Abstract
Background/Aim
Retinoic acid-inducible gene (RIG)-I like receptors (RLRs) are expressed on renal proximal tubular epithelial cells (RPTECs) in viral nephropathy, indicating the presence of RLR-mediated innate immune responses in RPTECs. Hypoxia is also known to affect innate immunity. This study investigated the effects of hypoxia, and hypoxia-inducible factor (HIF) on innate immunity in RPTECs.
Materials and Methods
Primary human RPTECs were cultured under normoxic or hypoxic conditions and treated with a synthetic analog of double-stranded RNA (polyIC). The expression levels of RIG-I and MDA5, as RLRs, and IFNβ, IL6, and TNFα, as inflammatory mediators were evaluated using quantitative reverse transcription-polymerase chain reaction, western blotting, and lactate dehydrogenase activity (LDH) assays. To further investigate the role of hypoxia, a small interfering RNA was used to knockdown HIF1α.
Results
Under normoxic conditions, polyIC increased RIG-I, MDA5, and IFNβ mRNA expression in RPTECs by, 9.4±0.4-, 10.8±0.5-, and 4.0±0.1-fold, respectively, compared to control, and by 5.4±0.1-, 7.4±0.1-, and 2.4±0.3-fold, respectively, under hypoxic conditions, the rate of increase was lower than that under normoxic conditions (p<0.01). Protein expression showed a similar trend. Under hypoxic conditions, polyIC treatment with HIF1α knockdown in RPTECs increased RIG-I, MDA5, and IFNβ mRNA expression by 3.1±0.5-, 2.9±0.4-, and 6.1±0.4-fold, respectively, and cytotoxicity, demonstrated by LDH assay, was increased compared to that without knockdown (all p<0.01).
Conclusion
Hypoxia suppresses polyIC-induced RLRs mediated innate immune responses in RPTECs via HIF1α.
Keywords: Renal proximal tubular epithelial cells, innate immune response, retinoic acid-inducible gene (RIG)-I-like receptors, hypoxia-inducible factor gene (RIG)-I-like receptors, hypoxiainducible factor
The innate immune system expresses various pattern-recognition receptors, including Toll-like receptors (TLRs), retinoic acid-inducible gene (RIG)-I-like receptors (RLRs), and cytoplasmic DNA receptors, which recognize infection by viruses and other microbes, and the resulting innate immune response serves as a host defense (1). Both RIG-I and melanoma differentiation-associated protein 5 (MDA5) are RLRs that identify dsRNA and play important roles in the early immune response to viral infection (2). In virus-infected cells, dsRNA detection activates nuclear factor-kappa B (NF-κB) and promotes type I interferon (IFN) production, resulting in activation of antiviral immune response signaling (3).
One of the complications of renal transplantation is viral infection that causes renal transplant dysfunction. Adults are often subclinically infected with cytomegalovirus (CMV) and BK virus (4,5), and reactivation following immunosuppression after transplantation can induce tubulointerstitial nephritis. Although renal disease and RLRs have received little attention, there is a significant increase in RIG-I and MDA5 mRNA expression in CMV and BKV-infected renal tissues after kidney transplantation (6). In immunohistological staining, RIG-I and MDA5 are expressed by the tubular epithelium during viral infection. RIG-I expression has been reported to be increased in renal collecting duct epithelial cells after BK virus infection (7).
PolyIC is a synthetic double-stranded RNA (dsRNA) that is widely used in cellular experiments to mimic viral infection. Several cells have been found to express dsRNA receptors after polyIC stimulation (8). In cultured renal proximal tubular epithelial cells (RPTECs), the expression of RLRs was higher than that of TLR3 upon polyIC stimulation (9). Thus, an RLR-mediated immune response system is thought to exist in RPTECs.
Hypoxia is closely associated with chronic kidney disease (CKD) as it contributes to the progression of CKD. The hypoxia-inducible factor (HIF) gene is expressed by living organisms to adapt to hypoxic environments. HIF1α is one of three isoforms of HIF, and it is implicated in the systemic hypoxic response. In the kidney, HIF1α is expressed in tubular epithelial cells in response to hypoxia (10). Under hypoxic conditions, HIFα is not hydroxylated, because the activity of prolyl hydroxylase domain-containing protein (PHD) is reduced in such conditions. Moreover, HIFα migrates from the cytoplasm to the nucleus to form a heterodimer with HIFβ, which further binds to the HIF target gene enhancer and increases transcription. Under normoxic condition, HIFα is hydroxylated by PHD and degraded by proteasomes. Prolyl hydroxylation requires iron as a cofactor, causing iron antagonists, such as cobalt chloride (CoCl2), to exhibit hypoxia-like effects by inhibiting the degradation of HIFα even under normal oxygen pressure. Notably, the activation of HIF affects innate immunity (11). HIF mediates cell-type specific effects on the cells of the innate immune system; therefore, the effects of HIF activation on epithelial cells, granulocytes, lymphocytes, macrophages, and dendritic cells are different (12). HIF1α activation in airway epithelial cells enhances pro-barrier function and suppresses inflammatory responses to pathogens (13,14). Although HIF activation has several biological functions in epithelial cells, the effects of HIF activation on the innate immune response in the kidney have not been studied. This study aimed to investigate the effects of hypoxia and HIF activation on the RLR-mediated immune response system of renal tubular epithelial cells. Based on the inconsistent reports of HIF1α activation in epithelial cells suppressing inflammation, we hypothesized that hypoxia suppresses polyIC-stimulated immune responses in renal tubular epithelial cells and that HIF1α mediates these effects.
