Abstract
Background/Aim
Patients with radiation sensitive Fanconi anemia (FA) are presenting with cancers of the oral cavity, oropharynx, and other anatomic locations.
Materials and Methods
Animal models for cancer in FA mice used orthotopic tumors from wild type mice. We derived a cancer cell line from Fanca–/– mice by topical application of the chemical carcinogen dimethyl benzanthracene (DMBA).
Results
A Fanca–/– mouse rhabdomyosarcoma was derived from a Fanca–/– (129/Sv) mouse. The in vitro clonogenic survival of the Fanca–/– clone 6 cancer cell line was consistent with the FA genotype. Transplanted tumors demonstrated hypoxic centers surrounded by senescent cells.
Conclusion
This Fanca–/– mouse syngeneic cancer should provide a valuable resource for discovery and development of new normal tissue radioprotectors for patients with FA and cancer.
Keywords: Fanconi anemia, carcinogen, transplantable tumor
Patients with Fanconi anemia (FA) demonstrate absence or functional defects in one or more of the gene products associated with the DNA-repair protein scaffold, which facilitates binding of repair enzymes to DNA double strand breaks (1,2). Many patients with FA suffer from anemia, which results from a hyper-responsiveness of their hematopoietic stem cells to the suppressive cytokine TGF-β (3-5) that is naturally produced by cells of the hematopoietic microenvironment. In the past decades, a significant number of patients with FA have been successfully treated with bone marrow transplantation (6-9). Successful bone marrow transplantation has been attributed in part to the availability of less toxic transplantation preparatory regimens (6-9). New preparation methods for bone marrow transplantation in patients with FA have included partial body irradiation, and delivery of chemotherapy drugs such as fludarabine (7) that are less toxic to normal tissue. Based upon the success of bone marrow transplantation, many patients with FA now live longer and have more productive lives (7,8,10-12).
A recent serious concern for both marrow-transplanted and non-transplanted patients with FA is the increasing frequency of cancer (13-16). Patients with FA are presenting with squamous cell carcinomas of the head and neck region, commonly at the base of tongue and oropharynx (17,18), but also at other sites in the aerodigestive tract including those in the esophagus and vulvovaginal region (15-18). Patients with FA may be sensitive to both ionizing irradiation (19) and DNA cross-linking chemotherapy agents such as mitomycin-C or cis-platinum (10).
The management of cancer in patients with FA has been complicated not only by their sensitivity for radiotherapy, but also by the locally aggressive invasiveness of the tumors, and their propensity for both local recurrence and distant metastasis (14). At the time of initial diagnosis, cancers in patients with FA show a high frequency of microscopic invasion beyond surgical resection margins (14), significant spread to regional lymph nodes, and distant metastasis (20-22). Recent research has focused on methods to reduce the toxicity of chemoradiotherapy in patients with FA (20-27). The development of new agents to protect normal tissues, while compromising the viability of residual tumor cells has already led to the discovery of novel radioprotective agents including the mitochondrial targeted nitroxide JP4-039 (23-29).
One potential problem with the discovery of new radioprotective agents to manage cancer in patients with FA is that current animal models that are used to define the therapeutic ratio (tumor reduction relative to normal tissue toxicity) use orthotopic tumors of a different genotype (26). A transplantable cancer in FA mice using cells of the same genotype would facilitate a better analysis of the magnitude of tumor cytoreduction by chemoradiotherapy relative to normal tissue toxicity. We here report a carcinogen (DMBA)-induced transplantable cancer cell line developed in Fanca–/– mice. This cell line, when injected subcutaneously, will develop an orthotopic tumor. The initial radiobiology studies demonstrate the potential value of this cancer cell line to form orthotopic tumors, which are suitable for evaluation of the toxicity of fractionated radiotherapy.
Materials and Methods
Mice and animal care. Fanca+/– mice on the 129/Sv background were originally obtained from Markus Grompe, PhD, Oregon Health Sciences Center. Colonies of Fanca+/+ and Fanca–/– mice were derived by breeding Fanca+/– mice to yield Fanca+/+ and Fanca–/– mice. The homozygous Fanca+/+ males were bred with Fanca+/+ females, and then male Fanca–/– with female Fanca–/– mice to yield homozygous Fanca+/+ and Fanca–/– colonies (27). The mouse colonies were maintained at the University of Pittsburgh. Animals were housed 4 per cage and managed according to the University of Pittsburgh Institutional Animal Care and Use Committee (IACUC) regulations. All animal protocols were approved by the University of Pittsburgh IACUC. Animals were maintained on deionized water and fed standard laboratory chow. Veterinary care was provided by the Division of Laboratory Animal Resources of the University of Pittsburgh.
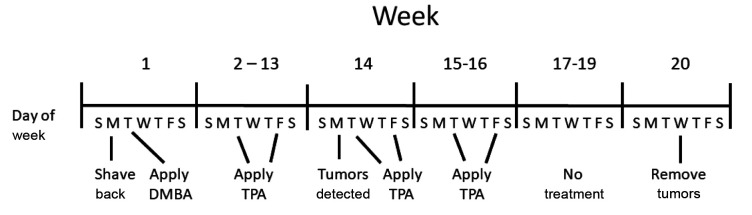
DMBA carcinogen induced tumors. The intrascapular region of the dorsum of the back of each mouse of groups of 10 six- to eight-week-old Fanca+/+ and Fanca–/– mice were shaved. Twenty-four hours later, 30 μg of dimethyl-benzanthracene (DMBA) (Millipore Sigma, Burlington, MA, USA) was dissolved in 200 μl of acetone (Millipore Sigma) and applied to the backs of each animal. Beginning one week later 12.34 μg of PMA [Phorbol 12-myristate 13-acetate, also known as tissue plasminogen activator (TPA); Millipore Sigma]. TPA was dissolved in 200 μl of acetone and applied bi-weekly to the same area of the skin of the back for 20 weeks. Tumor nodules formed at the site of both Fanca+/+ mice and Fanca–/– mice. The tumor nodules were removed, prepared into a single cell suspension by mincing with scissors and filtered through progressively smaller gauge needles down to 30 gauge. The cells were grown in vitro in Dulbecco’s Modified Eagles medium containing 20% fetal calf serum and penicillin/streptomycin (5,000 U/ml). The DMBA induced Fanca–/– tumor cells were passaged weekly at 1:2 and 1:5, then aliquots of 1×106 of the Fanca–/– or Fanca+/+ tumor cells were frozen in dimethyl sulfoxide (DMSO) (Millipore Sigma) at –80˚. Cells (106 cells in 100 μl saline) were injected subcutaneously in the dorsum of the back of Fanca–/– or Fanca+/+ mice. Three weeks later palpable tumors were detected on the back of the Fanca–/– mice. By 6 weeks after injection, tumors of 1 cm diameter were removed and prepared as single cell suspensions Aliquots of resuspended cells at 1×107 cells were injected subcutaneously into Fanca–/– or Fanca+/+ mice. The remaining resuspended cells were expanded in vitro for cloning. A tumor from a Fanca+/+ mouse that was treated with DMBA and TPA was also established, as a cell line. Th in vitro cell line was passaged three times. An aliquot of 1×107 Fanca+/+ tumor cells was injected subcutaneously in the back of each of 5 Fanca+/+ mice. The schema of the tumor induction process is shown in Figure 1.
