Abstract
A mutant of fast milk-coagulating (Fmc+) Lactococcus lactis subsp. lactis C2, designated L. lactis KB4, was identified. Although possessing the known components essential for utilizing casein as a nitrogen source, which include functional proteinase (PrtP) activity and oligopeptide, di- and tripeptide, and amino acid transport systems, KB4 exhibited a slow milk coagulation (Fmc−) phenotype. When the amino acid requirements of L. lactis C2 were compared with those of KB4 by use of a chemically defined medium, it was found that KB4 was unable to grow in the absence of aspartic acid. This aspartic acid requirement could also be met by aspartate-containing peptides. The addition of aspartic acid to milk restored the Fmc+ phenotype of KB4. KB4 was found to be defective in pyruvate carboxylase and thus was deficient in the ability to form oxaloacetate and hence aspartic acid from pyruvate and carbon dioxide. The results suggest that when lactococci are propagated in milk, aspartate derived from casein is unable to meet fully the nutritional demands of the lactococci, and they become dependent upon aspartate biosynthesis.
Lactococci are widely used as starters in cheese and other fermented milk products, and their rapid growth in milk is essential to yield an appropriate rate of lactic acid production, optimum curd formation, required flavor, and texture development in the final products and for the inhibition of undesirable microorganisms (28). The ability of a lactococcal strain to coagulate milk within 16 to 18 h at 21°C with a 1% inoculum defines a fast milk-coagulating (Fmc+) strain used in cheese manufacture (45). An important metabolic function that influences the rapid growth of lactococci in milk is their proteolytic system, which is required to obtain the amino acids needed for growth to high cell densities. If components of this caseinolytic system are missing, the result can be a slow milk coagulation (Fmc−) phenotype requiring up to 48 h or longer to coagulate milk, because the limited availability of utilizable amino acids does not allow growth to reach the prerequisite high cell densities.
The hydrolysis of casein by lactococci is initiated by a cell wall-associated proteinase (PrtP) which is responsible for the degradation of casein into oligopeptides. PrtP is synthesized as an inactive pre-pro-protein and requires processing by a maturation protein (PrtM) to exhibit activity (18). Three distinct amino acid transport systems, two di- and tripeptide transport systems, and an oligopeptide permease system are available for the uptake of amino acids and peptides by the cell. Intracellular peptidases then degrade the peptides into amino acids for growth. Lactococcal strains without the Prt plasmid, encoding prtP and prtM, exhibit an Fmc− phenotype. The oligopeptide transport (Opp) system is also essential for the Fmc+ phenotype (24, 38, 45). Although it has been speculated that an extracellular peptidase(s) may be involved in further hydrolysis of the large oligopeptides released by the action of PrtP on casein into smaller units utilizable by the available transport systems (19, 32), recent data indicate that the existence of an extracellular peptidase is not likely (14, 15). The latter studies found that a large fraction of the oligopeptides generated by PrtP degradation of casein were in the range of 4 to 8 amino acid residues, small enough to be transported into the cell through the Opp system. While an Opp− mutant of L. lactis was unable to utilize oligopeptides and grew poorly in milk, a di- and tripeptide transport-deficient mutant (DtpT−) grew as well as the wild-type strain in milk (15). It was further demonstrated that the wild-type strain and the DtpT− mutant accumulated β-casein-derived amino acids inside the cells when β-casein served as a protein source, but no significant accumulation of amino acids occurred with Opp− and DtpT− Opp− mutants (21). These observations indicated that oligopeptides are the main nitrogen source for lactococcal growth in milk and that there is no necessity to postulate the existence of an extracellular peptidase (15).
Based on these results and the intracellular locations of the peptidases identified so far, reasonable doubts have arisen concerning the existence of a putative extracellular peptidase in lactococci. There is also no evidence for the existence of other elements involved in a functional proteolytic system in lactococci. Previous work (44) in our laboratory on L. lactis C2 and its Fmc− derivatives provided putative genetic evidence for the possible requirement of another component for the Fmc+ phenotype. In this communication, we demonstrate that, in addition to a functional proteolytic system, pyruvate carboxylase activity is required to supply the cell with sufficient aspartate for the Fmc+ phenotype in L. lactis C2.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
Lactococcal strains and the plasmid used in this study are listed in Table 1. Cultures were propagated at 32°C in M17 broth containing 0.5% glucose (M17-G) or 0.5% lactose (M17-L) as the sole carbohydrate source (36). Cultures were stored at 4°C and maintained by biweekly transfers in M17 broth. The Fmc+ phenotype was determined as previously desribed (45). Fmc− (Prt+ Opp+) KB4 was originally isolated as a Lac+ Fmc− variant by growth of the parental strain, L. lactis C2, in lactose broth containing 6 μg of acriflavine per ml at 32°C for 20 h. The treated culture was plated on lactose indicator agar, and Lac+ colonies were screened for the inability to coagulate milk within 16 to 18 h at 21°C (27, 45).
TABLE 1.
Bacterial strains and plasmid
| Strain or plasmid | Plasmid content (MDa) | Descriptiona | Reference |
|---|---|---|---|
| Lactococcus lactis subsp. lactis strains | |||
| C2 | 30, 18, 12, 5, 2, and 1 | Lac+ Fmc+ (Prt+ Opp+), wild-type multiplasmid strain | 17 |
| KB4 | 30, 18, 5, 2, and 1 | Lac+ Fmc− (Prt+ Opp+), derivative of C2 missing the 12-MDa plasmid | 44 |
| LM0230 | Lac− Fmc− (Prt− Opp+), plasmid-free derivative of C2 (Opp in chromosome) | 7 | |
| Plasmid pKMP1 | 13 | Lac− Opp+ Emr | 11 |
Lac+ and Lac−, lactose fermenting and non-lactose fermenting, respectively; Fmc+ and Fmc−, ability and inability to coagulate 11% NFM (supplemented with 0.5% glucose when the strain was Lac−) at 21°C in 18 to 20 h, respectively; Prt+ and Prt−, ability and inability to hydrolyze casein by PrtP, the cell-wall associated proteinase, respectively; Opp+ and Opp−, presence and absence of the oligopeptide transport system, respectively; Emr, plasmid-mediated resistance to erythromycin.