Materials and Methods
Cell culture. RPTECs were purchased from Lonza. The cells were cultured in a renal epithelial cell-based medium supplemented with growth factors, such as hydrocortisone, human epidermal growth factor, fetal bovine serum, epinephrine, insulin, triiodothyronine, transferrin, and gentamicin/amphotericin B (all from Lonza), at 37˚C with 5% CO2. RPTECs between passages three and eight were used for the experiment. The hypoxic condition with 2% oxygen atmosphere was established using a hypoxia chamber (MCO-50M; PHC Corporation, Tokyo, Japan). For experiments under hypoxic conditions or CoCl2, cells were pre-incubated under hypoxic conditions or CoCl2 (150 μM) for 2 h prior to polyIC (500 nM) treatment.
Cytotoxicity assessment. The cytotoxicity under each condition was assessed by measuring lactate dehydrogenase (LDH) release using an LDH assay kit (Dojindo Laboratories, Kumamoto, Japan). RPTECs were grown in 96-well plates and incubated for 2 h under normoxic, hypoxic, or CoCl2 conditions, followed by 24 h of polyIC treatment. The LDH levels in the cell supernatant were measured colorimetrically.
Small interfering RNA. Cultured RPTECs were transfected with HIF1α-targeting small interfering RNAs (siRNAs). A siRNA targeting an irrelevant mRNA was used as a nonspecific control. Cells were transfected with 50 nM siRNA for 48 h in antibiotic-free media using the DharmaFECT transfection reagent (GE Healthcare, Little Chalfont, UK), according to the manufacturer’s protocol. Cells were then incubated for 6 and 24 h under normoxic or hypoxic conditions, with or without polyIC treatment, respectively. The target gene expression was assessed using quantitative reverse transcription-polymerase chain reaction (qRT-PCR).
Immunofluorescence study. RPTECs were grown on glass-bottom culture dishes (Matsunami Glass Ind., Osaka, Japan) and incubated under normoxic, hypoxic, CoCl2, or polyIC conditions. For immunofluorescence staining, cells were fixed with ice-cold methanol for 30 min, permeabilized with 0.1% Triton X-100 in phosphate-buffered saline for 20 min, incubated with an anti-human HIF1α mouse monoclonal antibody (Abcam) for 1 h, and then incubated with an AlexaFluor 488 goat anti-mouse antibody for 2 h.
Western blotting analysis. The cells were separated using a radioimmunoprecipitation (RIPA) lysis buffer (Santa Cruz Biotechnology). The protein concentration of the samples was measured using the bicinchoninic acid assay (Thermo Scientific). For sodium dodecyl sulfate–polyacrylamide gel electrophoresis (10% w/v) analysis, 20 μg of total protein was loaded per lane and transferred to polyvinylidene difluoride membranes. Membranes were treated with anti-RIG-I and anti-MDA5 antibodies (Immuno-Biological Laboratories, Takasaki, Japan) or anti-human glyceraldehyde-3-phosphatasedehydrogenase (GAPDH) antibody (Cell Signaling, Danvers, MA, USA) and visualized using a horseradish peroxidase (HRP) secondary antibody (Abcam, Cambridge, UK). The protein bands were identified using the Amersham Enhanced chemiluminescence Prime Western Blotting Detection Reagents (GE Healthcare, Buckinghamshire, UK). Densitometric analysis was performed using ImageJ software and blots were analyzed for GAPDH as a loading control.
Assessment of mRNA expression by qRT-PCR. Total RNA was extracted from cells using a QIA shredder and the RNeasy Protect Mini Kit (QIAGEN, Valencia, CA, USA) and then transcribed into the first-strand cDNA using the Omniscript RT kit (QIAGEN), according to the manufacturer’s protocol. The qRT-PCR was performed on a Sequence Detector (Applied Biosystems, Foster City, CA, USA) using a TaqMan Universal PCR Master Mix (Applied Biosystems) as directed by the manufacturer. Specific primers and probes (Applied Biosystems) were designed to assess GAPDH (Assay ID: Hs99999905_m1), RIG-I (Hs00204833_m1), MDA5 (Hs01070332_m1), interleukin 6 (IL6) (Hs00985638_g1), tumor necrosis factor-alpha (TNFα) (Hs1113624_g1), HIF1α (Hs00468869_m1), and interferon-beta (IFNβ) (Hs0107958_s1) expression. The target gene expression level was normalized with GAPDH expression levels.
Statistical analysis. All data were analyzed using Student’s t-test (two-sided) or analysis of variance with Bonferroni’s adjustment for experiments with more than two subgroups. The results were considered statistically significant if the p-value was less than 0.05. All statistical analyses were performed using the GraphPad Prism software (GraphPad Software, San Diego, CA, USA).