Figure 1. Time course of tumor induction. DMBA/TPA was administered for over 16 weeks and tumors appeared at week 14 for Fanca+/+ and week 15 for Fanca–/– mice. Fanca–/– and Fanca+/+ mice in groups of 10 were topically administered with DMBA (100 μM) in 100 μl saline as described in the Materials and Methods section. Beginning at week 2 and weekly for 13 weeks, mice received twice weekly skin applications of the promoter TPA as described in Materials and Methods.
Cloning of Fanca–/– tumor cell line. To obtain a homogenous Fanca–/– tumor cell line, the Fanca–/– tumor cell line was cloned after 4 weeks passage in vitro. Single cell suspensions were cloned by using a flow cytometer (Beckman Coulter MoFlo Astrios High Speed Sorter, Beckman Coulter, Brea, CA, USA) to place one cell in each well of each of three 96-well plates. Clonal cell lines were grown in medium containing DMBA, 20% fetal calf serum and antibiotics. Clonal cell lines were expanded and grown in medium containing 0 to 20% fetal calf serum. Clone 6 was chosen for study due to the speed of growth in 1% fetal calf serum. Aliquots of 108 cells of clone 6 were centrifuged to concentrate in 100 μl saline and injected subcutaneously into the back of each of 10 Fanca–/– and Fanca+/+ mice.
Other mouse cancer cell lines. The 3LL Lewis Lung squamous cell carcinoma cell line has been described (30,31). The 2F8cis mouse ovarian cancer cell line has been described (32,33).
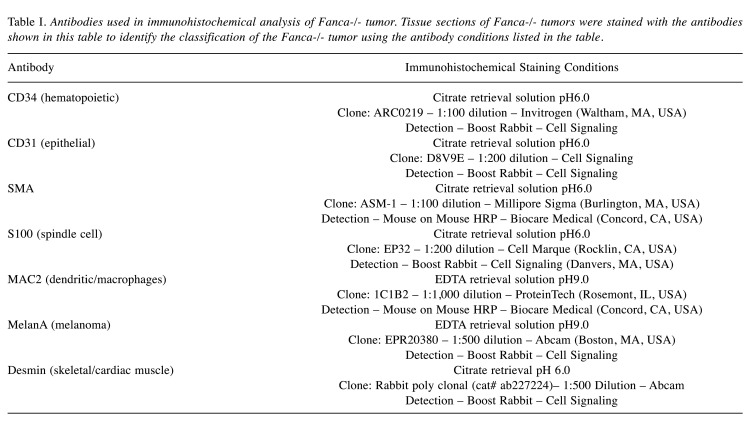
Histochemical and histopathologic staining. We analyzed the histopathology of the primary tumors and each of successive transplant generation tumors. A 2 cm tumor formed by injecting 108 Fanca–/– clone 6 cells was excised from a Fanca–/– mouse, fixed in 10% paraformaldehyde and parafilm embedded. The tumor was sectioned and stained using hematoxylin and eosin, as well as for markers to identify the phenotype. To further characterize tumors formed by Fanca–/– tumor cell lines, and clonal sublines, tumor cell sections were immunocytochemically stained for SMA, S100 (spindle cell markers), CD34 (hematopoietic stem cell), MAC2 (histiocytic marker), CD31 (endothelial), Melan-a (melanoma), and Desmin (smooth/cardiac muscle). Details on the antibodies such as supplier, dilution, and antigen retrieval are shown in Table I. The slides were deparaffinized and rehydrated using a standard histology protocol. Antigen retrieval was performed using a citrate retrieval solution pH 6.0 or EDTA retrieval solution pH 9.0 (ab93678 or ab93680, Abcam, Waltham, MA, USA) and a Decloaking chamber NXGEN (Biocare Medical, Pacheco, CA, USA) at 120˚C. The slides were stained using an Autostainer Plus (Dako, Carpenteria, CA, USA) with TBST rinse buffer (Dako). Each antibody was applied as described in Table I. The detection system was an HRP (Horse Radish Peroxidase) labeled polymer as listed in Table I. The substrate used was 3,3, Diaminobenzidine + (Dako). The slides were then counterstained with Hematoxylin (Dako).
Table I. Antibodies used in immunohistochemical analysis of Fanca-/- tumor. Tissue sections of Fanca-/- tumors were stained with the antibodies shown in this table to identify the classification of the Fanca-/- tumor using the antibody conditions listed in the table.
Assays for hypoxic regions in tumors and for senescent cells. Histopathological sections were stained for hypoxia inducible factor-1 (34), and simultaneously for p16 or beta-galactosidase, which are biomarkers of senescence (35) as previously described.
Absence of the Fanca gene and gene product in Fanca–/– tumor cells. To confirm that the DMBA-induced tumor cell line was derived from a Fanca–/– mouse, DNA was extracted from the Fanca–/– tumor cell line and a PCR reaction was performed using Fanca–/– gene specific primers used in genotyping of Fanca–/– mice (27).
Clonogenic radiation survival curves of Fanca–/– tumor cell lines. The methods for clonogenic radiation survival curves have been published previously (24). Briefly, clonal lines and pools of cancer cells were irradiated to doses between 0 and 8 Gy using a Cesium JL Shepherd Model 68A irradiator (JL Shepherd & Associates, San Fernando, CA, USA) at 275 cGy per minute. Cells were plated at varying densities ranging from 100 to 1,000 cells per plate and stained with crystal violet (Millipore Sigma) 7 days later. Quadruplicate plates at each radiation dose were scored on day 7 for colonies containing greater than 50 cells. The radiation survival curves were plotted using a computer program that calculates the D0 and ñ according to published methods using single-hit, multi-target, and linear quadratic models (24). Control cells included Lewis Lung Carcinoma (3LL) lung squamous cell carcinoma cells (30,31). Results were calculated by pooling triplicate experiments for data presentation.