DNA manipulations.
Plasmid DNA isolation, total cellular DNA purification, agarose gel electrophoresis, and electroporations were done as previously described (44). Southern transfer and probe hybridization were performed as described by Sambrook et al. (35). Genius system nonisotope digoxigenin labeling and detection were done according to the manufacturer’s directions (Boehringer Mannheim Biochemicals, Indianapolis, Ind.). PCR was used to make probe DNA with total cellular DNA or plasmid DNA of L. lactis C2 as a template. Oligoprimers were synthesized with a PCR-MATE EP DNA synthesizer (Applied Biosystems, Foster City, Calif.). These included oligonucleotides 5′-TCTCGCAGCAAACTAAGG-3′ and 5′-GGAAAGATTGGAAACTGG-3′ for amplifying a prtP subfragment (20), 5′-GTGTTGCCTTTCGGATTG-3′ and 5′-TTTTTCCAAGCCTGACCT-3′ for amplifying an oppD subfragment (44), and 5′-GGTTTCAGTCGCAGTAGC-3′ and 5′-CTTAGCAGTGTCCTCGTC-3′ for amplifying a prtM subfragment (39). PCR was conducted with Taq DNA polymerase according to the manufacturer’s instructions (Perkin-Elmer Corp., Norwalk, Conn.). Probes were labeled via 3′-end labeling with digoxigenin-11-ddUTP (Genius; Boehringer). Hybridizations and washings were performed at 65°C.
For PCR detection of certain genes from a specific strain, the synthesized gene-specific oligonucleotides referred to above were used as PCR primers. DNA from cells of an 18-h colony was used as a template for the reaction. The reaction cocktail (except for the Taq polymerase) was boiled for 10 min to release DNA from the cells before PCR.
Proteinase assay.
Cells were harvested, washed, and fractionated with a combination of lysozyme and mutanolysin in sucrose-MgCl2 (24% sucrose–10 mM MgCl2 in 50 mM Tris-HCl [pH 7.0]) without differentiating among loosely associated cell material, cell wall material, and cell wall components (4, 5). Proteinase was monitored with the substrate fluorescein isothiocyanate (FITC)-labeled β-casein (37) as described by Coolbear et al. (3). Fructose bisphosphate aldolase activity was determined by the method of Crow and Thomas (6).
Proteolytic assay.
Reconstituted nonfat milk (NFM) (11% [wt/vol] with 1% glucose) was inoculated (1%) with an overnight culture in M17-L or M17-G and incubated at 32°C for 12 h. A second 1% transfer was made into 11% NFM, and the mixture was incubated at 32°C for 36 h. Proteolysis of milk proteins was determined by the method of Hull (12).
Defined media and culture conditions for growth studies.
The chemically defined medium (CDM) described by Otto et al. (29) was used to study the utilization of casein, peptides, and amino acids by the lactococcal strains.
A simple synthetic medium (SSM) (1) containing glucose, potassium phosphate, basic vitamins, and nine essential amino acids (arginine, glutamate, histidine, isoleucine, leucine, methionine, serine, threonine, and valine) was used to determine the amino acid requirements of L. lactis C2 and the Fmc− derivatives L. lactis KB4 and LM0230. A modification was made to increase the amount of Mg2+ from 1 to 3 mM. Additional components were also provided per liter of medium as indicated: 0.42 g of l-aspartic acid, 0.275 g of l-phenylalanine, 0.05 g of l-tryptophan, 0.24 g of l-alanine, 0.675 g of l-proline, 0.44 g of l-lysine, 0.175 g of l-glycine, 0.2 g of l-tyrosine, 0.3 g of l-glutamine, 0.125 g of l-asparagine, 0.25 g of l-cysteine, 0.42 g of oxaloacetic acid (OAA), 0.42 g of fumaric acid, 0.42 g of malic acid, 0.42 g of pyruvate, 0.40 g of oligopeptide Asp-Ser-Asp-Pro-Arg, 0.40 g of oligopeptide Gly-Asp-Asp-Asp-Asp-Lys, or 0.84 g of Asp-Gly.
Strains were grown overnight in M17-G or M17-L. The 18-h cultures were then inoculated into M17 broth and propagated at 32°C for 6 h (optical density at 600 nm, about 0.7). Cells from 1 ml of the culture were harvested by centrifugation and washed twice with 1 volume of β-glycerophosphate buffer (50 mM β-glycerophosphate [pH 7.5], 20 mM CaCl2). The pellets were resuspended in 200 μl of 0.85% saline, and 30 μl was used to inoculate 3 ml of defined medium.
Assay for GOT.
Cell extract (CE) was prepared as described by Wahls (40). The activity of glutamate-oxaloacetate transaminase (GOT) was determined as described by Yagi et al. (43).
Incubation of cells with sodium [14C]bicarbonate.
L. lactis C2 and KB4 were grown in M17-L at 30°C for 18 h. Cells from 10 ml of each of the cultures were collected by centrifugation. The pellets were resuspended in 2.5 ml of M17 broth supplemented with 40 μl of sodium [14C]bicarbonate (0.5 mCi/ml; 2 to 10 mCi of crystalline solid per mmol) and incubated at room temperature for 1 h. The cells were then collected and washed twice with 1 volume of 0.85% NaCl. The pellets were suspended in 100 μl of saline, and 40 μl was used to determine fixed 14C activity by use of a model LS5000TD liquid scintillation spectrometer (Beckman Instruments, Inc., Fullerton, Calif.).
Assay for pyruvate carboxylase.