Results
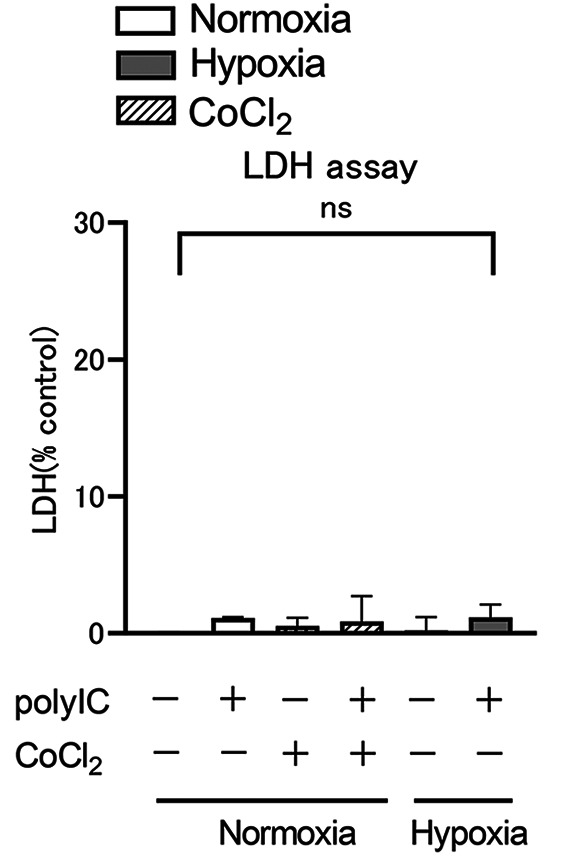
LDH assay. Figure 1 shows that LDH activity was measured in culture supernatants after RPTECs were incubated for 2 h under normoxic or hypoxic conditions, or with 150 μM CoCl2, and subsequently incubated for 24 h in the same environment with or without polyIC. There was no significant difference in LDH levels in the culture supernatant under hypoxic or CoCl2 conditions compared to normoxic conditions. In addition, there were no significant differences in LDH levels in the culture supernatants after polyIC treatment under each condition. Cytotoxicity was unaffected by 24-h exposure to hypoxia and CoCl2, as well as polyIC treatment.
Figure 1. LDH activity in the supernatant is expressed as a percentage of cytotoxicity, with all cells lysed as 100%. Renal proximal tubular epithelial cells (RPTECs) were cultured for 2 h under normoxic, hypoxic, or 150 μM CoCl2 conditions before being stimulated with polyIC (500 nM) for 24 h in the same environment. Data are presented as mean±standard error of the mean (SEM) (n=3/group).
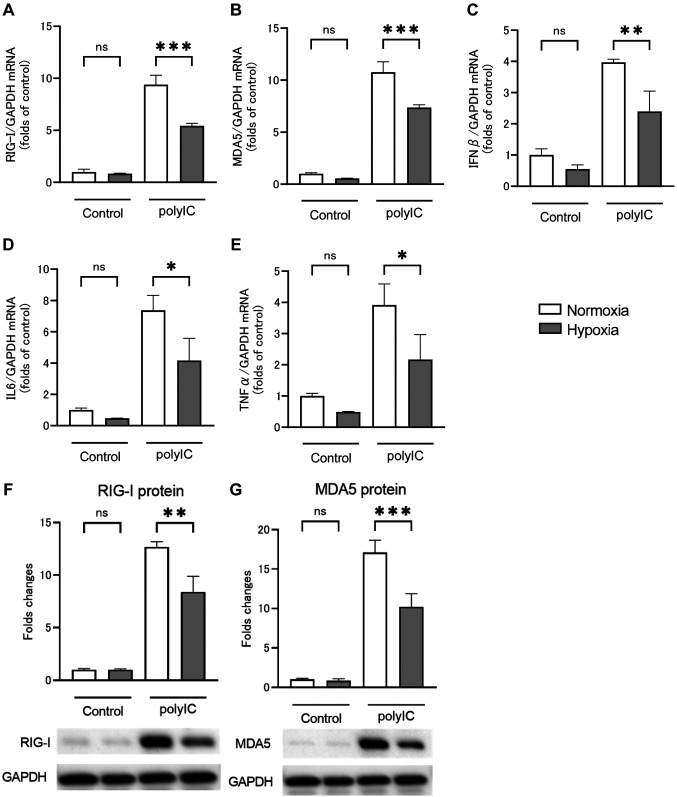
RLRs and inflammatory cytokine expression by polyIC treatment under hypoxic conditions. Figure 2 shows the results of qRT-PCR and western blotting analysis performed to assess the effect of polyIC treatment on RPTECs under hypoxic conditions. Hypoxia had no significant effect on the mRNA expression levels of RIG-I (Figure 2A), MDA5 (Figure 2B), IFNβ (Figure 2C), IL6 (Figure 2D), or TNFα (Figure 2E) in the absence of polyIC treatment. PolyIC (500 nM) treatment for 24 h increased RIG-I and MDA5 mRNA expression levels by 9.4±0.4- and 10.8±0.5-fold, respectively, compared to controls, while polyIC treatment under hypoxic conditions increased expression levels by 5.4±0.1- and 7.4±0.1-fold, respectively. The percentage of increase was significantly reduced in polyIC treatment under hypoxic conditions compared to that under normoxic conditions (Figure 2A and B). The RIG-I and MDA5 protein levels were increased by 12.7±0.3- and 17.1±0.9-fold, respectively, 24 h after polyIC treatment compared to control, whereas the percentage of increase was significantly decreased by 8.4±0.9- and 10.2±0.9-fold after polyIC treatment under hypoxic conditions compared to that under normoxic conditions (Figure 2F and G). The mRNA and protein expression levels showed similar trends. The IFNβ, IL6, and TNFα mRNA expression levels increased by 4.0±0.1-, 7.3±0.5-, and 3.9±0.3-fold, respectively, 6 h after polyIC treatment compared to control, while expression levels decreased by 2.4±0.3-, 4.2±0.7-, and 2.1±0.4-fold after polyIC treatment under hypoxic conditions compared to those under normoxic conditions. The percentage of increase was significantly reduced under hypoxic conditions compared to that under normoxic ones (Figure 2C-E).