Physics. The cells were irradiated using a JL Shepherd Model 68A cesium irradiator (JL Shepherd & Associates). The dose in cGy was determined using a cesium decay chart. The dose rate was verified each year by the Center for Medical Countermeasures Against Radiation Consortium (CMCRC) Physics Core at the University of Wisconsin who sends out phantoms for irradiation calibration using our Cesium Irradiator (26). The phantoms were sent back to the core to calculate the dose rate.
Statistics. All statistical calculations were performed using a Student t-test on the Graph Pad Prism (Boston, MA, USA) (23-27).
Results
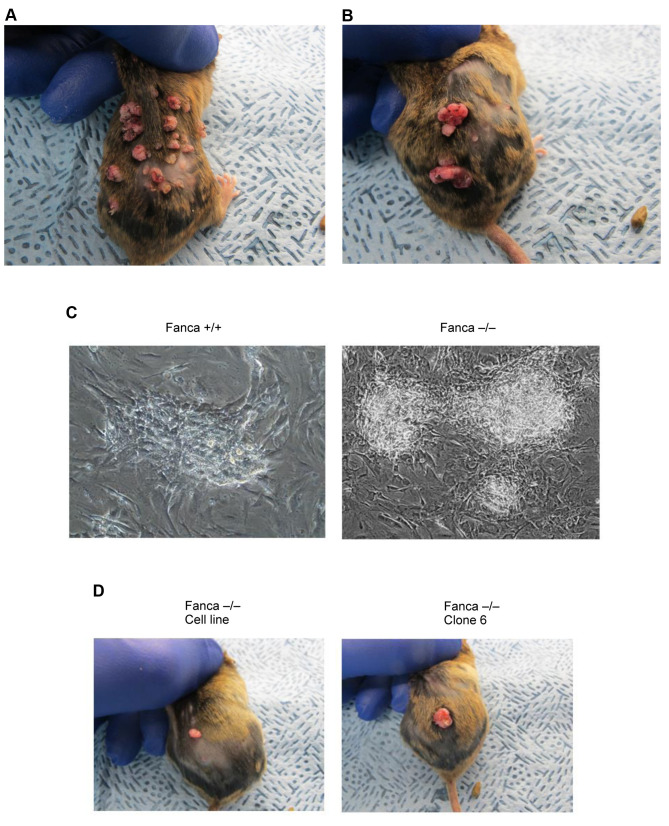
The formation of tumors at sites of DMBA injection. Tumors developed at the site of DMBA-application according to the protocol described in the Materials and Methods and are shown in Figure 1. The paradigm for DMBA and promoter application and time course are shown in Figure 1. Both Fanca–/– and Fanca+/+ mice had tumors by 14 weeks at the site of DMBA injection (Figure 2). The tumors were removed and made into single cell suspensions and cell lines developed (Figure 2C). The Fanca–/– cell line and clone 6 of the Fanca–/– cell line were injected into additional C57BL/6 mice, which we refer to as second generation mice and tumors developed 2 weeks after injection (Figure 2C).
Figure 2. Tumors at the site of topical administration of DMBA/TPA in Fanca–/– and Fanca+/+ mice (A and B). When tumors reached 1.0 cm in diameter, they were explanted to culture (×100) (C). Cells of Fanca–/– tumors or Fanca clone 6 were injected into the skin of the back of a second group of Fanca–/– and Fanca+/+ mice at 107 cells per 100 μl saline (D).
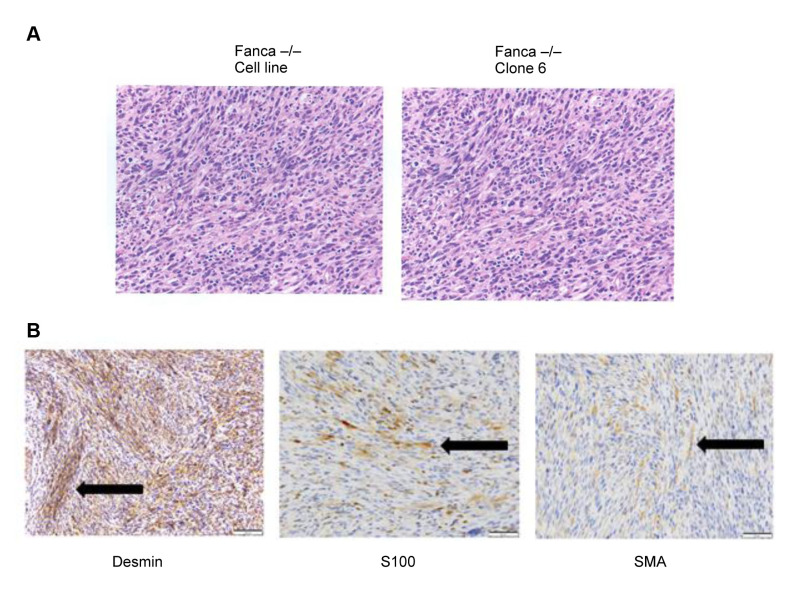
Histopathologic diagnosis and immunohistochemistry of tumors in Fanca–/– mice. Hematoxylin and Eosin staining of the Fanca–/– tumors (Figure 3A) showed moderately pleomorphic neoplastic spindle cells. There was moderate to high cellularity and poorly defined eosinophilic cytoplasm. The mitotic rate averaged two mitoses per 40 × high powered fields (HPF) (Figure 3A). The results of immunohisto-chemical (IHC) staining showed a strong positivity for the skeletal/cardiac muscle marker desmin, and slight positivity for SMA and S100 (Figure 3B), which is indicative of a primitive muscle tumor, most likely a rhabdomyosarcoma. The large spindle cells and high mitotic rate were suggestive of neoplasia. There was no evidence of glandular or epithelial morphology. This spindle cell morphology has been seen following injection of mice with breast cancer cell lines. The spindle cell morphology combined with a strong positivity for Desmin, moderate positivity for S100, and negative for SMA defines this as a malignant smooth muscle tumor. The IHC characteristics of the Fanca–/– clone 6 tumor were similar to those of the uncloned Fanca–/– tumor. There was strong immunoreactivity for desmin, and moderate immunoreactivity for SMA and S100. There was no immunoreactivity for CD31 (except vessels – endothelial cells), Melan A – melanoma, MAC2 – dendritic/macrophages, and CD34 hematopoietic/ endothelial. The results of IHC staining of tumor cells were consistent with the diagnosis of rhabdomyosarcoma.
Figure 3. Histopathology of Fanca–/– tumors. Fanca–/– and Fanca–/– Clone 6 tumors were excised, fixed in 10% formalin, sectioned, and stained with hematoxylin and eosin (H & E) (A). To identify the type of tumor, sections of Fanca–/– tumor were stained for multiple histochemical markers (B). Sections were strongly positive for Desmin and weakly positive for S100 and SMA. These are characteristics of a muscle tumor, most likely rhabdomyosarcoma. Black arrows point to areas of positive staining.