The pyruvate carboxylase activity of L. lactis was determined by the method of Renner and Bernlohr (34) with a GOT-coupled [14C]bicarbonate fixation assay. One milliliter of the reaction cocktail contained the following: CE, 0.3 ml; Tris-HCl (pH 7.5), 100 μmol; MnCl2, 5 μmol; sodium pyruvate, 10 μmol; ATP, 5 μmol; NaH14CO3, 1 μCi (0.5 mCi/ml; 2 to 10 mCi of crystalline solid per mmol); acetyl coenzyme A, 0.5 μmol; glutamate, 20 μmol; and GOT, 10 U. The reaction was terminated by the addition of 0.1 ml of 10% trichloroacetic acid. The activity of acid-stable 14C in the supernatant was measured by liquid scintillation counting. Ten micromoles of OAA or aspartate or 4 U of avidin was added to the reaction mixture to study the effects of each on pyruvate carboxylase activity. Ten micromoles of phosphoenolpyruvate (PEP) instead of pyruvate was used in the reaction mixture to study the substrate specificity of the carboxylase.
Protein assay.
Protein concentrations in CEs were determined by use of a protein assay according to the manufacturer’s instructions (Bio-Rad Laboratories, Hercules, Calif.).
Paper electrophoresis.
Pyruvate carboxylase assay products were analyzed by paper electrophoresis (41). Whatman no. 3 paper strips (1 cm wide) were used as a solid support, and electrophoresis was carried out with 2% pyridine–0.95% acetic acid (wt/wt) buffer (pH 5.2) for 60 min at 20 V/cm. About 20 μl of the reaction samples was subjected to electrophoresis, and unlabeled aspartate was added to the samples before loading as an internal control for detection. The amino acids were visualized by spraying with ninhydrin (41). The stained strip was then cut into pieces 1 cm long, and radioactivity was determined by scintillation counting.
RESULTS
L. lactis KB4 is an Fmc− derivative of L. lactis C2.
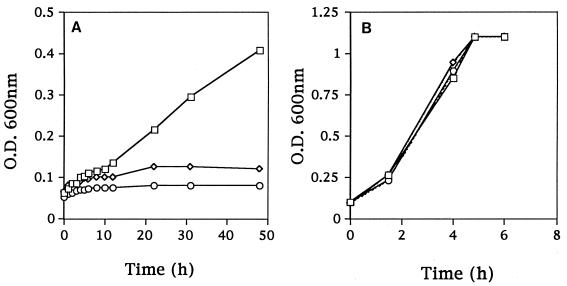
L. lactis KB4 required up to 40 h to coagulate milk at 21°C, compared with 18 to 20 h for Fmc+ L. lactis C2, resulting in its classification as an Fmc− derivative of C2. KB4 was also unable to grow in CDM with β-casein as the sole nitrogen source (Fig. 1A).
FIG. 1.
Growth in CDM of Fmc+ (Prt+ Opp+) L. lactis C2 (□), Fmc− (Prt− Opp+) L. lactis LM0230 (◊), and Fmc− (Prt+ Opp+) L. lactis KB4 (○). (A) CDM with casein as the sole amino acid source. (B) CDM with a pool of 20 amino acids. O.D., optical density.
Southern analysis and PCR detection of prtP, prtM, and oppD genes.
As the absence of PrtP or Opp components involved in casein utilization can result in the Fmc− phenotype, it was necessary to determine which, if any, of these components were missing in KB4. The prtP and prtM probes both hybridized to the 30-MDa Lac plasmid in KB4, indicating that these genes were located on this plasmid, as in L. lactis C2 (data not shown). PCR analysis also confirmed that KB4 carried the prtP and prtM genes. No corresponding prtM or prtP bands were detected in Fmc− (Prt− Opp+) L. lactis LM0230, the plasmid-free derivative of C2 (data not shown). PCR and Southern analyses of total cellular DNA indicated that the opp gene cluster was chromosomally located in KB4 (44).
L. lactis KB4 carries functional PrtP.
As prtP and prtM were located on the Lac plasmid in KB4, it was necessary to determine if these genes were functional in this derivative. To examine the functionality of the prtP and prtM genes, the Lac plasmid from KB4 was isolated and electroporated into Prt− Opp+ LM0230. The resulting transformants exhibited the Fmc+ phenotype, indicating that the prtP and prtM genes from KB4 were functional.
The ability of C2 and its derivatives to hydrolyze milk proteins was initially evaluated by the Hull (12) procedure. Fmc+ (Prt+ Opp+) C2 hydrolyzed milk protein, but unlike Fmc− (Prt− Opp+) LM0230, which did not hydrolyze casein, Fmc− KB4 appeared to hydrolyze this substrate (data not shown). This result suggested that KB4 possessed functional PrtP activity. To confirm this suggestion, FITC-labeled β-casein was used as the substrate to measure proteolytic activity (Table 2). Whole-cell preparations of strain KB4 were essentially free of lysed or leaky cells, as indicated by the absence of any detectable fructose bisphosphate aldolase activity, used as a marker for cytoplasmic enzymes, and clearly possessed significant levels of proteinase activity. Further, this proteinase activity was readily solubilized when lysozyme and mutanolysin were used to disrupt the cell wall, and the generated cell wall fraction was also shown to be free of cytoplasmic enzymes. These results indicated both the functionality of the proteinase and a cell surface location, rather than indicating that the activity possibly originated from intracellular proteolytic activity.
TABLE 2.
Proteinase activity of whole cells or cell fractions of lactococcal strainsa
| Fraction | Relative fluorescence units (% activityb) in strain:
|
||
|---|---|---|---|
| C2 | LM0230 | KB4 | |
| Whole cell | 61.6 | 2.4 | 22.8 |
| Cell surface | 25.4 (15.8) | 3.1 (6.0) | 19.6 (39.4) |
| Cytoplasmic | 108 (67.2) | 41.6 (80.0) | 24.5 (49.2) |
| Particulate | 27.3 (17.0) | 7.3 (14.0) | 5.7 (11.4) |
Detected with FITC-labeled β-casein.
One hundred percent activity is the sum of the activities of the three fractions for each strain.
KB4 carries functional amino acid transport, di- and tripeptide transport, and functional oligopeptide permease systems.
