Abstract
Background/Aim
The aim of this study was to analyze choroidal circulatory and structural changes using laser speckle flowgraphy (LSFG) and optical coherence tomography (OCT) in acute macular neuroretinopathy (AMN) after infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), respectively.
Case Report
A 24-year-old woman complained of distorted vision after SARS-CoV-2 infection oculi uterque (OU) and referred to our hospital because of ellipsoid zones (EZ) disruption on OCT. Her best-corrected visual acuity (BCVA) was 1.2 OU. Color fundus photographs revealed dark red lesions in the macula, and scanning laser ophthalmoscopy infrared images showed hypointensity consistent with dark red lesions OU. We diagnosed the patient with AMN after SARS-CoV-2 infection, and posterior sub-Tenon injections of triamcinolone acetonide were performed OU. Five months after the initial visit, her BCVA was 1.2 OU, and EZ disruption improved. The rate of change in macular blood flow assessed by the mean blur rate on LSFG was 20.4% and 29.6% increase oculus dexter (OD) and oculus sinister (OS) 5 months after the initial visit, respectively. The central choroidal thickness showed 13.5% increase OD and 16.1% increase OS. The binarization technique demonstrated that the ratio of luminal areas in choroidal area increased by 12.6% OD and 14.2% OS, and stromal areas increased by 7.3% OD and 16.9% OS.
Conclusion
Before and after treatment for AMN, the luminal component may have increased with improvement of acute choroidal circulatory disturbance caused by SARS-CoV-2, and increased stromal components may be due to chronic inflammation and tissue remodeling of the stroma.
Keywords: Acute macular neuroretinopathy, severe acute respiratory syndrome coronavirus 2, laser speckle flowgraphy, optical coherence tomography, binarization method
The coronavirus infection (COVID-19) with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has become a serious global problem that has threatened human health and vision since 2019. SARS-CoV-2, a ribonucleic acid (RNA) virus, causes not only respiratory disorders, but also multiple systemic complications including myocardial infarction, deep venous thromboembolism, and cerebrovascular events, which are associated with hyperinflammatory and coagulopathic conditions (1). In addition, 2% to 32% of COVID-19 cases have been found to lead to various ophthalmic manifestations (2). Conjunctivitis is a relatively common manifestation in patients with COVID-19, while it can cause various retinal vascular disorders (3-5).
Recently, a binarization using enhanced depth imaging-optical coherence tomography (OCT) images has made it possible to noninvasively quantify retinal structures as well as the luminal and stromal areas of the choroid over time (6,7). In general, the binarization method clinically allows quantitative evaluation of presumed histological intravascular and extravascular regions of the choroid, respectively (8). Laser speckle flowgraphy (LSFG) is a blood flow imaging device that uses laser scattering to noninvasively visualize the fundus circulation in two dimensions. We have used LSFG to observe fundus circulation in various retino-choroidal diseases, such as optic disc melanocytoma (9), choroidal macrovessel (10), sclerochoroidal calcification (11), juxtapapillary retinal capillary hemangioblastoma (12), and choroidal lymphoma (13). Recently, we reported a case of leukemic retinopathy and analyzed choroidal circulatory and structural changes using LSFG and OCT with the binarization method simultaneously, and there was a correlation between mean blur rate (MBR) as relative blood flow values and the ratio of luminal area (LA) /total choroidal area (CA) following the binarization (14). Acute macular neuroretinopathy (AMN) is a rare disease characterized by wedge-shaped dark reddish-brown lesions in the macula, which might be caused by oral contraceptive use, vasoconstrictor and sympathomimetic use, as well as a nonspecific viral illness or fever including SARS-CoV-2 infection (15). However, little is known about the alterations of choroidal circulatory changes and vascular structures in AMN following SARS-CoV-2 infection.
We herein present a case of AMN after infection with SARS-CoV-2 and analysis of the choroidal circulatory and structural changes using LSFG and OCT with the binarization method, respectively. We further reviewed the literature regarding COVID-19-related AMN and compared it with the current findings.
