Abstract
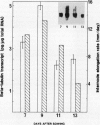
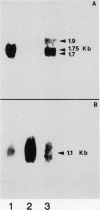
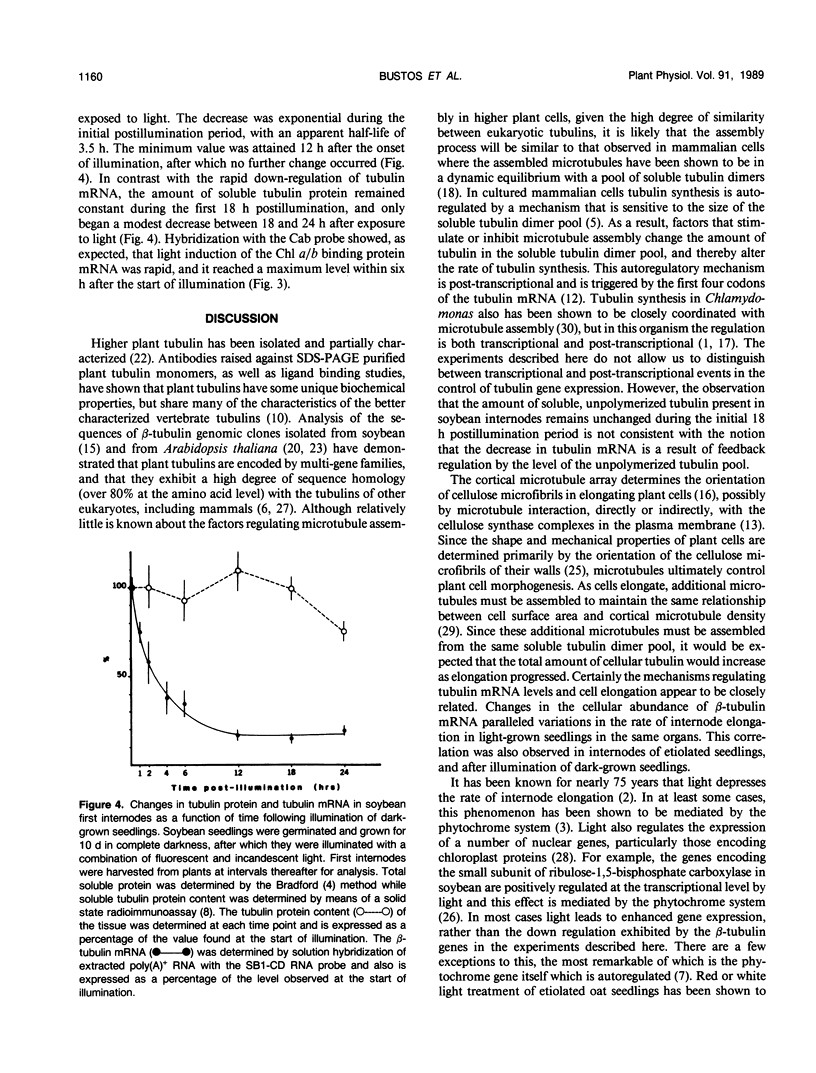
The relationship between tubulin gene expression and cell elongation was explored in developing internodes of Glycine max (L.) Merr., using light as a variable to alter the rate of elongation. First internodes of etiolated seedlings elongated two to three times more rapidly than did those of seedlings growing under a 12 hour diurnal light/dark cycle. Furthermore, light slowed or completely halted internode elongation in the etiolated seedlings, depending upon the age of the seedlings at the time of the light treatment. Steady state levels of β-tubulin mRNA were determined in Northern blots and by solution hybridization of poly(A)+RNA with a probe derived from the coding region of a previously characterized soybean β-tubulin gene. (MJ Guiltinan, DP Ma, RF Barker, MM Bustos, RJ Cyr, R Yadegari, DE Fosket [1987] Plant Mol Biol 10: 171-184). Internodes of light-grown seedlings exhibited levels of β-tubulin mRNA that differed by a factor of three, and varied concomitantly with the elongation rate. Illumination of 10-day-old etiolated seedlings not only stopped first internode elongation, but also brought about a 80% decrease in the steady state level of β-tubulin mRNA over the course of the subsequent 12 hours. This strong down regulation of β-tubulin mRNA occurred without significant changes in the size of the soluble tubulin pool and it was accompanied by a marked increase in chlorophyll a/b binding protein mRNA.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker E. J., Schloss J. A., Rosenbaum J. L. Rapid changes in tubulin RNA synthesis and stability induced by deflagellation in Chlamydomonas. J Cell Biol. 1984 Dec;99(6):2074–2081. doi: 10.1083/jcb.99.6.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleiss W., Smith H. Rapid suppression of extension growth in dark-grown wheat seedlings by red light. Plant Physiol. 1985 Mar;77(3):552–555. doi: 10.1104/pp.77.3.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W. Autoregulated instability of tubulin mRNAs: a novel eukaryotic regulatory mechanism. Trends Biochem Sci. 1988 Sep;13(9):339–343. doi: 10.1016/0968-0004(88)90103-x. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Sullivan K. F. Molecular biology and genetics of tubulin. Annu Rev Biochem. 1985;54:331–365. doi: 10.1146/annurev.bi.54.070185.001555. [DOI] [PubMed] [Google Scholar]