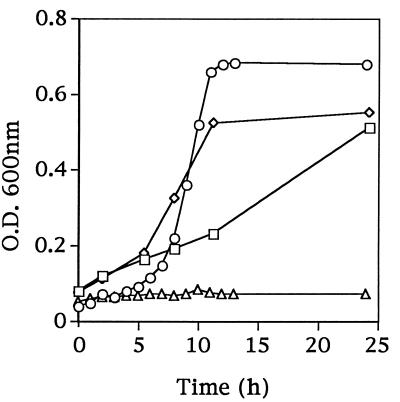
Growth studies showed that KB4 could grow in CDM containing a complete pool of amino acids (Fig. 1B) but not when leucine was omitted (Fig. 2). This result suggested that KB4 possessed functional amino acid transport systems and confirmed that Leu was an essential amino acid for the growth of KB4. When the leucine-deficient medium was supplemented with the leucine-containing dipeptide Leu-Gly or the tripeptide Leu-Gly-Gly, KB4 was also able to grow (Fig. 2). This result suggested that KB4 contained functional di- and tripeptide transport systems as well as functional intracellular peptidases to hydrolyze the peptides into free amino acids needed for growth.
FIG. 2.
Growth in CDM of Fmc− (Prt+ Opp+) L. lactis KB4 with various leucine sources: CDM without leucine (▵) and CDM without leucine but containing the dipeptide Leu-Gly (□), the tripeptide Leu-Gly-Gly (◊), or the pentapeptide Phe-Leu-Glu-Glu-Leu (○). O.D., optical density.
From the hybridization and PCR data, it was established that KB4 possessed the opp gene cluster on its chromosome. To exclude the possibility that the Fmc− phenotype of KB4 was due to mutations within the opp gene cluster, plasmid pKMP1, containing a functional opp gene cluster, was introduced into KB4. The transformants retained the Fmc− phenotype, which implied that a factor other than Opp was needed for the Fmc+ phenotype in KB4.
Further growth studies showed that KB4 was able to grow in the leucine-deficient medium when supplemented with the oligopeptide Phe-Leu-Glu-Glu-Leu (Fig. 2). This result supported the conclusion that KB4 possessed a functional Opp system as well as the peptidases to utilize this oligopeptide as a Leu source.
KB4 is an aspartate auxotrophic mutant.
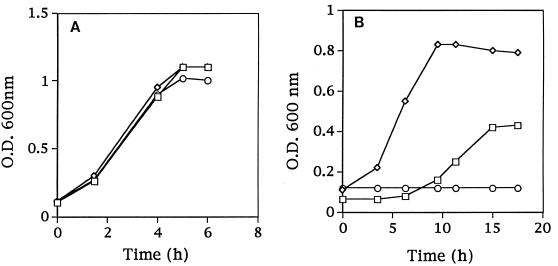
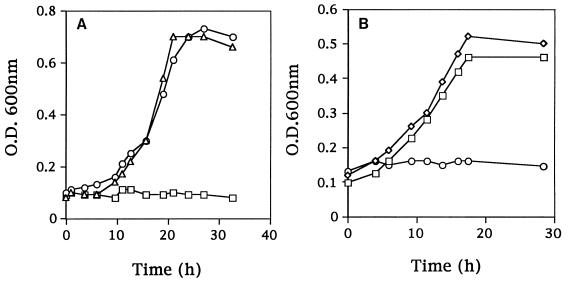
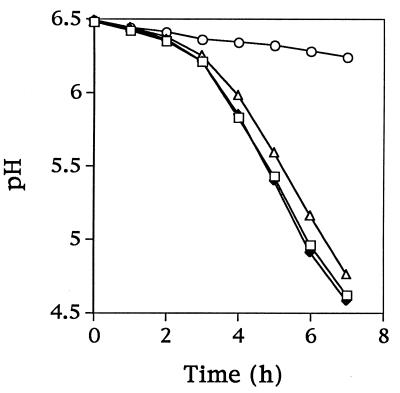
The amino acid requirements were compared for Fmc+ (Prt+ Opp+) C2, the Fmc− (Prt+ Opp+) derivative KB4, and the Fmc− (Prt− Opp+) derivative LM0230. All three strains grew in SSM when supplemented with the complete amino acid pool (Fig. 3A). C2 and LM0230 were also able to grow in SSM containing just nine essential amino acids. KB4, however, was unable to initiate growth in this medium (Fig. 3B). To further characterize the specific amino acid requirements of KB4, the remaining 11 amino acids were divided into five groups according to common biosynthetic pathways (group 1, Gln and Pro; group 2, Asp, Asn, and Lys; group 3, Cys and Gly; group 4, Ala; and group 5, Phe, Tyr, and Trp). KB4 was inoculated into SSM supplemented with each of the five groups of amino acids. Supplementation with group 2 resulted in the growth of KB4 (Fig. 4A). Supplementation with aspartic acid or asparagine was then found to meet the nutritional requirement of KB4 (the cell density reached a level similar to that of the parental strain C2 in SSM) (Fig. 4B). Thus, KB4 appeared unable to meet its aspartate requirement from casein hydrolyzed by PrtP. This conclusion was further supported by the observation that KB4 was able to coagulate milk within 18 h when the milk was supplemented with aspartic acid or asparagine. The pH decrease for KB4 in aspartate-supplemented milk was comparable to that for L. lactis C2 with or without aspartate (Fig. 5).
FIG. 3.
Growth of L. lactis C2 (□), LM0230 (◊), and KB4 (○) in SSM with all 20 amino acids (A) or with nine essential amino acids (Arg, Glu, His, Ile, Leu, Met, Ser, Thr, and Val) (B). O.D., optical density.
FIG. 4.
Growth of L. lactis KB4 in SSM supplemented with different groups of amino acids. (A) Group 1, Gln and Pro (□); group 2, Asp, Asn, and Lys (○). For SSM supplemented with group 3 (Cys and Gly), group 4 (Ala), and group 5 (Phe, Tyr, and Trp), the growth of KB4 resembled that exhibited with group 1 amino acids. The growth of L. lactis C2 in SSM supplemented with group 2 amino acids (▵) was included as a control. (B) Individual amino acid components from group 2: Asp (◊), Asn (□), and Lys (○). O.D., optical density.
FIG. 5.
Acid development by L. lactis C2 and KB4 in milk with or without added aspartate. Symbols: ○, KB4 in milk without added Asp; ▵, KB4 in milk supplemented with Asp; ⧫, C2 in milk without added Asp; □, C2 in milk supplemented with Asp.