Case Report
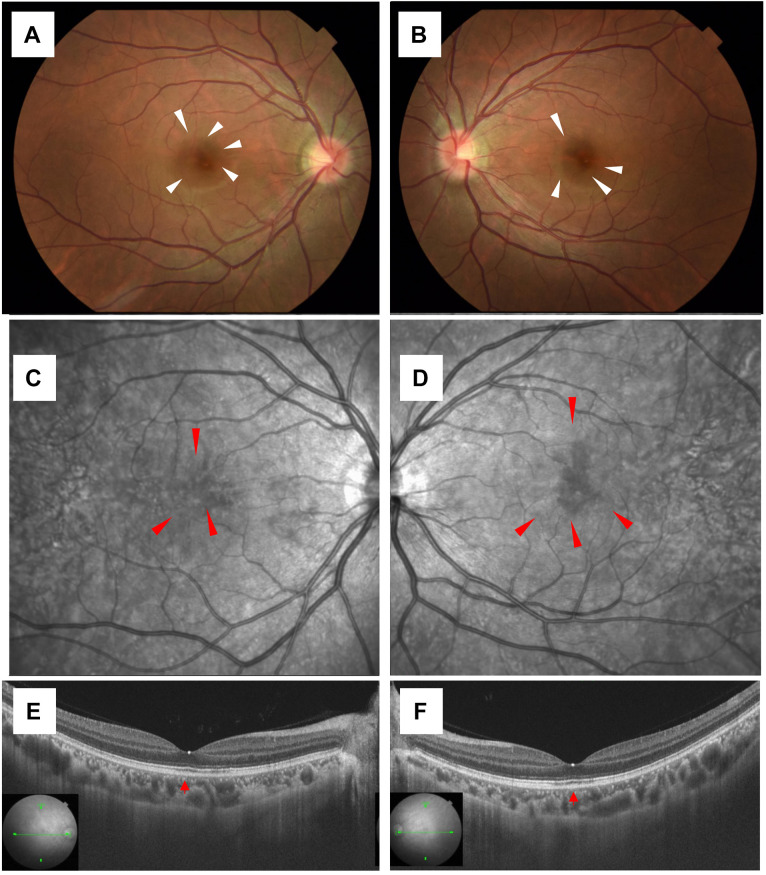
A 24-year-old female complained of distorted vision one day after a fever due to SARS-CoV-2 infection oculi uterque (OU). There were no special notes in her medical or family history. She had received mRNA vaccination against SARS-CoV-2 twice, and no adverse reactions were observed at the time of vaccination. Her smoking history was 4 years of 15 cigarettes per day. Physical history included childhood asthma, atopic dermatitis, and influenza pneumonia. She was referred to our clinic 14 days after infection because of distorted vision and ellipsoid zones (EZ) disruption on OCT. At an initial examination, her best-corrected visual acuity (BCVA) was 1.2 OU with myopia, and intraocular pressure was normal OU. Slit-lamp microscopy did not detect any findings OU. Color fundus photography showed dark red lesions in the macula (white arrowheads, Figure 1A and B), and scanning laser ophthalmoscopy (SLO) infrared images showed hypointensity consistent with dark red lesions OU (red arrowheads, Figure 1C and D). Swept-source (SS)-OCT on horizontal scans through the fovea showed minor irregularity of EZ and expansion of interdigitation zone (IZ) loss (red arrowhead, Figure 1E and F). Fluorescein angiography (FA) showed no obvious abnormality, and indocyanine green angiography (ICGA) showed no obvious abnormality in the early phase, but dark red lesions in the macula were slightly hypofluorescent in the late phase. Humphrey field analyzer 10-2 showed paracentral scotoma OU. Based on the clinical findings, this patient was diagnosed with AMN that developed after SARS-CoV-2 infection. Since her ocular symptoms did not improve, sub-Tenon injections of triamcinolone acetonide (STTA) of 40 mg were performed in the right and left eyes at 14 and 29 days following infection, respectively, after informed consent was given. Twenty weeks after the initial visit, her BCVA was 1.2 OU, the ocular symptom of distorted vision improved, and EZ and IZ disruption on OCT resolved. The institutional review board of Hokkaido University waived ethical assessment of this clinical study because it was a single case report with a non-invasive study. This study adhered to the tenets of the Declaration of Helsinki.
Figure 1. Initial findings on color fundus photography (CFP), scanning laser ophthalmoscopy (SLO) infrared images, and swept-source optical coherence tomography (SS-OCT) in the present case of leukemic retinopathy. A, B) CFP showed dark red lesions in the macula (white arrowheads). C, D) SLO infrared images showed hypointensity consistent with dark red lesions oculi uterque (OU) (red arrowheads). E, F) SS-OCT on horizontal scans through the fovea revealed minor irregularity of ellipsoid zones and expansion of interdigitation zone loss OU (red arrowheads).
Methods.