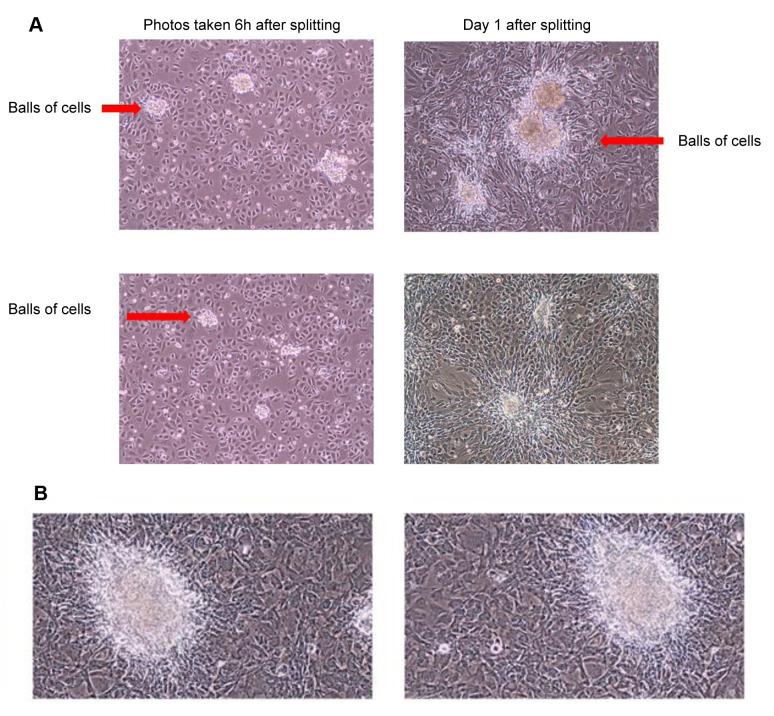
Results of serial passage of tumor cell line. Cell lines were grown in vitro as nonadherent cells mixed with adherent cells and piled up in clusters of cells on plates (Figure 4). The cells formed colonies when grown in agar. The results of serial in vitro passage of Fanca–/– and Fanca+/+ tumor cell lines revealed a highly reproducible morphology and growth pattern of adherent and clumped cells. The Fanca–/– tumor cells grew as adherent cells as well as spheres of 50 to 100 cells (Figure 4A). The Fanca+/+ cells grew as adherent cells; however did not produce spheres, but formed nodules when they became confluent (Figure 4B). Clonal lines displayed the same pattern.
Figure 4. Morphology of tumor cell lines in vitro on flat surfaces. Explanted tumors were passaged weekly at 1:10 and 1:50 dilutions. When culture plates became confluent, they were photographed (×100). Fanca–/– tumor cells grew as flattened cells with some of the cells growing in spheres of 100 cells (A) (red arrows). Fanca+/+ tumor cells do not form spheres of cells (B).
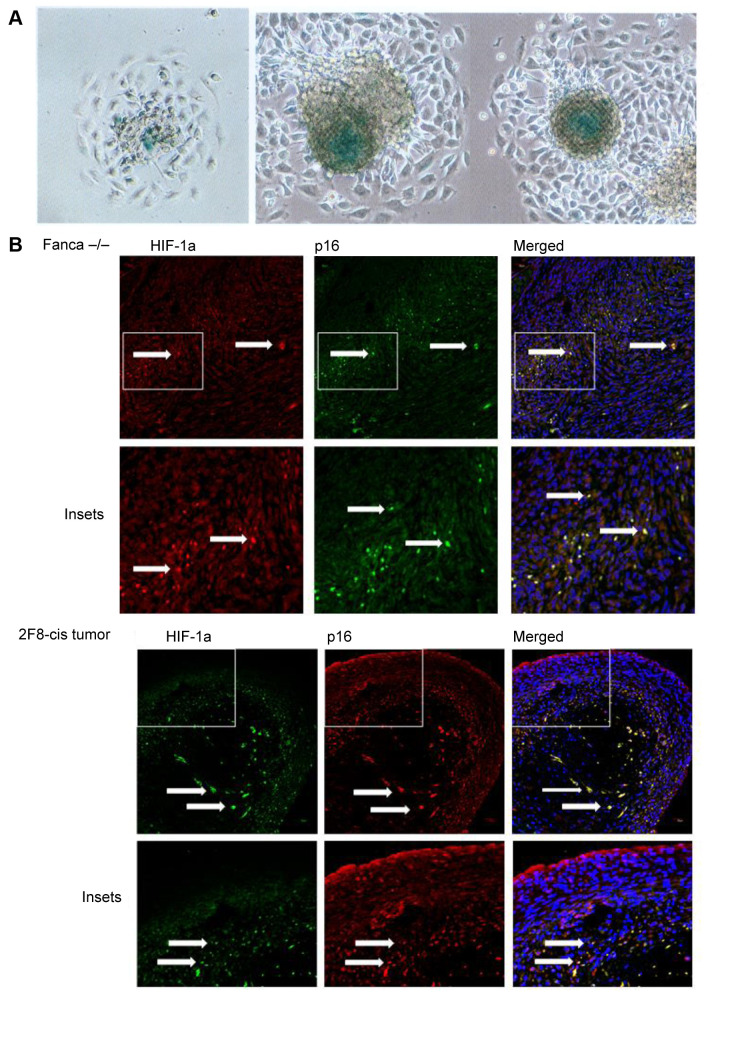
Presence of hypoxic centers in tumors from Fanca–/– Clone 6 transplantation. We next quantified the presence of senescent cells in the tumors produced by the transplanted Fanca–/– and Fanca+/+ tumor cell lines. The tumor cells demonstrated β-Gal positive tumor spheroids or tumor nodules when grown in vitro (Figure 5A). The tumors derived from Fanca–/– clone 6 cells contained hypoxic areas stained for HIF-1 and senescent cells that were stained for p16 (Figure 5B). As shown in Figure 5B, tumors demonstrated hypoxic centers with positive cells stained for hypoxia-inducible factor (HIF-1). The appearance of senescent cells in areas of hypoxia was uniformly observed in each transplant generation. Staining of tumor sections for senescent biomarkers, p16 and HIF-1, revealed senescent cells in areas of hypoxia (Figure 5B). These results are consistent with the rapid growth of cancer cells in orthotopic tumors. The results were compared with a tumor developed by a cell line derived from mouse ovarian cancer, 2F8cis (which also showed hypoxic centers stained for HIF-1 and senescent cells stained for p16) (Figure 5B).