KB4 uses various sources of aspartic acid.
Because casein contains about 7% (wt/wt) aspartate or asparagine and KB4 was shown to possess a functional proteolytic system able to utilize casein as a nitrogen source, it was necessary to confirm that KB4 possessed the essential components to utilize aspartate-containing peptides, assuming that they were produced during PrtP hydrolysis of casein. KB4 was grown in SSM supplemented with an aspartate-containing dipeptide or oligopeptide. The results indicated that the aspartate-containing peptides Asp-Gly and Asp-Ser-Asp-Pro-Arg were able to support the growth of KB4 and implied that KB4 had functional peptide transport systems as well as intracellular peptidases necessary for their utilization (Table 3). KB4 could not use the oligopeptide Gly-Asp-Asp-Asp-Asp-Lys as an aspartate source (Table 3). This result could have been due to its inability to transport the oligopeptide into the cell or to hydrolyze the oligopeptide within the cell. Common biosynthetic precursors of aspartate were also added to SSM, and their effects on KB4 growth were examined (Table 3). The data suggested that none of these precursors was able to replace aspartate as a growth requirement for KB4.
TABLE 3.
Growth of L. lactis KB4 in SSM supplemented with various aspartic acid sources or precursors of aspartic acida
| Supplementing component(s) | Growthb |
|---|---|
| Asp-Gly | + |
| Asp-Ser-Asp-Pro-Arg | + |
| Gly-Asp-Asp-Asp-Asp-Lys | − |
| OAA | − |
| Fumaric acid | − |
| Malic acid | − |
| Pyruvate | − |
According to possible biosynthetic pathways.
+, absorbance at 600 nm greater than 0.45 after 45 h of incubation at 32°C; −, absorbance at 600 nm less than 0.05 after 45 h of incubation at 32°C.
KB4 is deficient in CO2 fixation.
To further identify the defect in KB4, activities were determined for two key enzymes directly linked to aspartate biosynthesis in lactococci. GOT is responsible for transferring the NH2 group from glutamate to OAA to form aspartate. It was possible that this enzyme was defective in KB4. However, it was found that the GOT activities of L. lactis C2 and KB4 were similar (Table 4).
TABLE 4.
Assay for enzymes involved in aspartic acid biosynthesisa
| L. lactis strain | Enzyme | Sp act |
|---|---|---|
| C2 | GOT | 1.8 U/mg of protein |
| Pyruvate carboxylase | 13,663 cpm/mg of protein | |
| KB4 | GOT | 1.5 U/mg of protein |
| Pyruvate carboxylase | 740 cpm/mg of protein |
According to known biosynthetic pathways in lactococci (9).
In the late 1970s, Hillier and Jago (9) studied CO2 fixation in L. lactis C10 and concluded that pyruvate carboxylase was the major enzyme involved. The product of the fixation reaction was OAA, the precursor of aspartic acid (8–10). It was possible that KB4 was an aspartate auxotroph because of a defect in this enzyme. Preliminary results indicated that whole cells of C2 were 16 times more efficient in fixing [14C]bicarbonate than whole cells of KB4 when incubated with 14C-labeled NaHCO3. The GOT-coupled pyruvate carboxylase activity assay of CEs of C2 and KB4 revealed that the activity of this enzyme in KB4 was about 20 times lower than that in C2 (Table 4), suggesting that KB4 was defective in pyruvate carboxylase activity.
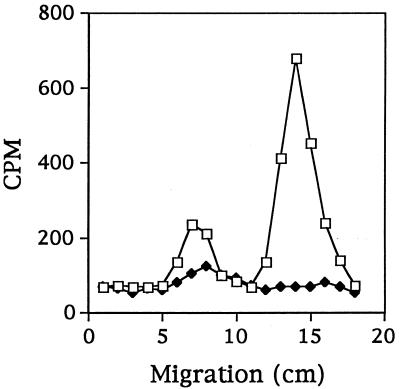
The 14C-labeled products of the enzyme reaction were subjected to paper electrophoresis. The isotope label in the reaction products from C2 comigrated with a cold aspartate standard. This result indicated that aspartate was the major product of the [14C]bicarbonate fixation reaction. No similar labeled component was observed for KB4 (Fig. 6), suggesting that KB4 was unable to synthesize aspartate from CO2 and pyruvate. An unidentified minor peak that was observed in the reaction products from both C2 and KB4 could account for the minor incorporation of isotope by KB4.
FIG. 6.
Distribution of acid-stable 14C in pyruvate carboxylase activity assay products from L. lactis C2 (□) and KB4 (⧫), as determined by paper electrophoresis.
Pyruvate carboxylase activity in CE of C2 could be inhibited by OAA and aspartate. Avidin also inhibited this enzyme activity, probably because the enzyme is biotin dependent (9). When PEP replaced pyruvate as the substrate, no significant amount of 14C-labeled product could be detected in L. lactis C2 (Table 5), suggesting that PEP was not the primary substrate in the enzyme reaction.
TABLE 5.
Effects of various substrates and cofactors on pyruvate carboxylase activity in L. lactis C2
| Substrate + cofactor | % Activitya |
|---|---|
| Pyruvateb | 100 |
| Pyruvate + avidin | 30 |
| Pyruvate + aspartate | 31.5 |
| Pyruvate + OAA | 38.2 |
| PEPc | 18.1 |
Enzyme activity was measured as acid-stable 14C counts per minute in 0.4-ml samples from 1-ml reaction mixtures in the GOT-coupled reaction. Results reported are averages of the percent activity because the individual counts per minute varied in each experiment.
The average pyruvate carboxylase activity was 13,666 cpm/mg of protein. For the negative control, trichloroacetic acid was added to the reaction mixture before incubation. The average activity detected was 4.1%.
The number of moles of PEP used was the same as that for pyruvate.