Figure 2. Hypoxia suppresses the release of inflammatory cytokines and retinoic acid-inducible gene (RIG)-I like receptors (RLRs) by renal proximal tubular epithelial cells (RPTECs) in response to polyIC treatment. RPTECs were cultured under normoxic or hypoxic (2% O2) conditions for 2 h and stimulated with polyIC for an additional 6 and 24 h, respectively. Quantitative reverse transcription-polymerase chain reaction (A-E) and western blotting analysis (E, F) showed that hypoxia markedly reduced RLRs mRNA and protein expression levels and inflammatory mediator mRNA expression levels. Data are presented as mean±SEM (n=3/group). Bars indicate significant differences of **p<0.01 and ***p<0.001.
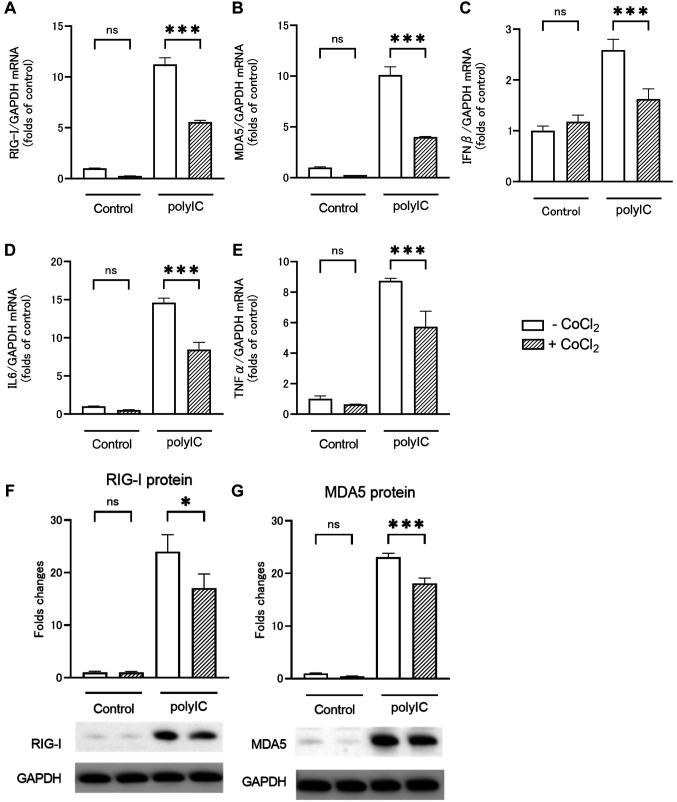
RLRs and inflammatory cytokine expression by polyIC treatment under CoCl2. CoCl2 is a hypoxia mimic that stabilizes HIF. We investigated whether the changes in RLRs and inflammatory cytokine expression levels after polyIC treatment on RPTECs under hypoxic conditions were caused by HIF expression. Figure 3 shows that qRT-PCR and western blotting analysis was performed to assess the effect of polyIC treatment on RPTECs under CoCl2-induced hypoxic conditions. RIG-I (Figure 3A), MDA5 (Figure 3B), IFNβ (Figure 3C), IL6 (Figure 3D), and TNFα (Figure 3E) mRNA expression levels were increased after polyIC treatment, but the percentage of increase was significantly reduced under CoCl2-induced hypoxic conditions. RIG-I and MDA5 protein levels were increased after polyIC treatment, but the percentage of increase was significantly reduced under CoCl2-induced hypoxic conditions (Figure 3F and G). The experimental results with CoCl2 were comparable to those obtained under hypoxia. Therefore, HIF stabilization had an effect on RLRs and inflammatory cytokine expression levels in RPTECs after polyIC treatment.
Figure 3. CoCl2 decreases the release of inflammatory cytokines and retinoic acid-inducible gene (RIG)-I like receptors (RLRs) by renal proximal tubular epithelial cells (RPTECs) in response to polyIC treatment. RPTECs were cultured under normoxic or CoCl2 (150 μM) conditions for 2 h and stimulated with polyIC for an additional 6 and 24 h. Quantitative reverse transcription-polymerase chain reaction (A-E) and western blotting analysis (E, F) showed that CoCl2 markedly reduced RLRs mRNA and protein expression levels and inflammatory mediator mRNA expression levels. Data are presented as mean±SEM (n=3/group). Bars indicate significant differences of *p<0.05 and ***p<0.001.
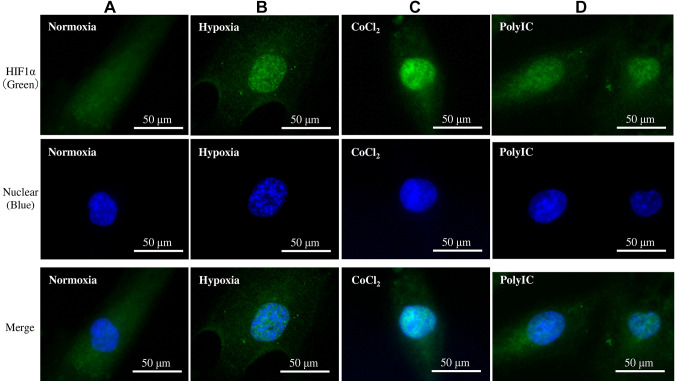
Immunofluorescence study. To confirm that HIF is expressed in the presence of hypoxia and CoCl2, immunostaining was performed. Immunofluorescence revealed that HIF1α was strongly stained in the cell nuclei of RPTECs cultured under hypoxic or CoCl2-induced hypoxic conditions for 6 h compared to those cultured under normoxic conditions (Figure 4A-C). HIF1α was weakly stained in the nuclei of RPTECs treated with polyIC for 6 h (Figure 4D).