Figure 5. Hypoxia and senescent cells in Fanca–/– tumors. HIF-1 positive cells and senescent cells in Fanca–/– spheres in vitro. Cell cultures were stained for biomarkers of hypoxia (HIF-1) and senescence (Beta-galactosidase) as described in the Materials and Methods (×100) (A): Tumor sections from Fanca–/– and 2F8cis ovarian cancer cell lines demonstrating senescent cells (p16 cells positive), which are also positive for HIF-1α. Arrows point to p16 positive, HIF-1α positive, or positive for both p16 and HIF-1α cells (B).
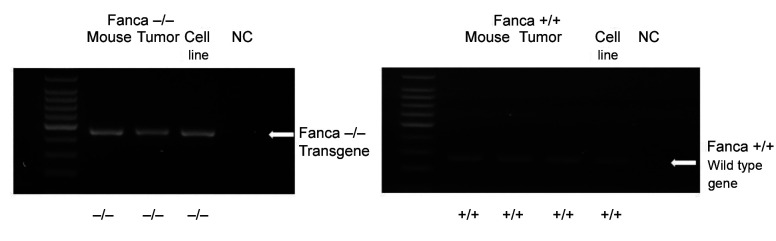
Genotype of Fanca–/– carcinogen-induced cancer cells. To confirm the genotype of cancer cell lines, RT-PCR performed for the detection of the Fanca gene revealed the absence of Fanca gene in the Fanca–/– mouse derived cell lines (Figure 6). These results indicate that the cancer cell line maintained the genotype of the original mouse from which the tumor was derived. In contrast, cells from the Fanca+/+ mouse derived tumor showed an intact Fanca gene (Figure 6).
Figure 6. Absence of Fanca gene in Fanca–/– clone 6 tumor cell line. DNA extracted from Fanca–/– clone 6 and control Fanca+/+ tumor cell lines was subjected to polymerase chain reaction (PCR) using primers specific for Fanca–/– transgene or Fanca+/+ gene. The PCR products were electrophoresed, gels stained with Ethidium Bromide, and observed under a UV light. Fanca–/– PCR reaction is shown on the left and Fanca+/+ PCR reaction shown on the right. Fanca–/– clone 6 cells had the Fanca–/– transgene, but not the Fanca+/+ gene, while the Fanca+/+ cells contained only the Fanca+/+ gene. NC: Negative control.
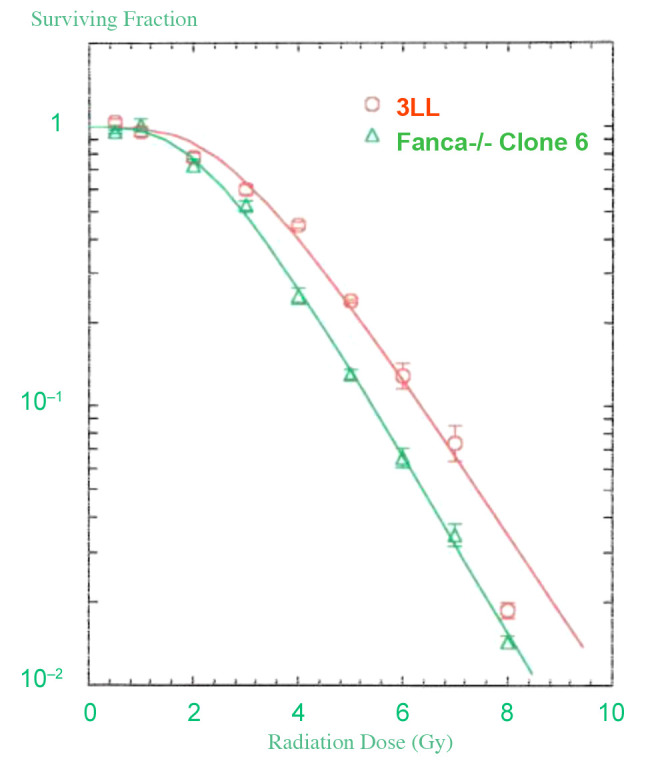
Clonogenic radiation survival of Fanca–/– tumor cell lines. As shown in Figure 7, the clonogenic radiation survival curve for Fanca–/– clone 6 revealed D0=1.57±0.11 and ñ=3.3±1.3 similar to those of the 3LL (Lewis Lung Carcinoma) cell line: D0=1.56±0.08 and ñ=3.6±1.6. The clonogenic radiation survival curves of Fanca–/– clone 6 tumor cells in culture were similar to those of wild type 3LL tumor cells.
Figure 7. Radiation survival curves in vitro. Clonogenic radiation survival curves were carried out as published (24) and are described in Materials and Methods. The Fanca-/- and 3LL mouse lung cancer cell lines were irradiated at doses ranging from 0 to 8 Gy and plated at 100, 500, or 1000 cells per culture dish. Seven days after irradiation, the cells were stained with crystal violet and colonies greater than 50 cells were counted. The data was analyzed using a single-hit, multi-target, and linear quadratic models. The data are the results of at least 3 separate experiments. The survival curve for Fanca-/- and 3LL cells is shown.
Discussion
The success of bone marrow transplantation in patients with FA has resulted in improved survival and quality of life for most children and young adults presenting with FA (6-8,11,18-19,36). The increased number of patients with FA, worldwide, is the result of the earlier diagnosis of many patients with anemia of unknown origin and also of the discovery of now over 26 genes associated with FA (1,2). The first reported patients with FA presented as children with short stature, absent thumbs, and anemia (10). The first gene associated with FA was Fanca, which was followed by the discovery of multiple other genes in the FA pathway (1). The anemia in FA has been shown to be the result of the hypersensitivity of bone marrow stem cells to TGF-β, which is a suppressor of hematopoiesis (4). The complexity of the evolution of FA during embryogenesis, growth, and development after birth have been elucidated with studies of double knockout FA mice that have simultaneous deletion of TGF-β (5). Currently, patients with FA live into their 40s and 50s. They reveal sensitivity to DNA crosslinking agents that are required for management of other diseases. The sensitivity of adults as well as children to DNA crosslinking agents remains a primary diagnostic criterion for FA (10).