DISCUSSION
Fast milk-coagulating ability is one of the characteristics of a successful cheese starter. Recent findings have shown that the loss not only of PrtP but also of Opp can cause the Fmc− phenotype (22, 30, 45). In addition, it was reported that an aminopeptidase pepA mutant had a lower acidification rate in milk (13), which might also contribute to an Fmc− phenotype. Evidence has also been presented that an extracellular peptidase is not required for the hydrolysis of oligopeptides into di- and tripeptides in order for lactococci to utilize casein as a nitrogen source (21).
During the examination of L. lactis C2 derivatives for the opp gene cluster, we observed that KB4 possessed a Lac+ Fmc− phenotype. KB4 was unable to grow in CDM containing casein as the sole nitrogen source but grew if the medium was supplemented with a pool of 20 amino acids. This result was initially misleading, because it suggested that KB4 was missing an unidentified component necessary for the cell to further break down casein hydrolytic products into smaller peptides for the available transport systems. It is believed that casein contains all of the amino acids essential for lactococcal growth (22) and should be able to provide the nitrogen requirement for KB4. However, upon examining the amino acid requirements, we found that, unlike C2, KB4 required aspartate for growth and pyruvate carboxylase activity was defective. Being deficient in CO2 fixation, OAA formation, and, hence, aspartic acid formation, KB4 became an aspartate auxotroph. KB4 was able to grow when SSM was supplemented with the dipeptide Asp-Gly or the pentapeptide Asp-Ser-Asp-Pro-Arg, demonstrating that KB4 carried functional transport systems and intracellular peptidases. KB4 was not able to grow in OAA-supplemented SSM, possibly because it lacked the proper transport system or because this compound was unstable in solution (26, 42).
Surprisingly, LM0230 grew to a higher cell density in SSM containing nine amino acids than did C2 or KB4. The reason for this phenomenon remains to be elucidated. However, supplementing SSM with aspartate or asparagine complemented the growth defect in KB4 and allowed this mutant to grow to cell densities comparable to those of the parental strain C2.
It is interesting that milk casein contains approximately 7% aspartate or asparagine but is still insufficient to meet the aspartate growth requirement of KB4. Several possibilities could account for this phenomenon. KB4 may be unable to utilize some of the casein-derived aspartate-containing oligopeptides due to the absence of appropriate transport systems (the transport systems may have preference for certain types of peptides) or may lack the intracellular peptidases needed to hydrolyze these peptides. The inability of KB4 to utilize aspartate from the oligopeptide Gly-Asp-Asp-Asp-Asp-Lys (Table 3) agrees with the assessment given above. Recently, Kunji et al. (23) also demonstrated that some peptidase-deficient mutants could not use certain Leu-containing tripeptides or oligopeptides because of the inability to hydrolyze these peptides intracellularly. This finding also supports the assumptions made above.
Another possibility is that the amount of aspartate derived from casein hydrolysis is insufficient to support lactococcal growth. As aspartate can be deaminated in vivo to form OAA, the amount of exogenous aspartate needed for KB4 actually reflects the sum of requirements for both aspartate and OAA in vivo. Further studies are needed to determine the quantity of aspartate required for the growth of lactococci. Previous reports demonstrated that aspartate may not be actively transported into L. lactis (10) or Leuconostoc mesenteroides (25). The mechanism by which KB4 is able to take up aspartate for growth needs to be investigated. The influence of pH on the transport of aspartate and asparagine in C2 and KB4 should also be considered (31).
It is known that CO2 is required for the optimum growth of lactococci in milk (16, 33). During a study of [14C]bicarbonate incorporation into L. lactis C10, Hillier and Jago (8) demonstrated that radioactivity was incorporated into the protein and nucleic acid fractions of the cell as well as into compounds which were excreted by the organism into the medium. The fixation of [14C]bicarbonate by L. lactis C10 was achieved by the combined reactions of pyruvate carboxylase and GOT to form aspartate. Resting cells of L. lactis C10 were able to synthesize aspartate de novo but could not actively transport aspartate into the cell (8–10). Our data on the pyruvate carboxylase-deficient L. lactis KB4 further illustrate the importance of this in vivo OAA biosynthesis pathway in lactococci. We are interested in KB4 because of the possible role of pyruvate carboxylase in coordinating metabolism in lactococci. OAA and aspartate not only are precursors for five other amino acids and thus critical for protein synthesis but also are involved in the biosynthesis of purines and pyrimidines. OAA is also a common intermediate for carbon metabolism, although lactococci do not have a complete tricarboxylic acid cycle. Thus, theoretically, pyruvate carboxylase is in a key position for these critical metabolic activities. Further studies of the pyruvate carboxylase gene and its expression are currently under way and might provide insights into metabolic cooperation and regulatory mechanisms in lactococci.
Study of the pyruvate carboxylase-deficient mutant L. lactis KB4 may also lead to information having industrial significance. Pyruvate is a key metabolic intermediate in lactic acid bacteria, and the flow of pyruvate into various pathways is tightly controlled. Because of the central position of pyruvate in sugar metabolism and especially its involvement in the production of flavor compounds such as diacetyl, studies of pyruvate metabolism recently have been greatly expanded (2). However, among these studies of pyruvate metabolic pathways, the drainage of pyruvate through OAA biosynthesis has been ignored. Thus, knowledge of the significance of pyruvate carboxylase will further benefit our understanding of the pyruvate pool in lactococci and may stimulate new ideas for bioengineering starter strains with enhanced diacetyl production. The unusual property of KB4 is that it is defective in pyruvate carboxylase yet can take up sufficient aspartate from the medium to fulfill its growth requirements. Thus, genetically it provides a model for generating a bioengineered strain in which one of the pathways for pyruvate drainage is blocked but which is still able to maintain other regular physiological properties in the presence of aspartate. Continuing the study of the biochemistry and molecular biology of pyruvate carboxylase in lactococci could have potential industrial applications.
ACKNOWLEDGMENTS
This research was supported in part by the Minnesota-South Dakota Dairy Foods Research Center, a National Institute of General Medical Sciences training grant in biotechnology, and the Kraft General Foods Chair in Food Science.
We thank D. Twomey for discussions and suggestions and Kathleen Baldwin for technical assistance and helpful comments on the manuscript.
Footnotes
Published as paper no. 971180027 of the contribution series of the Minnesota Agricultural Experimental Station and based on research conducted under project 18-62.
