Abstract
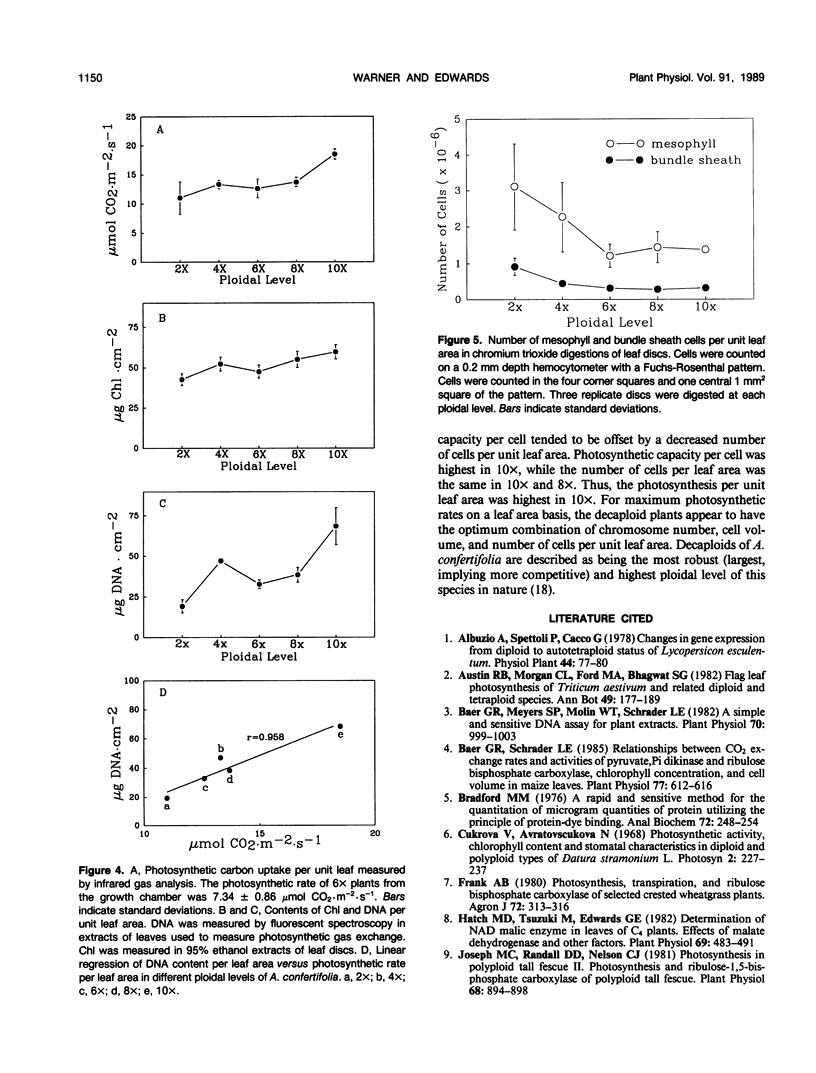
Photosynthetic rates, chlorophyll content, and activities of several photosynthetic enzymes were determined per cell, per unit DNA, and per unit leaf area in five ploidal levels of the C4 dicot Atriplex confertifolia. Volumes of bundle sheath and mesophyll protoplasts were measured in enzymatic digestions of leaf tissue. Photosynthetic rates per cell, contents of DNA per cell, and activities of the bundle sheath enzymes ribulose 1,5-bisphosphate carboxylase (RuBPC) and NAD-malic enzyme per cell were correlated with ploidal level at 99% or 95% confidence levels, and the results suggested a near proportional relationship between gene dosage and gene products. There was also a high correlation between volume of mesophyll and bundle sheath cells and the ploidal level. Contents of DNA per cell, activity of RuBPC per cell, and volumes of cells were correlated with photosynthetic rate per cell at the 95% confidence level. The mesophyll cells did not respond to changes in ploidy like the bundle sheath cells. In the mesophyll cells the chlorophyll content per cell was constant at different ploidal levels, there was less increase in cell volume than in bundle sheath cells with an increase in ploidy, and there was not a significant correlation (at 95% level) of phosphoenolpyruvate carboxylase activity or content and pyruvate,Pi dikinase activity with increase in ploidy. The number of photosynthetic cells per unit leaf area progressively decreased with increasing ploidy from diploid to hexaploid, but thereafter remained constant in octaploid and decaploid plants. Numbers of cells per leaf area were not correlated with cell volumes. The mean photosynthetic rates per unit leaf area were lowest in the diploid, similar in 4×, 6×, and 8×, and highest in the decaploid. The photosynthetic rate per leaf area was highly correlated with the DNA content per leaf area.