Figure 4. Immunofluorescence study. Renal proximal tubular epithelial cells (RPTECs) were incubated under normoxic conditions (A), hypoxic conditions (B), CoCl2 (C), or polyIC treatment (D) for 6 h. Hypoxia and CoCl2 resulted in HIF1α migration into the nucleus. The fluorescence was weaker than the others, but similar results were obtained with polyIC treatment.
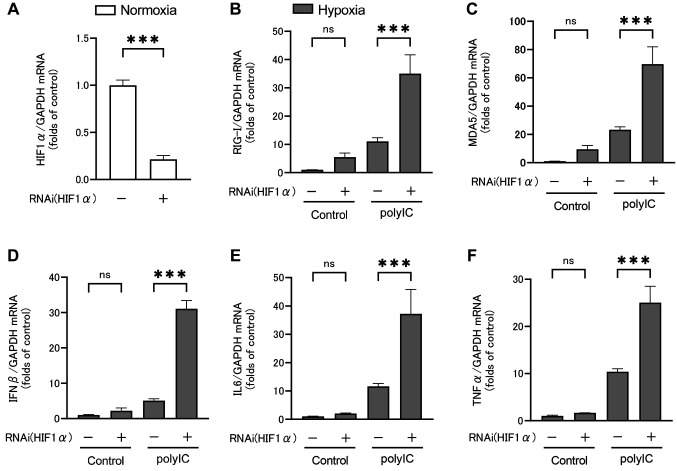
Effect of HIF1α small interfering RNA. The siRNA was used to investigate the effect of HIF1α activation on polyIC-induced RLRs and inflammatory cytokine expression under hypoxic conditions. HIF1α expression was knocked down in RPTECs using siRNA. Subsequently, cells were treated with polyIC under hypoxic conditions, and qRT-PCR was performed to assess RLRs and inflammatory cytokine expression, as shown in Figure 5. HIF1α mRNA expression was reduced by siRNA, indicating that target gene knockdown was achieved (Figure 5A). Under hypoxic conditions, HIF1α knockdown with no polyIC treatment had no significant effect on RIG-I (Figure 5B), MDA5 (Figure 5C), IFNβ (Figure 5D), IL6 (Figure 5E), or TNFα (Figure 5F) mRNA expression levels. However, RIG-I and MDA5 mRNA expression increased upon HIF1α knockdown. After 24 h of polyIC treatment and HIF1α knockdown, RIG-I and MDA5 mRNA expression levels were significantly increased by 3.1±0.5- and 2.9±0.4-fold, respectively (Figure 5B and C). After 6 h of polyIC treatment and HIF1α knockdown, the IFNβ, IL6, and TNFα mRNA expression levels were significantly increased by 6.1±0.4-, 3.2±0.6-, and 2.4±0.3-fold, respectively (Figure 5D-F).
Figure 5. HIF1α mRNA expression was reduced by siRNA, indicating that target gene knockdown was achieved (A). HIF1α knockdown increased RIG-I (B), MDA5 (C), IFNβ (D), IL6 (E), and TNFα (F) mRNA expression levels by renal proximal tubular epithelial cells (RPTECs) under hypoxic conditions. Data are presented as mean±SEM (n=3/group). Bars indicate significant differences at ***p<0.001.
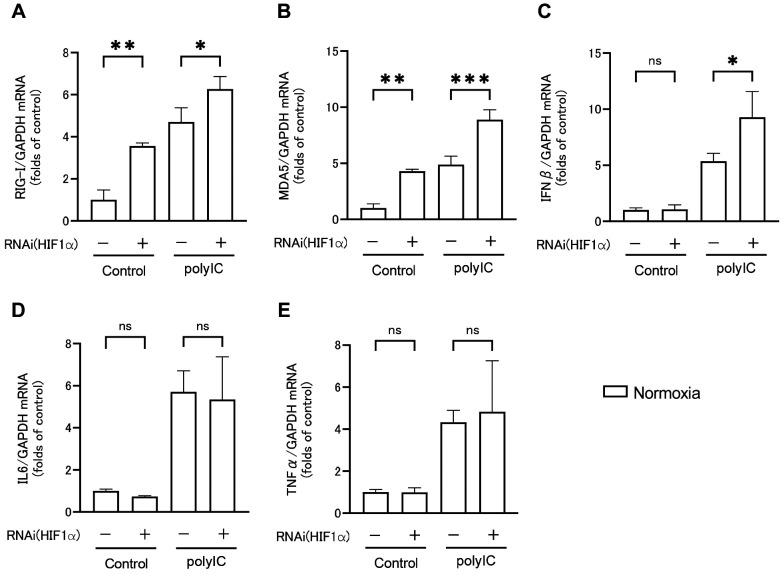
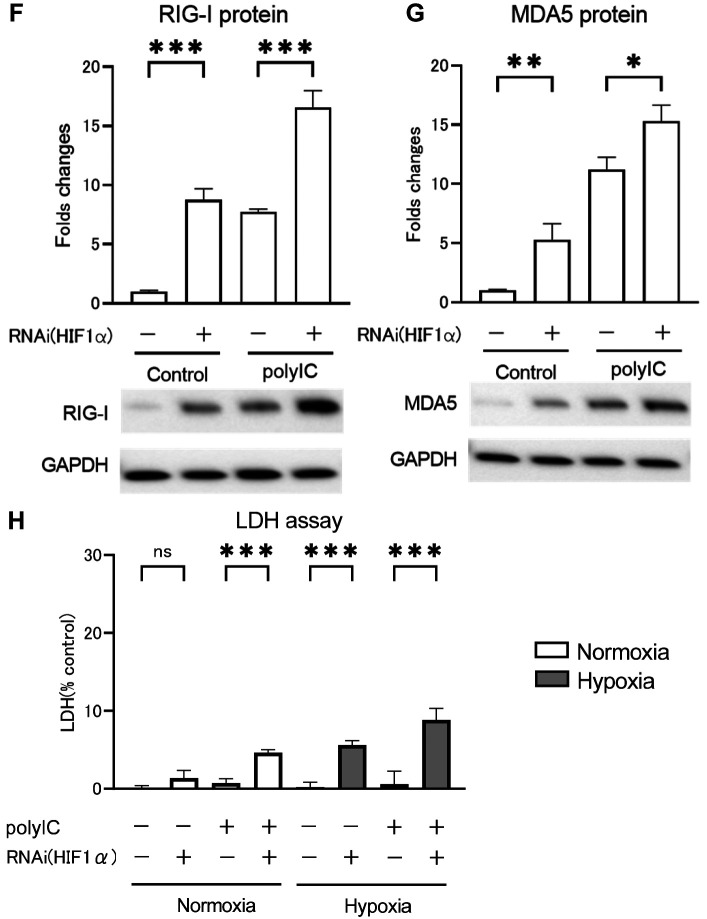
Next, the effect of polyIC-induced HIF1α activation under normoxic conditions on RLRs and inflammatory cytokine expression was investigated. Following HIF1α knockdown, RPTECs were treated with polyIC under normoxic conditions, and RLRs and inflammatory cytokine expression were assessed by qRT-PCR (Figure 6A-E) and western blotting analysis (Figure 6F and G), as well as cytotoxicity by LDH assay (Figure 6H). Under normoxic conditions, HIF1α knockdown with no polyIC treatment significantly increased RIG-I (Figure 6A) and MDA5 (Figure 6B) mRNA expression levels compared to controls, with no significant changes in IFNβ (Figure 6C), IL6 (Figure 6D), and TNFα (Figure 6F) mRNA expression levels. However, when the cells were treated with polyIC, HIF1α knockdown increased RIG-I, MDA5, and IFNβ mRNA expression levels, whereas IL6 and TNFα mRNA expression levels did not significantly differ with or without HIF1α knockdown. RIG-I and MDA5 protein levels followed a similar pattern to mRNA expression levels (Figure 6F and G). Therefore, HIF1α knockdown significantly increased RIG-I and MDA5 protein levels both with and without polyIC treatment.
