagy.
Figure 1.
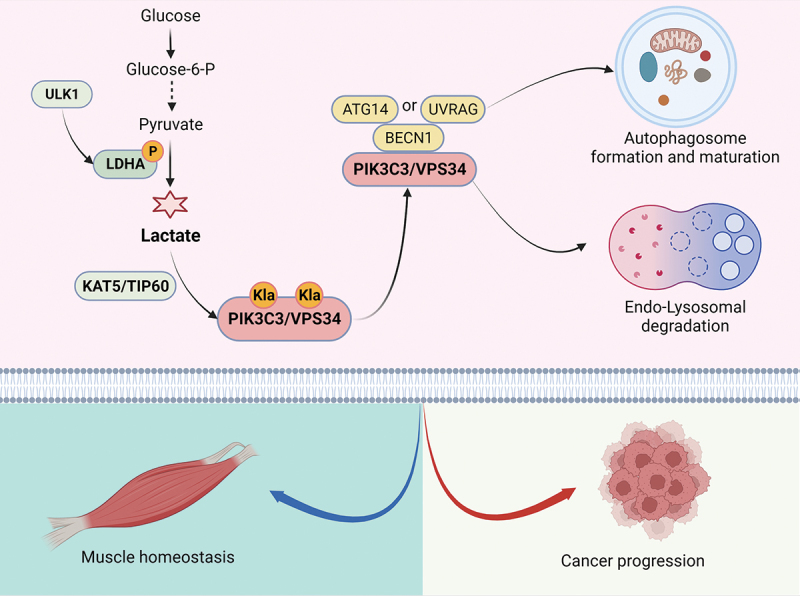
Schematic model summarizing the findings of our study. ULK1 phosphorylates LDHA serine 196 under serum-deprivation conditions, increases LDHA enzyme activity, and promotes lactate production. Lactate-mediated PIK3C3/VPS34 lactylation via the acetyltransferase KAT5/TIP60 further promotes its lipid kinase activity and activates autophagy in muscle cells and cancer cells.
Funding Statement
This study was supported by the National Key Research and Development Program of China (2021YFC2101100 to X.C.) and the National Nature Science Foundation of China (32170764 to X.C.).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Reference
- [1].Jia M, Yue X, Sun W, et al. ULK1-mediated metabolic reprogramming regulates Vps34 lipid kinase activity by its lactylation. Sci Adv. 2023;9(22):eadg4993. Epub 2023 Jun 2. PMID: 37267363. doi: 10.1126/sciadv.adg4993 [DOI] [PMC free article] [PubMed] [Google Scholar]