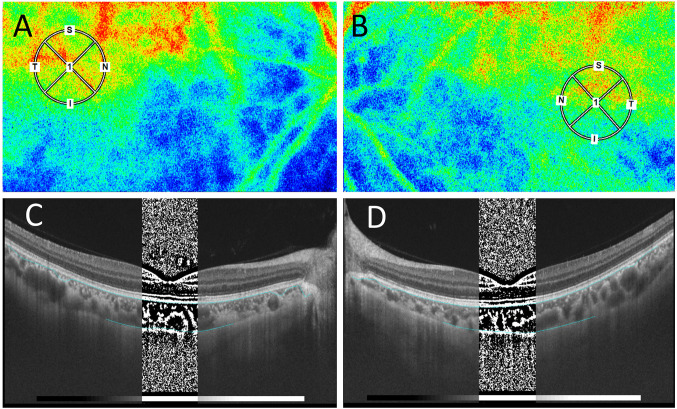
Analysis of LSFG measurement. In the present study, LSFG was used to evaluate changes in choroidal blood flow in AMN. Blood flow velocity was measured quantitatively by LSFG software (LSFG-NAVI, version 3.1.39.2, Softcare Ltd., Fukuoka, Japan), and relative blood flow values were determined as the MBR in accordance with previous reports (16). The pupils of the patient were dilated with 0.4% tropicamide (Mydrin-M; Santen Pharmaceutical Co., Ltd., Osaka, Japan) before examination. Ophthalmologic examinations were performed after the complete disappearance of light reflex in the pupils of both eyes. The macular area in the LSFG image was marked manually, and the vessels were automatically segmented using threshold values defined in the system’s software (LSFG Analyzer, version 3.0.47.0). A circle with a diameter of 1,500 μm to the fossa was defined as the region of interest on the LSFG (Figure 2A and B, small circle). Four to five consecutive measurements were taken for each circle, and the mean values were used for analysis. All tests were performed by an experienced operator. Ocular perfusion pressure (OPP) was calculated using the patient’s blood pressure and intraocular pressure, in accordance with previous reports (16).
Figure 2. Initial findings on laser speckle flowgraphy (LSFG), and optical coherence tomography (OCT) images with the binarization method in the present case of leukemic retinopathy. A, B) LSFG showed a mild warm-color blood flow signal corresponding to the small circles OU. C, D) In OCT images with the binarization method, bright and dark pixels correspond to the stromal and luminal regions of the choroid, respectively.
Analysis of SS-OCT-based choroidal structural parameters. The central choroidal thickness (CCT) in SS-OCT was measured manually by two experienced examiners from the lower edge of the retinal pigment epithelium layer to the scleral border. The choroidal structure of SS-OCT images (DRI OCT Triton; Topcon Inc., Tokyo, Japan) was analyzed by selecting a 1,500 μm region of the OCT image as the region of interest corresponding to the LSFG analysis range, using the semi-automatic analysis software EyeGround developed by Sonoda et al. (6). The brightness of each pixel in the OCT image was binarized using the Niblack method in EyeGround. The bright and dark pixels in the generated images corresponded to the choroidal stromal and luminal regions, respectively (Figure 2C and D; at initial visit). The areas of both regions were measured and quantitatively evaluated using a binarization method. The primary measurements were CA, LA, stromal area (SA), and the ratios of the luminal area/choroidal area (L/C). Two experienced examiners measured the parameters, and the mean values were shown.
Results.
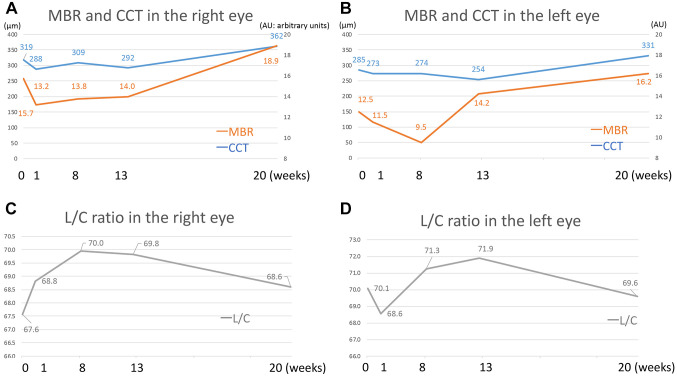
MBR and OPP assessed by LSFG. The MBR values (arbitrary unit) oculus dexter (OD) are shown in Figure 3A as follows: 15.7, 13.2, 13.8, 14.0, and 18.9 at the initial visit, 1, 8, 13, and 20 weeks after the initial visit, respectively. The rate of change assessed by MBR was 20.4% increase OD 20 weeks after the initial visit. The MBR values oculus sinister (OS) are shown in Figure 3B as follows: 12.5, 11.5, 9.5, 14.2, and 16.2 at the initial visit, 1, 8, 13, and 20 weeks after the initial visit, respectively. The rate of change assessed by MBR was 29.6% increase OS 20 weeks after treatment. MBR of both eyes decreased temporarily but gradually increased. OPP was 49.0, 51.4, 60.0, 56.7, and 66.7 mmHg OD and 51.1, 55.0, 59.7, 56.0 and 63.5 mmHg OS at the initial visit, 1, 8, 13, and 20 weeks after the initial visit, respectively, revealing no significant changes in either eye.