Figure 6. HIF1α knockdown increased RIG-I (A), MDA5 (B), and IFNβ (C) mRNA expression levels in renal proximal tubular epithelial cells (RPTECs) after polyIC treatment compared to those under normoxic conditions, with no significant difference in IL6 (D) and TNFα (F) mRNA expression levels. RIG-I (F) and MDA5 (G) protein expression levels were measured using western blotting analysis, and their results were comparable to that of qRT-PCR. LDH in the supernatant was presented as a percentage of cytotoxicity (H). HIF1α knockdown increased LDH activity in the supernatant of RPTECs under hypoxic conditions or after polyIC treatment. Data are presented as mean±SEM (n=3/group). Bars indicate significant differences at *p<0.05, **p<0.01, and ***p<0.001.
LDH levels were measured in the supernatants of RPTECs cultured under normoxic or hypoxic conditions, with or without HIF1α knockdown, and with or without polyIC treatment (Figure 6H). Under normoxic conditions with no polyIC treatment, LDH levels in the culture supernatant did not differ whether HIF1α was knocked down or not. However, under hypoxic conditions, HIF1α knockdown led to a significant increase in LDH levels. In contrast, HIF1α knockdown with polyIC treatment led to a significant increase in LDH levels under both normoxic and hypoxic conditions. Thus, HIF1α knockdown enhanced cytotoxicity under polyIC treatment or hypoxic conditions, and polyIC treatment under hypoxic conditions enhanced cytotoxicity even more.
Discussion
In this study, we evaluated the effects of hypoxia on the immune response mediated by RLRs of RPTECs. We treated RPTECs with polyIC and observed an increase in RIG-I and MDA5 as RLRs, and IFNβ, IL6, and TNFα as inflammatory cytokines using qRT-PCR and WB. The percentage of increase in RLRs and inflammatory cytokines was significantly reduced during polyIC treatment under hypoxic conditions compared with that under normoxic conditions, which constitute the main findings of this study. Similar results were obtained when the HIF stabilizer CoCl2 was used instead of hypoxia. Furthermore, immunofluorescence staining showed HIF1α activation in the nuclei of RPTECs treated with hypoxia and CoCl2, following which we evaluated the effects of HIF1α activation on RLRs and inflammatory cytokines using HIF1α siRNA. Accordingly, knockdown of HIF1α led to a significant increase in the expression of RLRs and inflammatory cytokines by polyIC treatment. Therefore, this indicated that the effects of hypoxia on RPTECs were primarily mediated by HIF1α. Although we did not identify the detailed mechanism in this study, RIG-I and MDA5 as well as their downstream antiviral signals induce activation of the transcription factors interferon regulatory factor 3 (IRF3) and NF-κB (3). HIF1α has been reported to suppress IRF3 transcription and NF-κB; however, the results obtained in the experiments are considered to be influenced by these factors (15,16).