REFERENCES
- 1.Cocaign-Bousquet M, Garrigues C, Novak L, Lindley N D, Loubiere P. Rational development of a simple synthetic medium for the sustained growth of Lactococcus lactis. J Appl Bacteriol. 1995;79:108–116. [Google Scholar]
- 2.Cocaign-Bousquet M, Garrigues C, Novak L, Loubiere P, Lindley N D. Physiology of pyruvate metabolism in Lactococcus lactis. In: Venema G, Huis in’t Veld J H J, Hugenholts J, editors. Lactic acid bacteria: genetics, metabolism and application. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1996. pp. 157–171. [Google Scholar]
- 3.Coolbear T, Holland R, Crow V L. Parameters affecting the release of cell surface components and lysis of Lactococcus lactis subsp. cremoris. Int Dairy J. 1992;2:213–232. [Google Scholar]
- 4.Coolbear T, Pillidge C J, Crow V L. The diversity of potential cheese ripening characteristics of lactic acid starter bacteria. 1. Resistance to cell lysis and levels and cellular distribution of proteinase activities. Int Dairy J. 1994;4:697–721. [Google Scholar]
- 5.Crow V L, Holland R, Coolbear T. Comparison of subcellular fractionation methods for Lactococcus lactis subsp. lactis and L. lactis subsp. cremoris. Int Dairy J. 1993;3:599–611. [Google Scholar]
- 6.Crow V L, Thomas T D. d-Tagatose 1,6-diphosphate aldolase from lactic streptococci: purification, properties, and use in measuring intracellular tagatose 1,6-diphosphate. J Bacteriol. 1982;151:600–608. doi: 10.1128/jb.151.2.600-608.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Efstathiou J D, McKay L L. Inorganic salts resistance associated with a lactose-fermenting plasmid in Streptococcus lactis. J Bacteriol. 1977;130:257–265. doi: 10.1128/jb.130.1.257-265.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hillier A J, Jago G R. Metabolism of [14C]bicarbonate by Streptococcus lactis: identification and distribution of labeled compounds. J Dairy Res. 1978;45:231–240. doi: 10.1017/s0022029900016654. [DOI] [PubMed] [Google Scholar]
- 9.Hillier A J, Jago G R. The metabolism of [14C]bicarbonate by Streptococcus lactis: the fixation of [14C]bicarbonate by pyruvate carboxylase. J Dairy Res. 1978;45:433–444. doi: 10.1017/s0022029900016654. [DOI] [PubMed] [Google Scholar]
- 10.Hillier A J, Rice G H, Jago G R. Metabolism of [14C]bicarbonate by Streptococcus lactis: the synthesis, uptake and excretion of aspartate by resting cells. J Dairy Res. 1978;45:241–246. [Google Scholar]
- 11.Horng J S, Polzin K M, McKay L L. Replication and temperature-sensitive maintenance functions of lactose plasmid pSK11L from Lactococcus lactis subsp. cremoris. J Bacteriol. 1991;173:7573–7581. doi: 10.1128/jb.173.23.7573-7581.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hull M E. Studies on milk proteins. II. Colorimetric determination of the partial hydrolysis of proteins in milk. J Dairy Sci. 1947;30:881–884. [Google Scholar]
- 13.I’Anson K J A, Movahedi S, Griffin H G, Gasson M J, Mulholland F. A non-essential glutamyl aminopeptidase is required for optimal growth of Lactococcus lactis MG1363 in milk. Microbiology. 1995;141:2873–2881. doi: 10.1099/13500872-141-11-2873. [DOI] [PubMed] [Google Scholar]
- 14.Juillard V, Laan H, Kunji E R S, Jeronimus-Stratingh C M, Bruins A P, Konings W N. The extracellular PI-type proteinase of Lactococcus lactis hydrolyzes β-casein into more than one hundred different oligopeptides. J Bacteriol. 1995;177:3472–3478. doi: 10.1128/jb.177.12.3472-3478.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Juillard V, Le Bars D, Kunji E R S, Konings W N, Gripon J-C, Richard J. Oligopeptides are the main source of nitrogen for Lactococcus lactis during growth in milk. Appl Environ Microbiol. 1995;61:3024–3030. doi: 10.1128/aem.61.8.3024-3030.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keen A R. Growth studies on the lactic streptococci. II. The effect of agitation on the growth characteristics of Streptococcus lactis ML3 in batch culture. J Dairy Res. 1972;39:141–150. doi: 10.1017/s0022029900013935. [DOI] [PubMed] [Google Scholar]
- 17.Klaenhammer T R, McKay L L, Baldwin K A. Improved lysis of group N streptococci for isolation and rapid characterization of plasmid deoxyribonucleic acid. Appl Environ Microbiol. 1978;35:592–600. doi: 10.1128/aem.35.3.592-600.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kok J. Genetics of the proteolytic system of lactic acid bacteria. FEMS Microbiol Rev. 1990;87:15–42. doi: 10.1111/j.1574-6968.1990.tb04877.x. [DOI] [PubMed] [Google Scholar]
- 19.Kok J, de Vos W M. The proteolytic system of lactic acid bacteria. In: Gasson M J, de Vos W M, editors. Genetics and biotechnology of lactic acid bacteria. Glasgow, United Kingdom: Blackie Academic & Professional; 1994. pp. 169–210. [Google Scholar]
- 20.Kok J, Leenhouts K J, Haandrikman A J, Ledeboer A M, Venema G. Nucleotide sequence of the cell wall proteinase gene of Streptococcus cremoris Wg2. Appl Environ Microbiol. 1988;54:231–238. doi: 10.1128/aem.54.1.231-238.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kunji E R S, Hagting A, de Vries C J, Juillard V, Haandrikman A J, Poolman B, Konings W N. Transport of β-casein-derived peptides by the oligopeptide transport system is a crucial step in the proteolytic pathway of Lactococcus lactis. J Biol Chem. 1995;270:1569–1574. doi: 10.1074/jbc.270.4.1569. [DOI] [PubMed] [Google Scholar]