Figure 3. Mean blur rate (MBR) by laser speckle flowgraphy and the central choroidal thickness (CCT) (A, B) and luminal area /choroidal area (L/C) ratio (C, D). A, B) MBR and CCT decreased temporarily in the acute phase, flat to slightly decreased in the subacute phase, and gradually increased in the chronic phase. C, D) L/C ratio increased in the right eye and decreased in the left eye in the acute phase, increased OU in the subacute phase, and gradually decreased OU in the chronic phase.
Alterations in OCT-based choroidal parameters. The CCT values OD were 319, 268, 309, 292, and 362 μm at the initial visit, 1, 8, 13, and 20 weeks after the initial visit, respectively (Figure 3C). The CCT values OS were 285, 273, 274, 254, and 331 μm at the initial visit, 1, 8, 13, and 20 weeks after the initial visit, respectively (Figure 3D). The rate change of CCT showed 13.5% increase OD and 16.1% increase OS 20 weeks after treatment. The CCT of both eyes decreased temporarily after the start of treatment, and then gradually increased thereafter.
The rates of change in choroidal structures by the binarization method in the designated 1,500 μm circle 20 weeks after treatment were as follows: L/C increased by 3.2% OD (Figure 3C) and decreased by 4.8% OS (Figure 3D); LA increased by 12.6% OD and 14.2% OS; SA increased by 7.3% OD and increased by 16.9% OS. Taken together, the L/C ratio increased for 1~8 weeks but then decreased. LA and SA decreased temporarily but gradually increased afterward.
Discussion
The present study demonstrated a case of AMN that developed after SARS-CoV-2 infection and analyzed choroidal circulatory and structural changes. MBR, CCT, LA, and SA decreased in the acute phase (1 week), slightly decreased around the subacute phase (after 8 weeks), and gradually increased in the chronic phase (after 13 weeks). In contrast, the L/C ratio increased OD and decreased OS in the acute phase, increased OU in the subacute phase, and gradually decreased OU in the chronic phase.
First, this study compared ophthalmic findings in the current patient with those reported on the choroidal circulation of AMN prior to the SARS-CoV-2 pandemic. Hashimoto et al. reported a 15-year-old male with AMN in both eyes, who recovered without treatment, and demonstrated that the thickened CCT in the acute phase decreased over time until 3 months (presumed subacute phase), whereas the MBR increased (17). Hashimoto et al. reported another case of AMN in a 41-year-old female who had taken oral contraceptives, and demonstrated that choroidal blood flow velocity determined by MBR on LSFG increased sequentially during systemic corticosteroid administration (18). The two cases by Hashimoto et al. (17,18) showed that the MBR increased till 3 months after the onset, in which the change in MBR was consistent with that in our case. The background of the two cases, however, was different from that of our case, since the first case (17) was a young male, and the second case (18) had a history of oral contraceptives use and of systemic corticosteroid treatment for 4 months. Hirooka et al. reported an 11-year-old female with AMN in the right eye, who recovered without treatment, and demonstrated that CCT did not show remarkable change, whereas the MBR continuously increased 6 months after the onset of the disease (19), which was consistent with changes in the chronic phase in our case. In particular, the acute decrease in MBR in our AMN case suggested that vascular damage might have preceded local inflammation, since SARS-CoV-2 is an infectious agent that causes vascular damages. In addition, as mentioned above, three reports in pre-COVID-19 era (17-19) have implicated the choroidal circulation impairments in the pathogenesis of AMN, but this was the first report of LSFG and OCT with a binarization method of AMN after infection with SARS-CoV-2.