The present study also reported two novel findings. First, immunofluorescence staining revealed that polyIC treatment weakly stained HIF1α in nuclei, indicating that polyIC treatment possibly activates HIF1α in RPTECs even under normoxic conditions. Since the local hypoxic environment in the infected tissues enhances the immune cells’ activity at the infection site via HIF1α, HIF is also known to be activated by inflammation (12). Although activation of HIF1α by IL-1β stimulation has been reported in RPTECs (17), there are no reports indicating that polyIC-induced inflammation activates HIF1α in RPTECs. The activation of HIF1α by polyIC stimulation needs to be quantitatively evaluated in the future. Second, HIF1α knockdown enhanced RLRs protein levels without increasing inflammatory cytokine levels, even without polyIC treatment. These observations imply that decreasing HIF1α levels may increase the expression of innate immune receptors in the noninfectious state without explicitly evoking an inflammatory response, although previous studies have not clarified the specific mechanism.
Although no cytotoxicity was observed after polyIC treatment in normoxic conditions, HIF1α knockdown increased cytotoxicity following polyIC treatment. Furthermore, a further increase in cytotoxicity was observed after polyIC treatment in hypoxic conditions. This finding suggests that insufficient activation of HIF1α leads to cytotoxicity. Moreover, the level of HIF1α activation and the intensity of cytotoxicity were found to be related. Regarding qualitative assessment, fluorescent immunostaining revealed that HIF1α staining was stronger in hypoxia than in the polyIC treatment condition, suggesting a higher level of HIF1α activation. Moreover, cytotoxicity was highest under hypoxic conditions with polyIC treatment, indicating that insufficient HIF1α activation is more cytotoxic, despite the required higher level of HIF1α activation.
To summarize, HIF1α activation suppressed the expression of RIG-I, MDA5, and inflammatory cytokines in polyIC treatment, whereas insufficient HIF1α activation increased the expression of RIG-I, MDA5, and inflammatory cytokines, leading to cytotoxicity. HIF activation suppressed excessive inflammation leading to cytotoxicity by antiviral responses, which may have resulted in cellular protection. Hypoxia is an important factor involved in the progression of CKD. Remarkably, in CKD, oxidative stress and uremic agents disrupt HIF1α activation (18,19); in addition, the tubular interstitium becomes relatively hypoxic. This study suggests that in CKD, relative HIF1α deficiency causes tubular epithelial cell damage and promotes inflammation during complications of viral interstitial nephritis, thereby exacerbating cell damage and contributing to tissue and organ damage.
The limitation of our study is that the TLR3-dependent signaling cascade and the effects of hypoxia on TLR signaling, TLR-RLR interaction, and RIG-I-MDA5 interaction were not examined. Further studies investigating the roles of TLR3, RIG-I, and MDA5 in RPTECs are needed. The activation of HIF1α by polyIC stimulation has only been evaluated qualitatively, but quantitative evaluation is needed. HIF-PHD inhibitors are newly available for the treatment of renal anemia in CKD, and the effect of HIF activation in tubular epithelial cells by these drugs on innate immune responses is of interest and a subject for future research.
In conclusion, hypoxia suppressed RIG-I and MDA5 expression in response to polyIC in cultured differentiated human RPTECs and reduced inflammatory cytokine expression. These were due to hypoxia-induced suppression of the innate immune response via HIF1α activation. This indicates that HIF1α may be involved in the immunological response of proximal tubular cells.
Conflicts of Interest
The Authors have no conflicts of interest directly relevant to the content of this article.
Authors’ Contributions
D.N. and M.S conceived of the presented idea. M.N., I.N., T.F. and R.M. carried out the experiments and performed the analytic calculations. N.N. and H.T. helped supervise the project. D.N. and M.S. wrote the manuscript. All Authors approved the final manuscript.