- Gay D. A., Yen T. J., Lau J. T., Cleveland D. W. Sequences that confer beta-tubulin autoregulation through modulated mRNA stability reside within exon 1 of a beta-tubulin mRNA. Cell. 1987 Aug 28;50(5):671–679. doi: 10.1016/0092-8674(87)90325-4. [DOI] [PubMed] [Google Scholar]
- Keller L. R., Schloss J. A., Silflow C. D., Rosenbaum J. L. Transcription of alpha- and beta-tubulin genes in vitro in isolated Chlamydomonas reinhardi nuclei. J Cell Biol. 1984 Mar;98(3):1138–1143. doi: 10.1083/jcb.98.3.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschner M., Mitchison T. Beyond self-assembly: from microtubules to morphogenesis. Cell. 1986 May 9;45(3):329–342. doi: 10.1016/0092-8674(86)90318-1. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morejohn L. C., Fosket D. E. Higher plant tubulin identified by self-assembly into microtubules in vitro. Nature. 1982 Jun 3;297(5865):426–428. doi: 10.1038/297426a0. [DOI] [PubMed] [Google Scholar]
- Oppenheimer D. G., Haas N., Silflow C. D., Snustad D. P. The beta-tubulin gene family of Arabidopsis thaliana: preferential accumulation of the beta 1 transcript in roots. Gene. 1988;63(1):87–102. doi: 10.1016/0378-1119(88)90548-3. [DOI] [PubMed] [Google Scholar]
- Shirley B. W., Berry-Lowe S. L., Rogers S. G., Flick J. S., Horsch R., Fraley R. T., Meagher R. B. 5' proximal sequences of a soybean ribulose-1,5-bisphosphate carboxylase small subunit gene direct light and phytochrome controlled transcription. Nucleic Acids Res. 1987 Aug 25;15(16):6501–6514. doi: 10.1093/nar/15.16.6501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traas J. A., Braat P., Derksen J. W. Changes in microtubule arrays during the differentiation of cortical root cells of Raphanus sativus. Eur J Cell Biol. 1984 Jul;34(2):229–238. [PubMed] [Google Scholar]
- Weeks D. P., Collis P. S. Induction of microtubule protein synthesis in Chlamydomonas reinhardi during flagellar regeneration. Cell. 1976 Sep;9(1):15–27. doi: 10.1016/0092-8674(76)90048-9. [DOI] [PubMed] [Google Scholar]