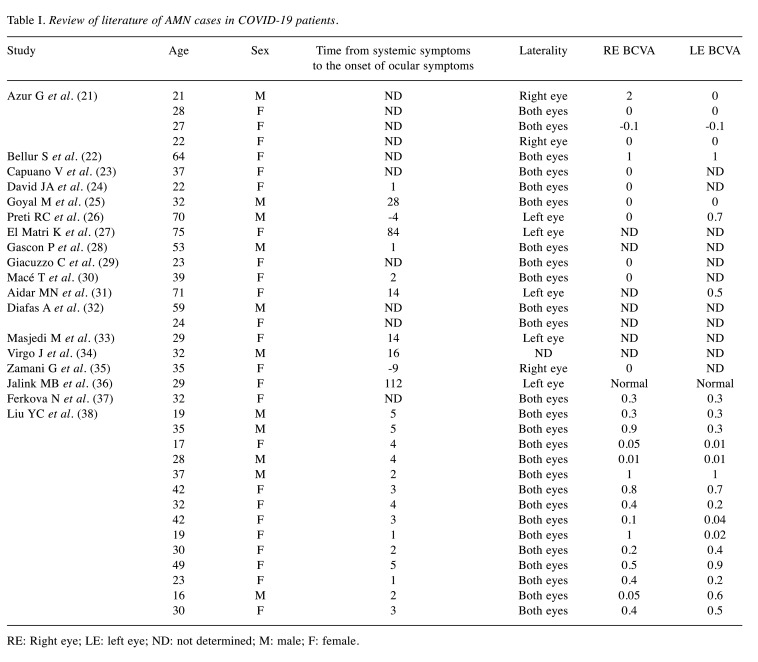
Next, this study reviewed a total of 36 cases of AMN following COVID-19 based on the published literature through the PubMed website (Table I). Majumder PD et al. (20) reviewed 18 AMN cases after SARS-Cov-2 infection in 2023; therefore, the present study added 17 reported cases including the current case. On the other hand, cases of paracentral acute middle maculopathy without ophthalmic findings of AMN, or cases of AMN after SARS-CoV-2 vaccination were excluded in this study.
Table I. Review of literature of AMN cases in COVID-19 patients.
RE: Right eye; LE: left eye; ND: not determined; M: male; F: female.
Mean age of all the patients included in this analysis was 35.5±15.7 years. Twenty-four cases (69%) were female, and 11 cases (31%) were male, and 26 cases had bilateral lesions. Ocular symptoms appeared an average of 12.1±26.6 days after SARS-CoV-2 infection. This case is a young woman, who did not take oral contraceptives, but had a history of smoking. In fact, since smoking has been reported to have a negative effect on the coagulation system in COVID-19 (39), it should be investigated in cases of COVID-19-related AMN reports as a future issue. In one case (21) with poor vision at onset, oral prednisolone was administered. In another case (37), low molecular weight heparin was administered in a preventive dose. All other cases had been observed without treatment. The visual prognosis was generally good, but the patient with poor vision at onset had persistent scotomas (21). The current patient developed AMN 1 day after infection with SARS-CoV-2, the duration of which was relatively short compared to previous reports. While the patient with poor vision in the previous report was treated, this patient had good vision but suffered from distorted vision. Therefore, STTA were performed in both eyes, and her ocular symptom of distorted vision eventually improved.
It is known that adhesion of SARS-CoV-2 has a close relation with receptors (angiotensin-converting enzyme 2: ACE2), which are histologically located in the ocular adnexal tissues (40) as well as vascular systems including the choroidal middle-large vessels and choriocapillaris (41). In fact, viral particles following infection with SARS-CoV-2 possibly cause choroidal vasculopathy (42). Bellur et al. and Liu et al. reported that the choriocapillaris vascular density was reduced in AMN after SARS-CoV-2 infection using OCT angiography (22,38). In our case, SARS-CoV-2 virion attached presumed ACE2-positive choroidal vessels, led to acute choroidal bloodstream congestion, and impaired choroidal circulation, which subsequently reduced stromal and luminal areas at the acute phase of infection. At this acute phase, there were no dark spots even on ICGA, suggesting that inflammation of the choroid had yet happened or the inflammation did not involve the choroidal vascular structures. Pathologically diffuse lymphocytic infiltration and fibrosis can occur in the chronic phase after SARS-CoV-2 infection of the ocular adnexa (40). In our case, since the vasculopathy improved as the infection recovered and the lumen was enlarged presented as increased L/C ratio, lymphocyte infiltration into the stroma caused inflammation and an increase in stromal components observed by OCT. In addition, STTA controlled inflammation, let to stromal remodeling, and maintained stromal area from the subacute to chronic phase.