References
- 1.Rosenberger C, Mandriota S, Jürgensen JS, Wiesener MS, Hörstrup JH, Frei U, Ratcliffe PJ, Maxwell PH, Bachmann S, Eckardt K. Expression of Hypoxia-Inducible Factor-1α and -2α in Hypoxic and Ischemic Rat Kidneys. J Am Soc Nephrol. 2002;13(7):1721–1732. doi: 10.1097/01.asn.0000017223.49823.2a. [DOI] [PubMed] [Google Scholar]
- 2.El Awad B, Kreft B, Wolber E, Hellwig-Bürgel T, Metzen E, Fandrey J, Jelkmann W. Hypoxia and interleukin-1β stimulate vascular endothelial growth factor production in human proximal tubular cells. Kidney Int. 2000;58(1):43–50. doi: 10.1046/j.1523-1755.2000.00139.x. [DOI] [PubMed] [Google Scholar]
- 3.Bowie AG, Unterholzner L. Viral evasion and subversion of pattern-recognition receptor signalling. Nat Rev Immunol. 2008;8(12):911–922. doi: 10.1038/nri2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boothpur R, Brennan DC. Human polyoma viruses and disease with emphasis on clinical BK and JC. J Clin Virol. 2010;47(4):306–312. doi: 10.1016/j.jcv.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Low J, Humes H, Szczypka M, Imperiale M. BKV and SV40 infection of human kidney tubular epithelial cells in vitro. Virology. 2004;323(2):182–188. doi: 10.1016/j.virol.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 6.Heutinck KM, Rowshani AT, Kassies J, Claessen N, Van Donselaar-van der Pant KA, Bemelman FJ, Eldering E, Van Lier RA, Florquin S, Ten Berge IJ, Hamann J. Viral double-stranded RNA sensors induce antiviral, pro-inflammatory, and pro-apoptotic responses in human renal tubular epithelial cells. Kidney Int. 2012;82(6):664–675. doi: 10.1038/ki.2012.206. [DOI] [PubMed] [Google Scholar]
- 7.Karikó K, Ni H, Capodici J, Lamphier M, Weissman D. mRNA Is an Endogenous Ligand for Toll-like Receptor 3. J Biol Chem. 2004;279(13):12542–12550. doi: 10.1074/jbc.m310175200. [DOI] [PubMed] [Google Scholar]
- 8.Perrot I, Deauvieau F, Massacrier C, Hughes N, Garrone P, Durand I, Demaria O, Viaud N, Gauthier L, Blery M, Bonnefoy-Berard N, Morel Y, Tschopp J, Alexopoulou L, Trinchieri G, Paturel C, Caux C. TLR3 and Rig-like receptor on myeloid dendritic cells and Rig-like receptor on human NK cells are both mandatory for production of IFN-gamma in response to double-stranded RNA. J Immunol. 2010;185(4):2080–2088. doi: 10.4049/jimmunol.1000532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heutinck KM, Kassies J, Florquin S, Ten Berge IJ, Hamann J, Rowshani AT. SerpinB9 expression in human renal tubular epithelial cells is induced by triggering of the viral dsRNA sensors TLR3, MDA5 and RIG-I. Nephrol Dial Transplant. 2012;27(7):2746–2754. doi: 10.1093/ndt/gfr690. [DOI] [PubMed] [Google Scholar]
- 10.Peyssonnaux C, Datta V, Cramer T, Doedens A, Theodorakis EA, Gallo RL, Hurtado-Ziola N, Nizet V, Johnson RS. HIF-1alpha expression regulates the bactericidal capacity of phagocytes. J Clin Invest. 2005;115(7):1806–1815. doi: 10.1172/JCI23865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenberger C, Khamaisi M, Abassi Z, Shilo V, Weksler-zangen S, Goldfarb M, Shina A, Zibertrest F, Eckardt K, Rosen S, Heyman S. Adaptation to hypoxia in the diabetic rat kidney. Kidney Int. 2008;73(1):34–42. doi: 10.1038/sj.ki.5002567. [DOI] [PubMed] [Google Scholar]
- 12.Eltzschig HK, Carmeliet P. Hypoxia and inflammation. N Engl J Med. 2011;364(7):656–665. doi: 10.1056/NEJMra0910283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Polke M, Seiler F, Lepper PM, Kamyschnikow A, Langer F, Monz D, Herr C, Bals R, Beisswenger C. Hypoxia and the hypoxia-regulated transcription factor HIF-1α suppress the host defence of airway epithelial cells. Innate Immun. 2017;23(4):373–380. doi: 10.1177/1753425917698032. [DOI] [PubMed] [Google Scholar]
- 14.Nangaku M. Chronic Hypoxia and Tubulointerstitial Injury. J Am Soc Nephrol. 2006;17(1):17–25. doi: 10.1681/ASN.2005070757. [DOI] [PubMed] [Google Scholar]
- 15.Miar A, Arnaiz E, Bridges E, Beedie S, Cribbs AP, Downes DJ, Beagrie RA, Rehwinkel J, Harris AL. Hypoxia Induces Transcriptional and Translational Downregulation of the Type I IFN Pathway in Multiple Cancer Cell Types. Cancer Res. 2020;80(23):5245–5256. doi: 10.1158/0008-5472.CAN-19-2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peng T, Du SY, Son M, Diamond B. HIF-1α is a negative regulator of interferon regulatory factors: Implications for interferon production by hypoxic monocytes. Proc Natl Acad Sci U.S.A. 2021;118(26):e2106017118. doi: 10.1073/pnas.2106017118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wen H, Ting J. Agitation by suffocation: How hypoxia activates innate immunity via the Warburg effect. Cell Metabol. 2013;17(6):814–815. doi: 10.1016/j.cmet.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 18.Ceradini DJ, Yao D, Grogan RH, Callaghan MJ, Edelstein D, Brownlee M, Gurtner GC. Decreasing intracellular superoxide corrects defective ischemia-induced new vessel formation in diabetic mice. J Biol Chem. 2008;283(16):10930–10938. doi: 10.1074/jbc.M707451200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Y, Chang F, Wu C, Chou Y, Hsu H, Chiang W, Shen J, Chen Y, Wu K, Tsai T, Duffield JS, Lin S. Platelet-derived growth factor receptor signaling activates pericyte–myofibroblast transition in obstructive and post-ischemic kidney fibrosis. Kidney Int. 2011;80(11):1170–1181. doi: 10.1038/ki.2011.208. [DOI] [PubMed] [Google Scholar]