Abstract
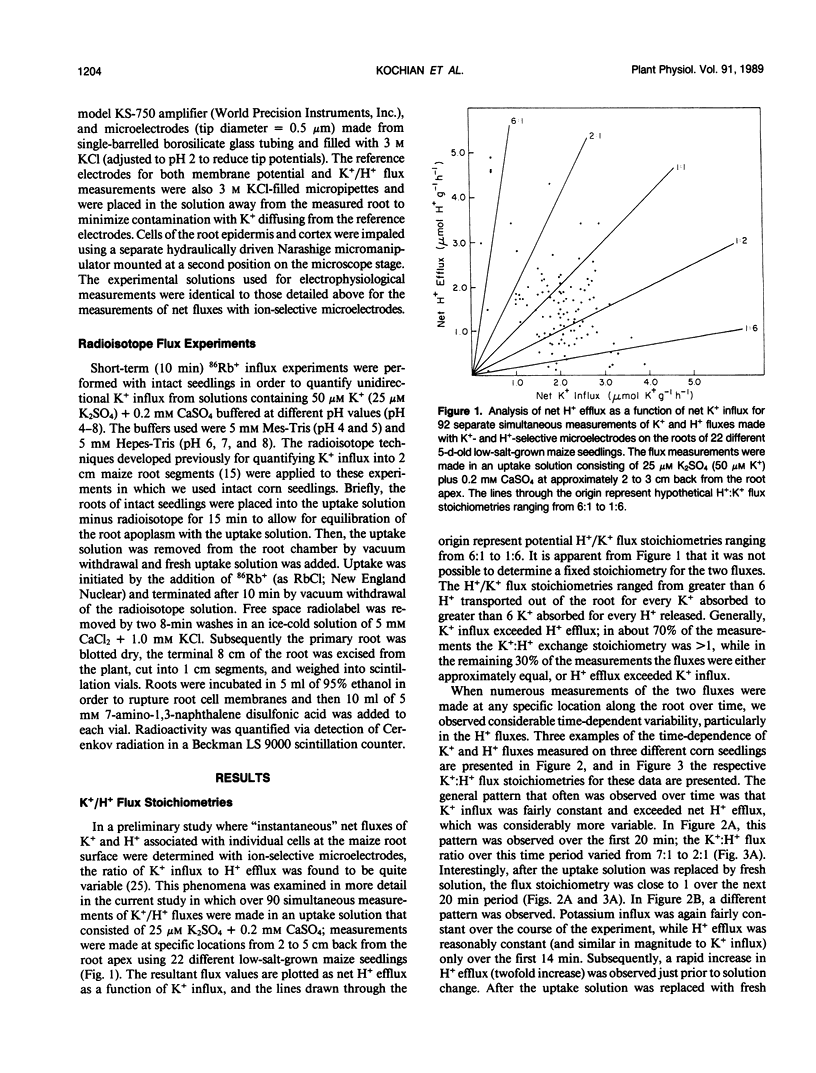
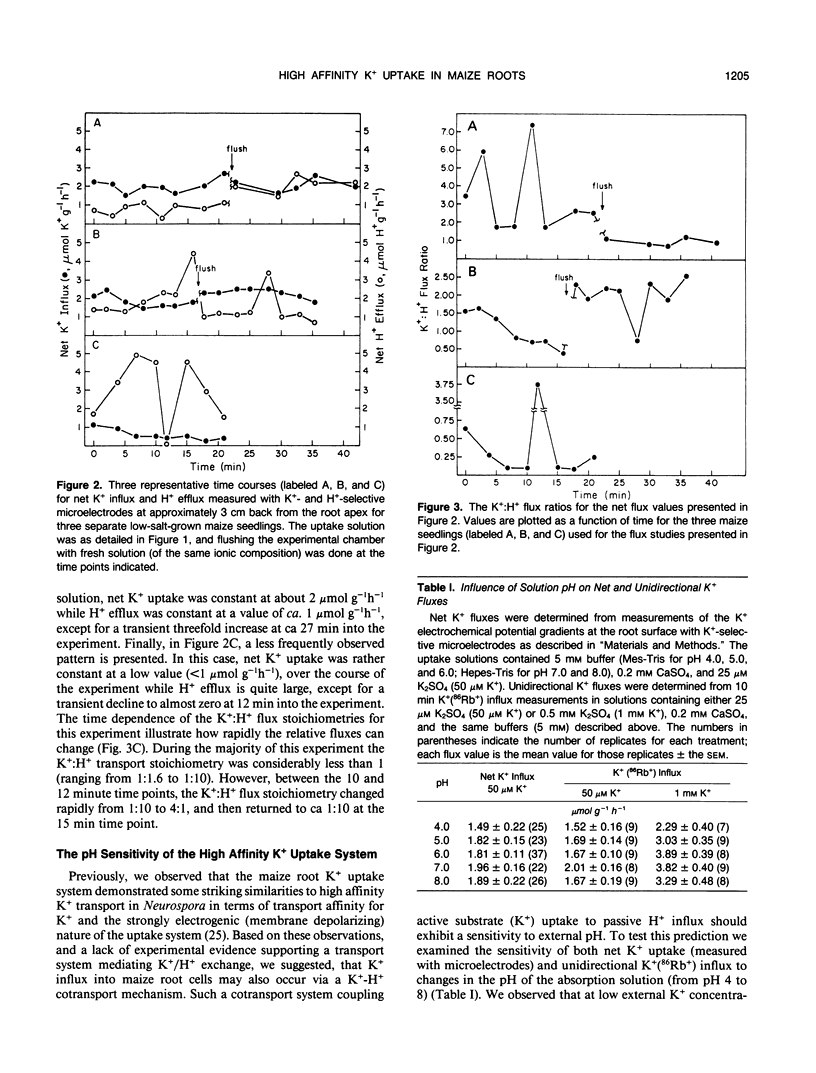
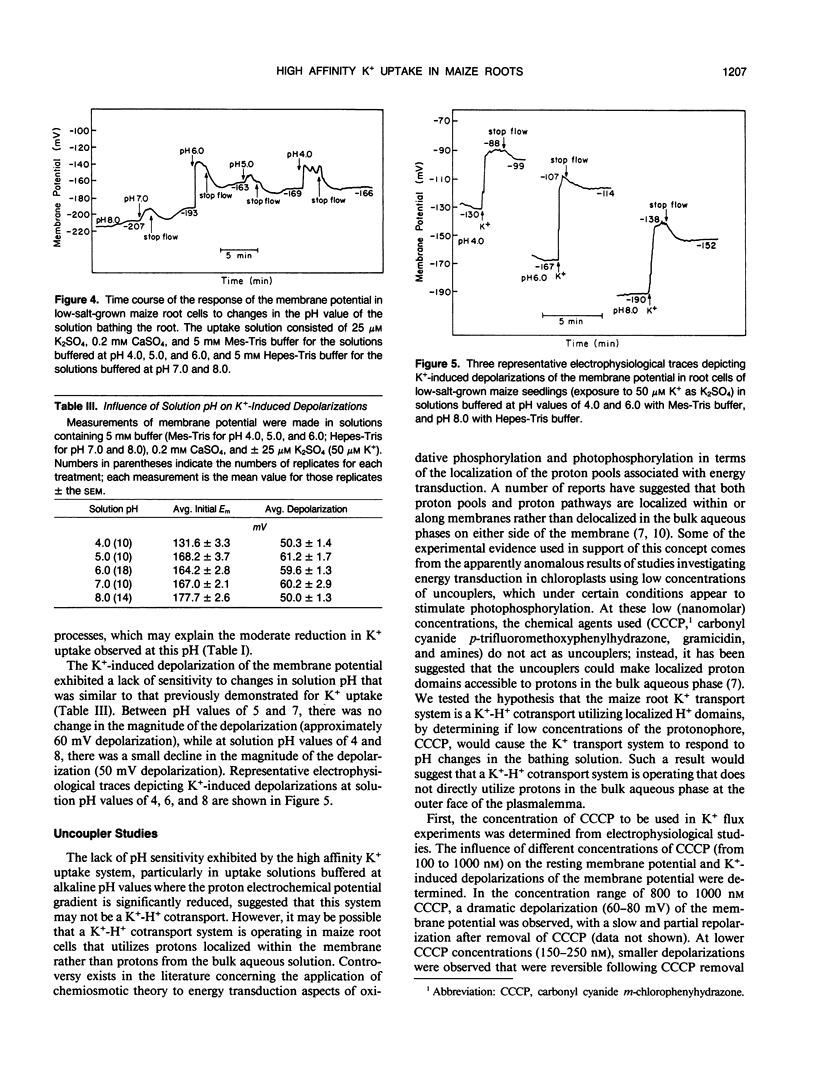
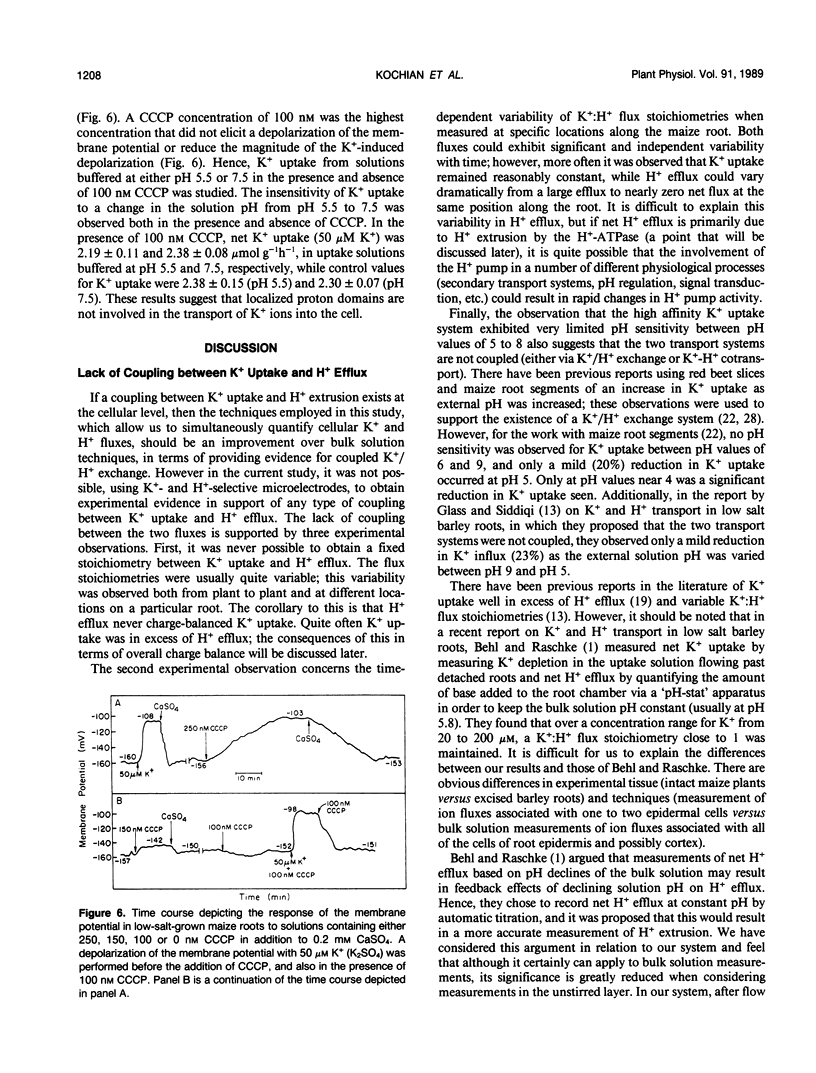
We report here on the putative coupling between a high affinity K+ uptake system which operates at low external K+ concentrations (Km = 10-20 micromolar), and H+ efflux in roots of intact, low-salt-grown maize plants. An experimental approach combining electrophysiological measurements, quantification of unidirectional K+(86Rb+) influx, and the simultaneous measurement of net K+ and H+ fluxes associated with individual cells at the root surface with K+- and H+-selective microelectrodes was utilized. A microelectrode system described previously (IA Newman, LV Kochian, MA Grusak, and WJ Lucas [1987] Plant Physiol 84: 1177-1184) was used to quantify net ion fluxes from the measurement of electrochemical potential gradients for K+ and H+ ions within the unstirred layer at the root surface. No evidence for coupling between K+ uptake and H+ efflux could be found based on: (a) extremely variable K+:H+ flux stoichiometries, with K+ uptake often well in excess of H+ efflux; (b) dramatic time-dependent variability in H+ extrusion when both fluxes were measured