In conclusion, before and after treatment of this case, the luminal component increased with improvement of acute choroidal circulatory disturbance due to SARS-CoV-2 infection, and the interstitial component may have increased due to chronic inflammation and remodeling of the stroma.
Conflicts of Interest
The Authors declare that they have no competing interests in relation to this study.
Authors’ Contributions
MM wrote the paper and acquired clinical data. SK reviewed the paper and interpreted the clinical data. KH analyzed LSFG data. HE and YI analyzed and verified the results of binarization. SI conducted clinical revision and supervised the data interpretation. All Authors have read and approved the manuscript.
Acknowledgements
The Authors would like to thank Nose Nanako for technical assistance with LSFG analysis.
References
- 1.Hanff TC, Mohareb AM, Giri J, Cohen JB, Chirinos JA. Thrombosis in COVID-19. Am J Hematol. 2020;95(12):1578–1589. doi: 10.1002/ajh.25982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ulhaq ZS, Soraya GV. The prevalence of ophthalmic manifestations in COVID-19 and the diagnostic value of ocular tissue/fluid. Graefes Arch Clin Exp Ophthalmol. 2020;258(6):1351–1352. doi: 10.1007/s00417-020-04695-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marinho PM, Marcos AAA, Romano AC, Nascimento H, Belfort R Jr. Retinal findings in patients with COVID-19. Lancet. 2020;395(10237):1610. doi: 10.1016/S0140-6736(20)31014-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walinjkar JA, Makhija SC, Sharma HR, Morekar SR, Natarajan S. Central retinal vein occlusion with COVID-19 infection as the presumptive etiology. Indian J Ophthalmol. 2020;68(11):2572–2574. doi: 10.4103/ijo.IJO_2575_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sheth JU, Narayanan R, Goyal J, Goyal V. Retinal vein occlusion in COVID-19: A novel entity. Indian J Ophthalmol. 2020;68(10):2291–2293. doi: 10.4103/ijo.IJO_2380_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sonoda S, Sakamoto T, Yamashita T, Uchino E, Kawano H, Yoshihara N, Terasaki H, Shirasawa M, Tomita M, Ishibashi T. Luminal and stromal areas of choroid determined by binarization method of optical coherence tomographic images. Am J Ophthalmol. 2015;159(6):1123–1131.e1. doi: 10.1016/j.ajo.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 7.Sonoda S, Sakamoto T, Kakiuchi N, Shiihara H, Sakoguchi T, Tomita M, Yamashita T, Uchino E. Semi-automated software to measure luminal and stromal areas of choroid in optical coherence tomographic images. Jpn J Ophthalmol. 2018;62(2):179–185. doi: 10.1007/s10384-017-0558-1. [DOI] [PubMed] [Google Scholar]
- 8.Branchini LA, Adhi M, Regatieri CV, Nandakumar N, Liu JJ, Laver N, Fujimoto JG, Duker JS. Analysis of choroidal morphologic features and vasculature in healthy eyes using spectral-domain optical coherence tomography. Ophthalmology. 2013;120(9):1901–1908. doi: 10.1016/j.ophtha.2013.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kikuchi I, Kase S, Hashimoto Y, Hirooka K, Ishida S. Involvement of circulatory disturbance in optic disk melanocytoma with visual dysfunction. Graefes Arch Clin Exp Ophthalmol. 2019;257(4):835–841. doi: 10.1007/s00417-019-04257-7. [DOI] [PubMed] [Google Scholar]
- 10.Kataoka K, Kase S, Noda K, Ishida S. Laser speckle flowgraphy findings in a patient with choroidal macrovessel. Ophthalmol Retina. 2020;4(11):1123–1124. doi: 10.1016/j.oret.2020.06.018. [DOI] [PubMed] [Google Scholar]