With the success of bone marrow transplantation for patients with FA, a new clinical problem has arisen. This is the increasing incidence of cancers, primarily squamous cell carcinomas of the aerodigestive tract and vulvovaginal region in both marrow transplanted and non-transplanted patients with FA (13-14,17,18). Cancer in patients with FA represents a major challenge for combined modality management due to several characteristics of the cancers and the patient’s sensitivity to both chemotherapy and radiotherapy (19). Patients with FA demonstrate cancers with marked invasiveness, a propensity for regional node metastasis, and local and distant recurrence (21). Radiotherapy in the management of cancer in patients with FA has been difficult due to the radiosensitivity of some patients with FA. Novel approaches have been designed to identify the radiosensitive patients with FA and, thereby, minimize the toxicity of radiotherapy (20-22). One such strategy is to apply a low dose per fraction to small tissue volume for the first weeks of a multiweek program and examine patients daily for signs of radiation toxicity: skin redness, mucositis, and neutropenia (7-9). Radiation protective drugs have been developed to increase the therapeutic ratio of irradiation in animal models using transplantation of orthotopic tumors. Orthotopic tumors have been derived from wild type mice that do not have the FA genotype (25-29). Furthermore, the radiobiology of a mouse FA cancer cell line has not been well characterized.
In the present study, we established a transplantable cancer cell line produced by treating Fanca–/– mice with a chemical carcinogen (DMBA). We demonstrated the skeletal/cardiac sarcoma histopathology, transplantability in vivo, and radiobiology in vitro. The cancer cell line that was maintained in the Fanca–/– mice had the Fanca–/– genotype and both the parent cell line and a clonal subline Fanca–/– clone 6 formed tumors in vivo that were characterized by a skeletal/cardiac sarcoma morphology. The tumor cells were histochemically positive for biomarkers of sarcomas. The radiobiology revealed a clonogenic survival of D0=1.57±0.11 Gy and ñ=3.3±1.3, which was similar to that of the squamous cell lung carcinoma 3LL Lewis Lung Carcinoma, which was developed in C57BL/6 mice (D0=1.56±0.08; ñ=3.6±1.6). That the Fanca–/– clone 6 mouse cancer cell line was not relatively radiosensitive may not translate to a relative radiation responsiveness of tumors in vivo. Further studies are required to establish the radiation response in vivo, and potential different responsiveness to added chemotherapy (30). Furthermore, the Fanca–/– clone 6 tumor cell line did not form tumors in unirradiated Fanca+/+ mice. Whether novel histocompatibility antigens that prompted immune rejection were induced in the tumors in Fanca–/– mice by DMBA treatment requires further investigation. At present we cannot explain the mechanism of tumor cell rejection of Fanca–/– clone 6 following transplantation into Fanca+/+ mice.
The presence of hypoxic cells in tumors that are formed by the Fanca–/– clone 6 tumor cell line presents an opportunity to test new, therapeutic agents, which target hypoxic cells in patients with FA. Furthermore, the detection of senescent cells in Fanca–/– clone 6 cell line derived tumors in vivo presents an opportunity to evaluate the therapeutic effect of new senolytic agents for potential therapeutic effect on tumor growth in vivo. Comparison of the senescence associated secretory proteins (SASP) produced by Fanca–/– clone 6 senescent cells with the SASP proteins produced by senescent cells in Fanca+/+ tumors may reveal mouse genotype specific differences. Such differences could facilitate identification of novel SASP (35) in Fanca–/– tumors; perhaps, explaining their radiosensitivity and tumor aggressiveness in vivo.
The present Fanca–/– cancer cell line and subclonal line could be valuable for studies of the genes associated with tumor invasiveness, regional lymph node metastasis, and propensity for local recurrence of cancers in patients with FA. These cell lines could also be valuable for studies of early activation of novel carcinogen-induced oncogenes (37).
There is an additional reason to study tumors formed by Fanca–/– clone 6. Patients with FA present with cancers that are known to be more invasive, aggressive, and have a propensity for local recurrence and metastasis, which appears to be greater than that in similar stage cancers in non-FA patients.
There is new interest in the role of senescent cells in tumors. Fanca–/– clone 6 transplanted tumors have been shown to have areas of HIF-1-positive cells and surrounding areas of senescent cells, which are p16+. Cell sorting and separation techniques are now available to remove senescent cells from normal tissues and cancer cells. The Fanca–/– clone 6 tumors may present an opportunity to sort and study the senescence secretory proteins (SASP) produced the different cell types found in the tumor. The SASP components of senescent cells that are specific to cancer may be valuable to elucidate the aggressiveness (invasiveness beyond resection margins) of the cancers, the propensity for local recurrence and metastasis. Senescent cells are known to increase in numbers following exposure to ionizing irradiation in both normal tissues and in cancer. If irradiation induced senescent cells within Fanca–/– clone 6 tumors are producing novel SASP the new data may provide an opportunity to isolate and evaluate those particular SASP components that are associated with aggressiveness, local recurrence, and metastasis.
The present method of induction of cancer by DMBA administration may be applicable to other FA mouse models including Fancd2–/– (C57BL/6 and 129/Sv background genetic strains), and Fancg–/– mice. Common elements of the SASP in senescent cells in control and irradiated tumors of other FA genotypes may provide valuable information that may lead to an understanding of the factors that are associated with the aggressiveness, propensity for local recurrence, and metastasis of squamous cell cancers in patients with FA.
Conflicts of Interest
None of the Authors have any conflicts of interest to declare in relation to this study.
Authors’ Contributions
JG designed experiments; ME and RF performed the animal experiments; DS made the cell lines, performed all in vitro experiments, including radiation survival experiments; RF passaged tumors; WH, LR and AG carried out animal tumor histopathology evaluations; SH assessed radiation dosimetry for in vitro survival curves; and HW carried out statistical analysis for all experiments.
Acknowledgements
This research was supported by a NCI/NIAID U19-AI068021 grant. This project used the Hillman Animal Facility, Cytometry Facility and Tissue and Research Pathology Services that are supported in part by award P30CA047904.