- 22.Kunji E R S, Mierau I, Hagting A, Poolman B, Konings W N. The proteolytic systems of lactic acid bacteria. In: Venema G, Huis in’t Veld J H J, Hugenholts J, editors. Lactic acid bacteria: genetics, metabolism and application. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1996. pp. 91–125. [Google Scholar]
- 23.Kunji E R S, Mierau I, Poolman B, Konings W N, Venema G, Kok J. Fate of peptides in peptidase mutants of Lactococcus lactis. Mol Microbiol. 1996;21:123–131. doi: 10.1046/j.1365-2958.1996.6231339.x. [DOI] [PubMed] [Google Scholar]
- 24.Kunji E R S, Smid E J, Plapp R, Poolman B, Konings W N. Di-tripeptides and oligopeptides are taken up via distinct transport mechanisms in Lactococcus lactis. J Bacteriol. 1993;175:2052–2059. doi: 10.1128/jb.175.7.2052-2059.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marty-Teysset C, Lolkema J S, Schmitt P, Divies C, Konings W N. The citrate metabolic pathway in Leuconostoc mesenteroides: expression, amino acid synthesis, and α-ketocarboxylate transport. J Bacteriol. 1996;178:6209–6215. doi: 10.1128/jb.178.21.6209-6215.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McFeeters R F, Chen K. Utilization of electron acceptors for anaerobic mannitol metabolism by Lactobacillus plantarum. Compounds which serve as electron acceptors. Food Microbiol. 1986;3:73–81. [Google Scholar]
- 27.McKay L L, Baldwin K A, Zottola E A. Loss of lactose metabolism in lactic streptococci. Appl Microbiol. 1972;23:1090–1096. doi: 10.1128/am.23.6.1090-1096.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mierau I. Peptide degradation in Lactococcus lactis in vivo: a first exploration. Ph.D. dissertation. Groningen, The Netherlands: University of Groningen; 1996. [Google Scholar]
- 29.Otto R, ten Brink B, Veldkamp H, Konings W N. The relation between growth rate and electrochemical proton gradient of Streptococcus cremoris. FEMS Microbiol Lett. 1983;16:69–74. [Google Scholar]
- 30.Poolman B, Juillard V, Kunji E R S, Hagting A, Konings W N. Casein-breakdown by Lactococcus lactis. In: Bozoglu T F, Ray B, editors. Lactic acid bacteria: current advances in metabolism, genetics and applications. Berlin, Germany: Springer-Verlag KG; 1996. pp. 304–326. [Google Scholar]
- 31.Poolman B, Konings W N. Relation of growth of Streptococcus lactis and Streptococcus cremoris to amino acid transport. J Bacteriol. 1988;170:700–707. doi: 10.1128/jb.170.2.700-707.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pritchard G G, Coolbear T. The physiology and biochemistry of the proteolytic system in lactic acid bacteria. FEMS Microbiol Rev. 1993;12:179–206. doi: 10.1111/j.1574-6976.1993.tb00018.x. [DOI] [PubMed] [Google Scholar]
- 33.Reiter B, Oram J D. A note on the carbon dioxide requirement of Streptococcus lactis strain ML3. J Dairy Res. 1961;28:175–176. [Google Scholar]
- 34.Renner E D, Bernlohr R W. Characterization and regulation of pyruvate carboxylase of Bacillus licheniformis. J Bacteriol. 1972;109:764–772. doi: 10.1128/jb.109.2.764-772.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 36.Terzaghi B E, Sandine W E. Improved medium for lactic streptococci and their bacteriophages. Appl Microbiol. 1975;29:807–813. doi: 10.1128/am.29.6.807-813.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Twining S S. Fluorescein isothiocyanate-labeled casein assay for proteolytic enzymes. Anal Biochem. 1984;143:30–34. doi: 10.1016/0003-2697(84)90553-0. [DOI] [PubMed] [Google Scholar]
- 38.Tynkkynen S, Buist G, Kunji E R S, Kok J, Poolman B, Venema G, Haandrikman A J. Genetic and biochemical characterization of the oligopeptide transport system of Lactococcus lactis. J Bacteriol. 1993;175:7523–7532. doi: 10.1128/jb.175.23.7523-7532.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vos P, van Asseldonk M, van Jeveren F, Siezen R, Simons G, de Vos W M. A maturation protein is essential for production of active forms of Lactococcus lactis SK11 serine proteinase located in or secreted from the cell envelope. J Bacteriol. 1989;171:2795–2802. doi: 10.1128/jb.171.5.2795-2802.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wahls K E. Construction of lactate dehydrogenase-deficient lactococcal variants for potential use in enhancing diacetyl production. M.S. thesis. St. Paul: University of Minnesota; 1994. [Google Scholar]
- 41.Whitaker J R. Amines, amino acids, and peptides. In: Zweig G, Whitaker J R, editors. Paper chromatography and electrophoresis. New York, N.Y: Academic Press, Inc.; 1967. pp. 50–101. [Google Scholar]
- 42.Wilcock A R, Goldberg D M. Kinetic determination of malate dehydrogenase activity eliminating problems due to spontaneous conversion of oxaloacetate to pyruvate. Biochem Med. 1972;6:116–126. doi: 10.1016/0006-2944(72)90029-4. [DOI] [PubMed] [Google Scholar]
- 43.Yagi T, Kagamiyama H, Nozaki M, Soda K. Glutamate-aspartate transaminase from microorganisms. Methods Enzymol. 1985;113:83–84. doi: 10.1016/s0076-6879(85)13020-x. [DOI] [PubMed] [Google Scholar]
- 44.Yu W. Sequence determination and characterization of regions flanking the replication origin of the plasmid pSK11L from Lactococcus lactis subsp. cremoris. Ph.D. dissertation. St. Paul: University of Minnesota; 1994. [Google Scholar]
- 45.Yu W, Gillies K, Kondo J K, Broadbent J R, McKay L L. Loss of plasmid-mediated oligopeptide transport system in lactococci: another reason for slow milk coagulation. Plasmid. 1996;35:145–155. doi: 10.1006/plas.1996.0017. [DOI] [PubMed] [Google Scholar]