- 11.Mitamura M, Kase S, Ishida S. Multimodal imaging in sclerochoroidal calcification: a case report and literature review. BMC Ophthalmol. 2020;20(1):248. doi: 10.1186/s12886-020-01520-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mitamura M, Kase S, Hirooka K, Ishida S. Laser speckle flowgraphy in juxtapapillary retinal capillary hemangioblastoma: a case report on natural course and therapeutic effect. Oncotarget. 2020;11(42):3800–3804. doi: 10.18632/oncotarget.27771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fukutsu K, Namba K, Iwata D, Mizuuchi K, Kase S, Suzuki K, Shimizu H, Shibata Y, Yamawaki F, Onozawa M, Ishida S. Pseudo-inflammatory manifestations of choroidal lymphoma resembling Vogt-Koyanagi-Harada disease: case report based on multimodal imaging. BMC Ophthalmol. 2020;20(1):94. doi: 10.1186/s12886-020-01353-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitamura M, Kase S, Hirooka K, Endo H, Ito Y, Cho Y, Ishida S. Alterations of choroidal circulation and vascular morphology in a patient with chronic myeloid leukemia before and after chemotherapy. BMC Ophthalmol. 2022;22(1):160. doi: 10.1186/s12886-022-02380-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhavsar KV, Lin S, Rahimy E, Joseph A, Freund KB, Sarraf D, Cunningham ET. Acute macular neuroretinopathy: A comprehensive review of the literature. Surv Ophthalmol. 2016;61(5):538–565. doi: 10.1016/j.survophthal.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 16.Hirooka K, Saito W, Namba K, Takemoto Y, Mizuuchi K, Uno T, Tagawa Y, Hashimoto Y, Ishida S. Relationship between choroidal blood flow velocity and choroidal thickness during systemic corticosteroid therapy for Vogt-Koyanagi-Harada disease. Graefes Arch Clin Exp Ophthalmol. 2015;253(4):609–617. doi: 10.1007/s00417-014-2927-5. [DOI] [PubMed] [Google Scholar]
- 17.Hashimoto Y, Saito W, Saito M, Hasegawa Y, Ishida S. Increased thickness and decreased blood flow velocity of the choroid in a patient with acute macular neuroretinopathy. BMC Ophthalmol. 2019;19(1):109. doi: 10.1186/s12886-019-1123-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hashimoto Y, Saito W, Mori S, Saito M, Ishida S. Increased macular choroidal blood flow velocity during systemic corticosteroid therapy in a patient with acute macular neuroretinopathy. Clin Ophthalmol. 2012;6:1645–1649. doi: 10.2147/OPTH.S35854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirooka K, Saito W, Noda K, Ishida S. Enhanced-depth imaging optical coherence tomography and laser speckle flowgraphy in a patient with acute macular neuroretinopathy. Ocul Immunol Inflamm. 2014;22(6):485–489. doi: 10.3109/09273948.2014.916305. [DOI] [PubMed] [Google Scholar]
- 20.Dutta Majumder P, Agarwal A. Acute macular neuroretinopathy and paracentral acute middle maculopathy during SARS-CoV-2 infection and vaccination. Vaccines (Basel) 2023;11(2):474. doi: 10.3390/vaccines11020474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Azar G, Bonnin S, Vasseur V, Faure C, Salviat F, Clermont CV, Titah C, Farès S, Boulanger E, Derrien S, Couturier A, Duvilliers A, Manassero A, Hage R, Tadayoni R, Behar-Cohen F, Mauget-Faÿsse M. Did the COVID-19 pandemic increase the incidence of acute macular neuroretinopathy. J Clin Med. 2021;10(21):5038. doi: 10.3390/jcm10215038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bellur S, Zeleny A, Patronas M, Jiramongkolchai K, Kodati S. Bilateral acute macular neuroretinopathy after COVID-19 vaccination and infection. Ocul Immunol Inflamm. 2023;31(6):1222–1225. doi: 10.1080/09273948.2022.2093753. [DOI] [PubMed] [Google Scholar]
- 23.Capuano V, Forte P, Sacconi R, Miere A, Mehanna CJ, Barone C, Bandello F, Souied EH, Querques G. Acute macular neuroretinopathy as the first stage of SARS-CoV-2 infection. Eur J Ophthalmol. 2023;33(3):NP105–NP111. doi: 10.1177/11206721221090697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.David JA, Fivgas GD. Acute macular neuroretinopathy associated with COVID-19 infection. Am J Ophthalmol Case Rep. 2021;24:101232. doi: 10.1016/j.ajoc.2021.101232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goyal M, Murthy SI, Annum S. Retinal manifestations in patients following COVID-19 infection: A consecutive case series. Indian J Ophthalmol. 2021;69(5):1275–1282. doi: 10.4103/ijo.IJO_403_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Preti RC, Zacharias LC, Cunha LP, Monteiro MLR. Acute macular neuroretinopathy as the presenting manifestation of Covid-19 infection. Retin Cases Brief Rep. 2022;16(1):12–15. doi: 10.1097/ICB.0000000000001050. [DOI] [PubMed] [Google Scholar]