References
- 1.Ceccaldi R, Sarangi P, D’Andrea AD. The Fanconi anaemia pathway: new players and new functions. Nat Rev Mol Cell Biol. 2016;17(6):337–349. doi: 10.1038/nrm.2016.48. [DOI] [PubMed] [Google Scholar]
- 2.Zhang H, Kozono DE, O’Connor KW, Vidal-Cardenas S, Rousseau A, Hamilton A, Moreau L, Gaudiano EF, Greenberger J, Bagby G, Soulier J, Grompe M, Parmar K, D’Andrea AD. TGF-β inhibition rescues hematopoietic stem cell defects and bone marrow failure in Fanconi anemia. Cell Stem Cell. 2016;18(5):668–681. doi: 10.1016/j.stem.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodríguez A, Zhang K, Färkkilä A, Filiatrault J, Yang C, Velázquez M, Furutani E, Goldman DC, García de Teresa B, Garza-Mayén G, McQueen K, Sambel LA, Molina B, Torres L, González M, Vadillo E, Pelayo R, Fleming WH, Grompe M, Shimamura A, Hautaniemi S, Greenberger J, Frías S, Parmar K, D’Andrea AD. MYC promotes bone marrow stem cell dysfunction in fanconi anemia. Cell Stem Cell. 2021;28(1):33–47.e8. doi: 10.1016/j.stem.2020.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodríguez A, Yang C, Furutani E, García de Teresa B, Velázquez M, Filiatrault J, Sambel LA, Phan T, Flores-Guzmán P, Sánchez S, Monsiváis Orozco A, Mayani H, Bolukbasi OV, Färkkilä A, Epperly M, Greenberger J, Shimamura A, Frías S, Grompe M, Parmar K, D’Andrea AD. Inhibition of TGFβ1 and TGFβ3 promotes hematopoiesis in Fanconi anemia. Exp Hematol. 2021;93:70–84.e4. doi: 10.1016/j.exphem.2020.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodríguez A, Epperly M, Filiatrault J, Velázquez M, Yang C, McQueen K, Sambel LA, Nguyen H, Iyer DR, Juárez U, Ayala-Zambrano C, Martignetti DB, Frías S, Fisher R, Parmar K, Greenberger JS, D’Andrea AD. TGFβ pathway is required for viable gestation of Fanconi anemia embryos. PLoS Genet. 2022;18(11):e1010459. doi: 10.1371/journal.pgen.1010459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ebens CL, MacMillan ML, Wagner JE. Hematopoietic cell transplantation in Fanconi anemia: current evidence, challenges and recommendations. Expert Rev Hematol. 2017;10(1):81–97. doi: 10.1080/17474086.2016.1268048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stepensky P, Shapira MY, Balashov D, Trakhtman P, Skorobogatova E, Rheingold L, Brooks R, Revel-Vilk S, Weintraub M, Stein J, Maschan A, Or R, Resnick IB. Bone marrow transplantation for Fanconi anemia using fludarabine-based conditioning. Biol Blood Marrow Transplant. 2011;17(9):1282–1288. doi: 10.1016/j.bbmt.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 8.Locatelli R, Zecca M, Pession A, Morreale G, Longoni D, Di Bartolomeo P, Porta F, Fagioli F, Nobili B, Bernardo ME, Messina C, Italian Pediatric Group The outcome of children with Fanconi anemia given hematopoietic stem cell transplantation and the influence of fludarabine in the conditioning regimen: a report from the Italian pediatric group. Haematologica. 2007;92(10):1381–1388. doi: 10.3324/haematol.11436. [DOI] [PubMed] [Google Scholar]
- 9.Gluckman E, Roha V, Ionescu I, Bierings M, Harris RE, Wagner J, Kurtzberg J, Champagne MA, Bonfim C, Bittencourt M, Darbyshire P, Fernandez M-N, Locatelli F, Pasquini R, Eurocord-Netcord and EBMT Results of unrelated cord blood transplant in Fanconi anemia patients: Risk factor analysis for engraftment and survival. Biol Blood Marrow Transplant. 2007;13(9):1073–1082. doi: 10.1016/j.bbmt.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 10.Auerbach AD. Fanconi anemia and its diagnosis. Mutat Res. 2009;668(1-2):4–10. doi: 10.1016/j.mrfmmm.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gluckman E, Auerbach AD, Horowitz MM, Sobocinski KA, Ash RC, Bortin MM, Butturini A, Camitta BM, Champlin RE, Friedrich W, Good RA, Gordon-Smith EC, Harris RE, Klein JP, Ortega JJ, Pasquini R, Ramsay NK, Speck B, Vowels MR, Zhang MJ, Gale RP. Bone marrow transplantation for Fanconi Anemia. Blood. 1995;86(7):2856–2862. [PubMed] [Google Scholar]
- 12.Kutler DI, Singh B, Satagopan J, Batish SD, Berwick M, Giampietro PF, Hanenberg H, Auerbach AD. A 20-year perspective on the International Fanconi Anemia Registry (IFAR) Blood. 2003;101(4):1249–1256. doi: 10.1182/blood-2002-07-2170. [DOI] [PubMed] [Google Scholar]
- 13.Kutler DI, Auerbach AD, Satagopan J, Giampietro PF, Batish SD, Huvos AG, Goberdhan A, Shah JP, Singh B. High incidence of head and neck squamous cell carcinoma in patients with Fanconi anemia. Arch Otolaryngol Head Neck Surg. 2003;129(1):106. doi: 10.1001/archotol.129.1.106. [DOI] [PubMed] [Google Scholar]
- 14.Rosenberg PS, Socié G, Alter BP, Gluckman E. Risk of head and neck squamous cell cancer and death in patients with Fanconi anemia who did and did not receive transplants. Blood. 2005;105(1):67–73. doi: 10.1182/blood-2004-04-1652. [DOI] [PubMed] [Google Scholar]
- 15.Rosenberg PS, Greene MH, Alter BP. Cancer incidence in persons with Fanconi anemia. Blood. 2003;101(3):822–826. doi: 10.1182/blood-2002-05-1498. [DOI] [PubMed] [Google Scholar]
- 16.Rosenberg PS, Alter BP, Ebell W. Cancer risks in Fanconi anemia: findings from the German Fanconi Anemia Registry. Haematologica. 2008;93(4):511–517. doi: 10.3324/haematol.12234. [DOI] [PubMed] [Google Scholar]
- 17.Nalepa G, Clapp DW. Fanconi anaemia and cancer: an intricate relationship. Nat Rev Cancer. 2018;18(3):168–185. doi: 10.1038/nrc.2017.116. [DOI] [PubMed] [Google Scholar]
- 18.Toptan T, Brusadelli MG, Turpin B, Witte DP, Surrallés J, Velleuer E, Schramm M, Dietrich R, Brakenhoff RH, Moore PS, Chang Y, Wells SI. Limited detection of human polyomaviruses in Fanconi anemia related squamous cell carcinoma. PLoS One. 2018;13(12):e0209235. doi: 10.1371/journal.pone.0209235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gluckman E, Devergie A, Dutreix J. Radiosensitivity in Fanconi anaemia: application to the conditioning regimen for bone marrow transplantation. Br J Haematol. 1983;54(3):431–440. doi: 10.1111/j.1365-2141.1983.tb02117.x. [DOI] [PubMed] [Google Scholar]