Full text
PDF


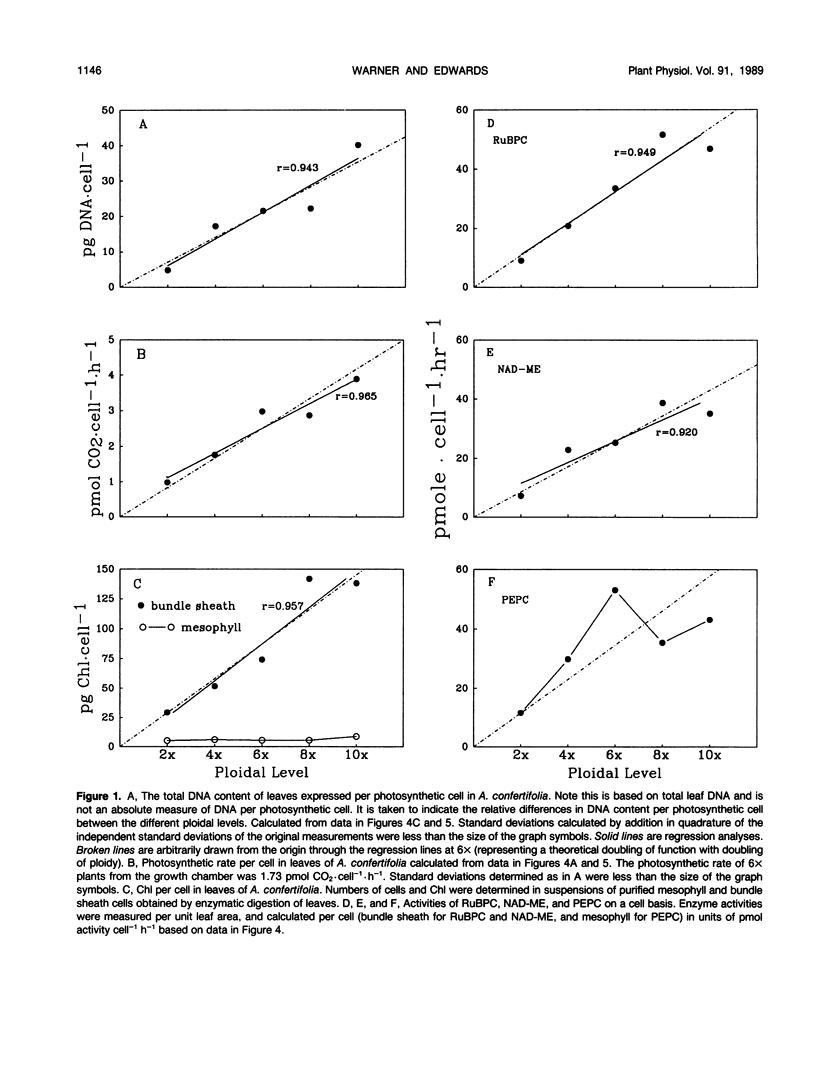

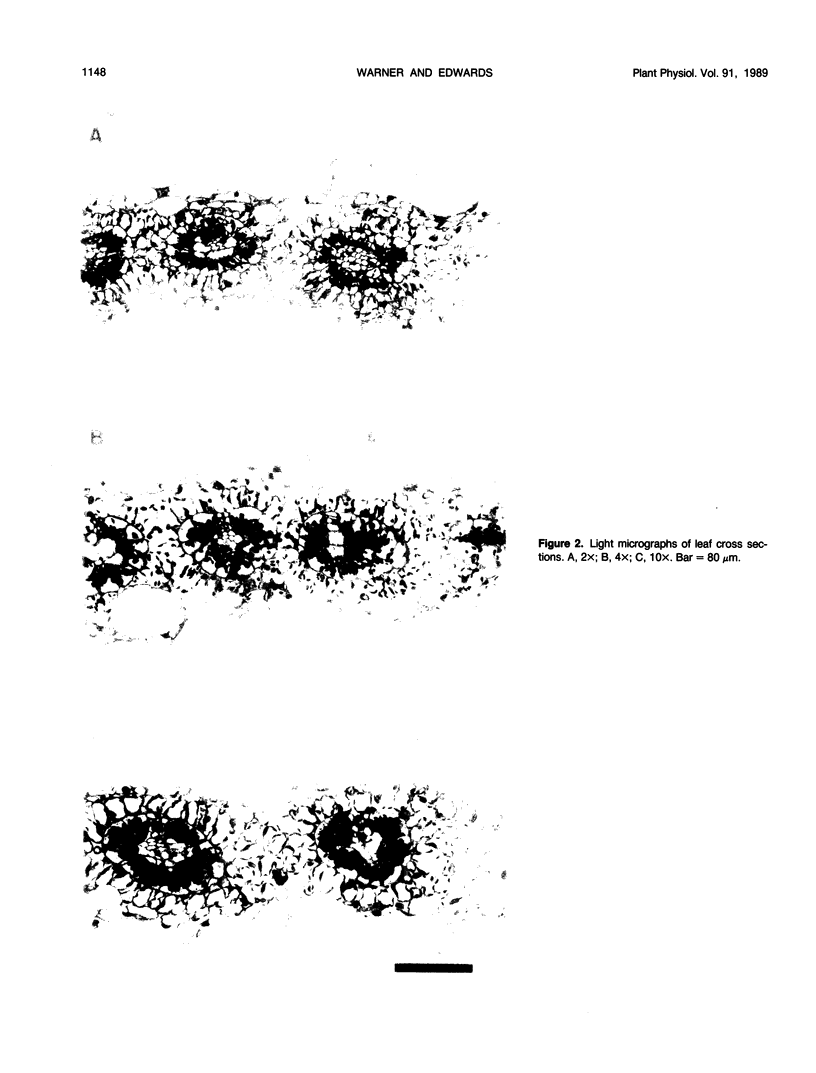
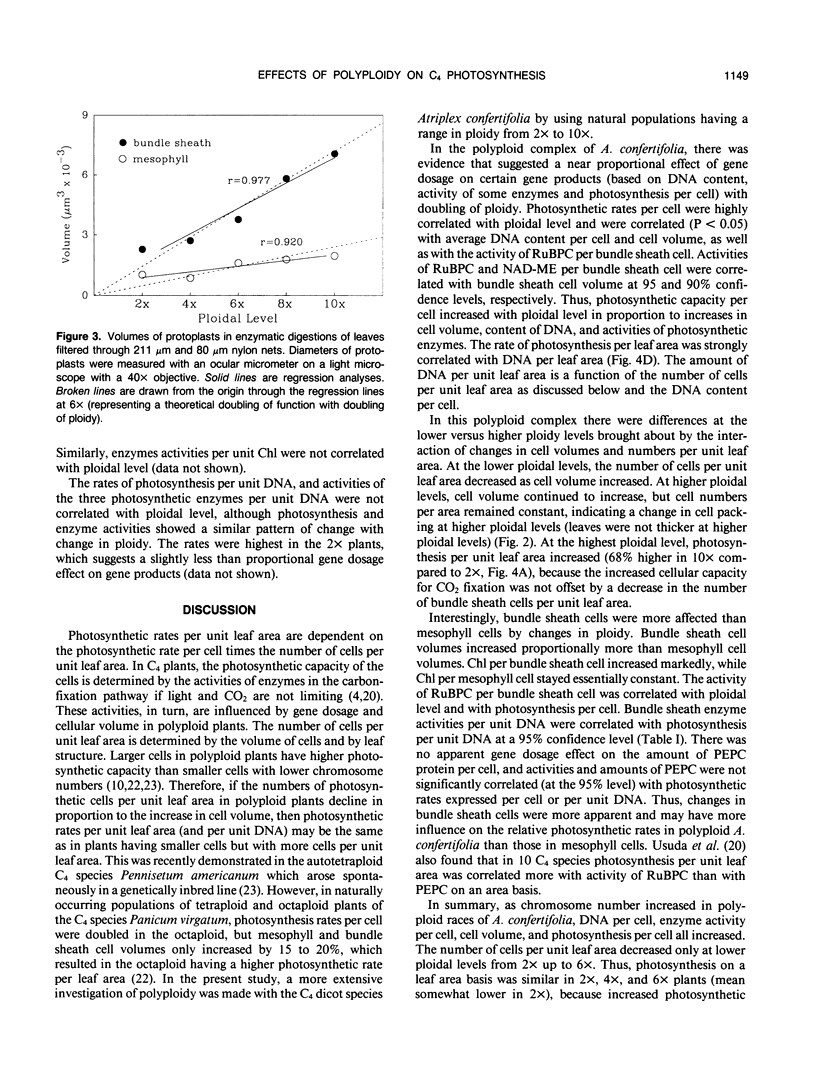

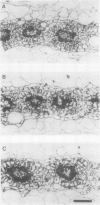
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baer G. R., Meyers S. P., Molin W. T., Schrader L. E. A simple and sensitive DNA assay for plant extracts. Plant Physiol. 1982 Oct;70(4):999–1003. doi: 10.1104/pp.70.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer G. R., Schrader L. E. Relationships between CO(2) Exchange Rates and Activities of Pyruvate,Pi Dikinase and Ribulose Bisphosphate Carboxylase, Chlorophyll Concentration, and Cell Volume in Maize Leaves. Plant Physiol. 1985 Mar;77(3):612–616. doi: 10.1104/pp.77.3.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Hatch M. D., Tsuzuki M., Edwards G. E. Determination of NAD Malic Enzyme in Leaves of C(4) Plants : EFFECTS OF MALATE DEHYDROGENASE AND OTHER FACTORS. Plant Physiol. 1982 Feb;69(2):483–491. doi: 10.1104/pp.69.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph M. C., Randall D. D. Photosynthesis in Polyploid Tall Fescue : II. PHOTOSYNTHESIS AND RIBULOSE-1, 5-BISPHOSPHATE CARBOXYLASE OF POLYPLOID TALL FESCUE. Plant Physiol. 1981 Oct;68(4):894–898. doi: 10.1104/pp.68.4.