- 27.El Matri K, Werda S, Chebil A, Falfoul Y, Hassairi A, Bouraoui R, Matri LE. Acute macular outer retinopathy as a presumed manifestation of COVID-19. J Fr Ophtalmol. 2021;44(8):1274–1277. doi: 10.1016/j.jfo.2021.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gascon P, Briantais A, Bertrand E, Ramtohul P, Comet A, Beylerian M, Sauvan L, Swiader L, Durand JM, Denis D. Covid-19-associated retinopathy: a case report. Ocul Immunol Inflamm. 2020;28(8):1293–1297. doi: 10.1080/09273948.2020.1825751. [DOI] [PubMed] [Google Scholar]
- 29.Giacuzzo C, Eandi CM, Kawasaki A. Bilateral acute macular neuroretinopathy following COVID-19 infection. Acta Ophthalmol. 2022;100(2):e611–e612. doi: 10.1111/aos.14913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Macé T, Pipelart V. Acute macular neuroretinopathy and SARS-CoV-2 infection: Case report. J Fr Ophtalmol. 2021;44(9):e519–e521. doi: 10.1016/j.jfo.2021.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aidar MN, Gomes TM, de Almeida MZH, de Andrade EP, Serracarbassa PD. Low visual acuity due to acute macular neuroretinopathy associated with COVID-19: a case report. Am J Case Rep. 2021;22:e931169. doi: 10.12659/AJCR.931169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diafas A, Ghadiri N, Beare N, Madhusudhan S, Pearce I, Tan SZ. Comment on: ‘Paracentral acute middle maculopathy and acute macular neuroretinopathy following SARS-CoV-2 infection’. Eye (Lond) 2022;36(7):1507–1509. doi: 10.1038/s41433-021-01709-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Masjedi M, Pourazizi M, Hosseini NS. Acute macular neuroretinopathy as a manifestation of coronavirus disease 2019: A case report. Clin Case Rep. 2021;9(10):e04976. doi: 10.1002/ccr3.4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Virgo J, Mohamed M. Paracentral acute middle maculopathy and acute macular neuroretinopathy following SARS-CoV-2 infection. Eye (Lond) 2020;34(12):2352–2353. doi: 10.1038/s41433-020-1069-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zamani G, Ataei Azimi S, Aminizadeh A, Shams Abadi E, Kamandi M, Mortazi H, Shariat S, Abrishami M. Acute macular neuroretinopathy in a patient with acute myeloid leukemia and deceased by COVID-19: a case report. J Ophthalmic Inflamm Infect. 2021;10(1):39. doi: 10.1186/s12348-020-00231-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jalink MB, Bronkhorst IHG. A sudden rise of patients with acute macular neuroretinopathy during the COVID-19 pandemic. Case Rep Ophthalmol. 2022;13(1):96–103. doi: 10.1159/000522080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferková N, Hudečková H, Barnau A. Bilateral acute macular neuroretinopathy in acute COVID-19 infection: a case study. Cesk Slov Oftalmol. 2023;79(3):150–154. doi: 10.31348/2023/21. [DOI] [PubMed] [Google Scholar]
- 38.Liu YC, Wu B, Wang Y, Chen S. Clinical and multimodal imaging features of acute macular neuroretinopathy lesions following recent SARS-CoV-2 infection. Int J Ophthalmol. 2023;16(5):755–761. doi: 10.18240/ijo.2023.05.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Esteban EMA, Ares AC, Rodríguez MJD. Increased healthcare costs in COVID-19 patients with unhealthy habits: The case of smoking. Tob Induc Dis. 2023;21:82. doi: 10.18332/tid/163301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kase S, Ishida S. COVID-19-related chronic bilateral dacryoadenitis: a clinicopathological study. JAMA Ophthalmol. 2022;140(4):312–318. doi: 10.1001/jamaophthalmol.2021.6364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reinhold A, Tzankov A, Matter MS, Mihic-Probst D, Scholl HPN, Meyer P. Ocular pathology and occasionally detectable intraocular severe acute respiratory syndrome Coronavirus-2 RNA in five fatal Coronavirus disease-19 cases. Ophthalmic Res. 2021;64(5):785–792. doi: 10.1159/000514573. [DOI] [PubMed] [Google Scholar]
- 42.Yan Y, Diao B, Liu Y, Zhang W, Wang G, Chen X. Severe acute respiratory syndrome Coronavirus 2 nucleocapsid protein in the ocular tissues of a patient previously infected with Coronavirus disease 2019. JAMA Ophthalmol. 2020;138(11):1201–1204. doi: 10.1001/jamaophthalmol.2020.3962. [DOI] [PMC free article] [PubMed] [Google Scholar]