- 20.Greenberger JS, Epperly MW. New York, NY, USA, Nova Science Publishers. 2016. Radiotherapy for the patient with Fanconi Anemia: A challenge for the radiation oncologist, Chapter 7. In: Horizons in Cancer Research, Volume 60. Watanabe HS (ed.) pp. pp. 63–76. [Google Scholar]
- 21.Lewis LM, Tang AL, Wise-Draper TM, Myers KC, Greenberger JS, Takiar V. Successful use of a therapeutic trial of graduated volume and dose escalation for postoperative head and neck radiotherapy in a Fanconi anemia patient. Head & Neck. 2020;42(10):E16–E22. doi: 10.1002/hed.26395. [DOI] [PubMed] [Google Scholar]
- 22.Gardner UG Jr, Wood SG, Chen EY, Greenberger JS, Grossberg AJ. Use of a therapeutic trial of graduated neoadjuvant radiation therapy for locally advanced esophageal cancer in a patient with Fanconi anemia. Adv Radiat Oncol. 2021;7(1):100810. doi: 10.1016/j.adro.2021.100810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bernard ME, Kim H, Berhane H, Epperly MW, Franicola D, Zhang X, Houghton F, Shields D, Wang H, Bakkenist CJ, Frantz MC, Forbeck EM, Goff JP, Wipf P, Greenberger JS. GS-nitroxide (JP4-039)-mediated radioprotection of human Fanconi anemia cell lines. Radiat Res. 2011;176(5):603–612. doi: 10.1667/rr2624.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berhane H, Epperly MW, Goff J, Kalash R, Cao S, Franicola D, Zhang X, Shields D, Houghton F, Wang H, Wipf P, Parmar K, Greenberger JS. Radiologic differences between bone marrow stromal and hematopoietic progenitor cell lines from Fanconi Anemia (Fancd2(–/–)) mice. Radiat Res. 2014;181(1):76–89. doi: 10.1667/RR13405.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berhane H, Shinde A, Kalash R, Xu K, Epperly MW, Goff J, Franicola D, Zhang X, Dixon T, Shields D, Wang H, Wipf P, Li S, Gao X, Greenberger JS. Amelioration of radiation-induced oral cavity mucositis and distant bone marrow suppression in fanconi anemia Fancd2–/– (FVB/N) mice by intraoral GS-nitroxide JP4-039. Radiat Res. 2014;182(1):35–49. doi: 10.1667/RR13633.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shinde A, Berhane H, Rhieu BH, Kalash R, Xu K, Goff J, Epperly MW, Franicola D, Zhang X, Dixon T, Shields D, Wang H, Wipf P, Parmar K, Guinan E, Kagan V, Tyurin V, Ferris RL, Zhang X, Li S, Greenberger JS. Intraoral mitochondrial-targeted GS-nitroxide, JP4-039, radioprotects normal tissue in tumor-bearing radiosensitive Fancd2(–/–) (C57BL/6) mice. Radiat Res. 2016;185(2):134–150. doi: 10.1667/RR14035.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Willis J, Epperly MW, Fisher R, Zhang X, Shields D, Hou W, Wang H, Li S, Wipf P, Parmar K, Guinan E, Steinman J, Greenberger JS. Amelioration of head and neck radiation-induced mucositis and distant marrow suppression in Fanca(–/–) and Fancg(–/–) mice by intraoral administration of GS-nitroxide (JP4-039) Radiat Res. 2018;189(6):560–578. doi: 10.1667/RR14878.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Epperly MW, Fisher R, Zhang X, Hou W, Shields D, Wipf P, Wang H, Thermozier S, Greenberger JS. Fanconi anemia mouse genotype-specific mitigation of total body irradiation by GS-nitroxide JP4-039. In Vivo. 2020;34(1):33–38. doi: 10.21873/invivo.11742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quinn TJ, Ding X, Li X, Wilson GD, Buelow K, Sivananthan A, Thermozier S, Henderson A, Epperly MW, Franicola D, Wipf P, Greenberger JS, Stevens CW, Kabolizadeh P. Amelioration of mucositis in proton therapy of Fanconi anemia Fanca(–/–) mice by JP4-039. In Vivo. 2019;33(6):1757–1766. doi: 10.21873/invivo.11666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greenberger JS, Epperly MW, Zeevi A, Brunson KW, Goltry KL, Pogue-Geile KL, Bray J, Berry LA. Stromal cell involvement in leukemogenesis and carcinogenesis. In Vivo. 1996;10(1):1–18. [PubMed] [Google Scholar]
- 31.Guo H, Epperly MW, Bernarding M, Nie S, Gretton J, Jefferson M, Greenberger JS. Manganese superoxide dismutase-plasmid/liposome (MnSOD-PL) intratracheal gene therapy reduction of irradiation-induced inflammatory cytokines does not protect orthotopic Lewis Lung carcinomas. In Vivo. 2003;17(1):13–22. [PubMed] [Google Scholar]
- 32.Espinal A, Epperly MW, Mukherjee A, Fisher R, Shields D, Wang H, Huq MS, Hamade DF, Vlad AM, Coffman L, Buckanovich R, Yu J, Leibowitz BJ, van Pijkeren JP, Patel RB, Stolz D, Watkins S, Ejaz A, Greenberger JS. Intestinal radiation protection and mitigation by second-generation probiotic Lactobacillus-reuteri engineered to deliver Interleukin-22. Int J Mol Sci. 2022;23(10):5616. doi: 10.3390/ijms23105616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hamade DF, Espinal A, Yu J, Leibowitz BJ, Fisher R, Hou W, Shields D, van Pijkeren JP, Mukherjee A, Epperly MW, Vlad AM, Coffman L, Wang H, Saiful Huq M, Patel R, Huang J, Greenberger JS. Lactobacillus reuteri releasing IL-22 (LR-IL-22) facilitates intestinal radioprotection for whole-abdomen irradiation (WAI) of ovarian cancer. Radiat Res. 2022;198(1):89–105. doi: 10.1667/RADE-21-00224.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ke Q, Costa M. Hypoxia-inducible Factor-1 (HIF-1) Mol Pharmacol. 2006;70(5):1469–1480. doi: 10.1124/mol.106.027029. [DOI] [PubMed] [Google Scholar]
- 35.Mukherjee A, Epperly MW, Fisher R, Hou W, Shields D, Saiful Huq M, Pifer PM, Mulherkar R, Wilhite TJ, Wang H, Wipf P, Greenberger JS. Inhibition of tyrosine kinase Fgr prevents radiation-induced pulmonary fibrosis (RIPF) Cell Death Discov. 2023;9(1):252. doi: 10.1038/s41420-023-01538-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bagby GC, Alter BP. Fanconi Anemia. Semin Hematol. 2006;43(3):147–156. doi: 10.1053/j.seminhematol.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 37.Mukherjee A, Epperly MW, Fisher R, Shields D, Hou W, Pennathur A, Luketich J, Wang H, Greenberger JS. Carcinogen 4-Nitroquinoline Oxide (4-NQO) induces Oncostatin-M (OSM) in esophageal cells. In Vivo. 2023;37(2):506–518. doi: 10.21873/invivo.13108. [DOI] [PMC free article] [PubMed] [Google Scholar]