at a particular location along the root over time; and (c) a lack of pH sensitivity by the high affinity K+ uptake system (to changes in external pH) when net K+ uptake, unidirectional K+(86Rb+) influx, and K+-induced depolarizations of the membrane potential were determined in uptake solutions buffered at pH values from pH 4 to 8. Based on the results presented here, we propose that high affinity active K+ absorption into maize root cells is not mediated by a K+/H+ exchange mechanism. Instead, it is either due to the operation of a K+-H+ cotransport system, as has been hypothesized for Neurospora, or based on the striking lack of sensitivity to changes in extracellular pH, uptake could be mediated by a K+-ATPase as reported for Escherichia coli and Saccharomyces.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blatt M. R., Rodriguez-Navarro A., Slayman C. L. Potassium-proton symport in Neurospora: kinetic control by pH and membrane potential. J Membr Biol. 1987;98(2):169–189. doi: 10.1007/BF01872129. [DOI] [PubMed] [Google Scholar]
- Cheeseman J. M., Hanson J. B. Energy-linked Potassium Influx as Related to Cell Potential in Corn Roots. Plant Physiol. 1979 Nov;64(5):842–845. doi: 10.1104/pp.64.5.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein E., Rains D. W., Elzam O. E. RESOLUTION OF DUAL MECHANISMS OF POTASSIUM ABSORPTION BY BARLEY ROOTS. Proc Natl Acad Sci U S A. 1963 May;49(5):684–692. doi: 10.1073/pnas.49.5.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein W., Wieczorek L., Siebers A., Altendorf K. Potassium transport in Escherichia coli: genetic and biochemical characterization of the K+-transporting ATPase. Biochem Soc Trans. 1984 Apr;12(2):235–236. doi: 10.1042/bst0120235. [DOI] [PubMed] [Google Scholar]
- Gaber R. F., Styles C. A., Fink G. R. TRK1 encodes a plasma membrane protein required for high-affinity potassium transport in Saccharomyces cerevisiae. Mol Cell Biol. 1988 Jul;8(7):2848–2859. doi: 10.1128/mcb.8.7.2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketchum K. A., Shrier A., Poole R. J. Characterization of potassium-dependent currents in protoplasts of corn suspension cells. Plant Physiol. 1989 Apr;89(4):1184–1192. doi: 10.1104/pp.89.4.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochian L. V., Lucas W. J. Potassium transport in corn roots : I. Resolution of kinetics into a saturable and linear component. Plant Physiol. 1982 Dec;70(6):1723–1731. doi: 10.1104/pp.70.6.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochian L. V., Xin-Zhi J., Lucas W. J. Potassium Transport in Corn Roots : IV. Characterization of the Linear Component. Plant Physiol. 1985 Nov;79(3):771–776. doi: 10.1104/pp.79.3.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W., Hanson J. B. Cell potentials, cell resistance, and proton fluxes in corn root tissue: effects of dithioerythritol. Plant Physiol. 1976 Sep;58(3):276–282. doi: 10.1104/pp.58.3.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W. Potassium and Phosphate Uptake in Corn Roots: Further Evidence for an Electrogenic H/K Exchanger and an OH/Pi Antiporter. Plant Physiol. 1979 May;63(5):952–955. doi: 10.1104/pp.63.5.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman I. A., Kochian L. V., Grusak M. A., Lucas W. J. Fluxes of h and k in corn roots : characterization and stoichiometries using ion-selective microelectrodes. Plant Physiol. 1987 Aug;84(4):1177–1184. doi: 10.1104/pp.84.4.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Navarro A., Blatt M. R., Slayman C. L. A potassium-proton symport in Neurospora crassa. J Gen Physiol. 1986 May;87(5):649–674. doi: 10.1085/jgp.87.5.649. [DOI] [PMC free article] [PubMed] [Google Scholar]