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molin W. T., Meyers S. P., Baer G. R., Schrader L. E. Ploidy Effects in Isogenic Populations of Alfalfa : II. Photosynthesis, Chloroplast Number, Ribulose-1,5-Bisphosphate Carboxylase, Chlorophyll, and DNA in Protoplasts. Plant Physiol. 1982 Dec;70(6):1710–1714. doi: 10.1104/pp.70.6.1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perchorowicz J. T., Raynes D. A., Jensen R. G. Measurement and preservation of the in vivo activation of ribulose 1,5-bisphosphate carboxylase in leaf extracts. Plant Physiol. 1982 May;69(5):1165–1168. doi: 10.1104/pp.69.5.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polson A., von Wechmar M. B., van Regenmortel M. H. Isolation of viral IgY antibodies from yolks of immunized hens. Immunol Commun. 1980;9(5):475–493. doi: 10.3109/08820138009066010. [DOI] [PubMed] [Google Scholar]
- Rathnam C. K., Chollet R. Photosynthetic and Photorespiratory Carbon Metabolism in Mesophyll Protoplasts and Chloroplasts Isolated from Isogenic Diploid and Tetraploid Cultivars of Ryegrass (Lolium perenne L.). Plant Physiol. 1980 Mar;65(3):489–494. doi: 10.1104/pp.65.3.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scrutton M. C., Fatebene F. An assay system for localisation of pyruvate and phosphoenolpyruvate carboxylase activity on polyacrylamide gels and its application to detection of these enzymes in tissue and cell extracts. Anal Biochem. 1975 Nov;69(1):247–260. doi: 10.1016/0003-2697(75)90584-9. [DOI] [PubMed] [Google Scholar]
- Timko M. P., Vasconcelos A. C. Euploidy in Ricinus: EUPLOIDY EFFECTS ON PHOTOSYNTHETIC ACTIVITY AND CONTENT OF CHLOROPHYLL-PROTEINS. Plant Physiol. 1981 Jun;67(6):1084–1089. doi: 10.1104/pp.67.6.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uedan K., Sugiyama T. Purification and characterization of phosphoenolpyruvate carboxylase from maize leaves. Plant Physiol. 1976 Jun;57(6):906–910. doi: 10.1104/pp.57.6.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner D. A., Ku M. S., Edwards G. E. Photosynthesis, Leaf Anatomy, and Cellular Constituents in the Polyploid C(4) Grass Panicum virgatum. Plant Physiol. 1987 Jun;84(2):461–466. doi: 10.1104/pp.84.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintermans J. F., de Mots A. Spectrophotometric characteristics of chlorophylls a and b and their pheophytins in ethanol. Biochim Biophys Acta. 1965 Nov 29;109(2):448–453. doi: 10.1016/0926-6585(65)90170-6. [DOI] [PubMed] [Google Scholar]